Clinically Explainable Prediction of Immunotherapy Response Integrating Radiomics and Clinico-Pathological Information in Non-Small Cell Lung Cancer †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Study Design
2.2.1. Feature Extraction from CT
2.2.2. Feature Selection
2.2.3. Model Training and Validation
2.2.4. Bayesian Network Structure Learning (BNSL)
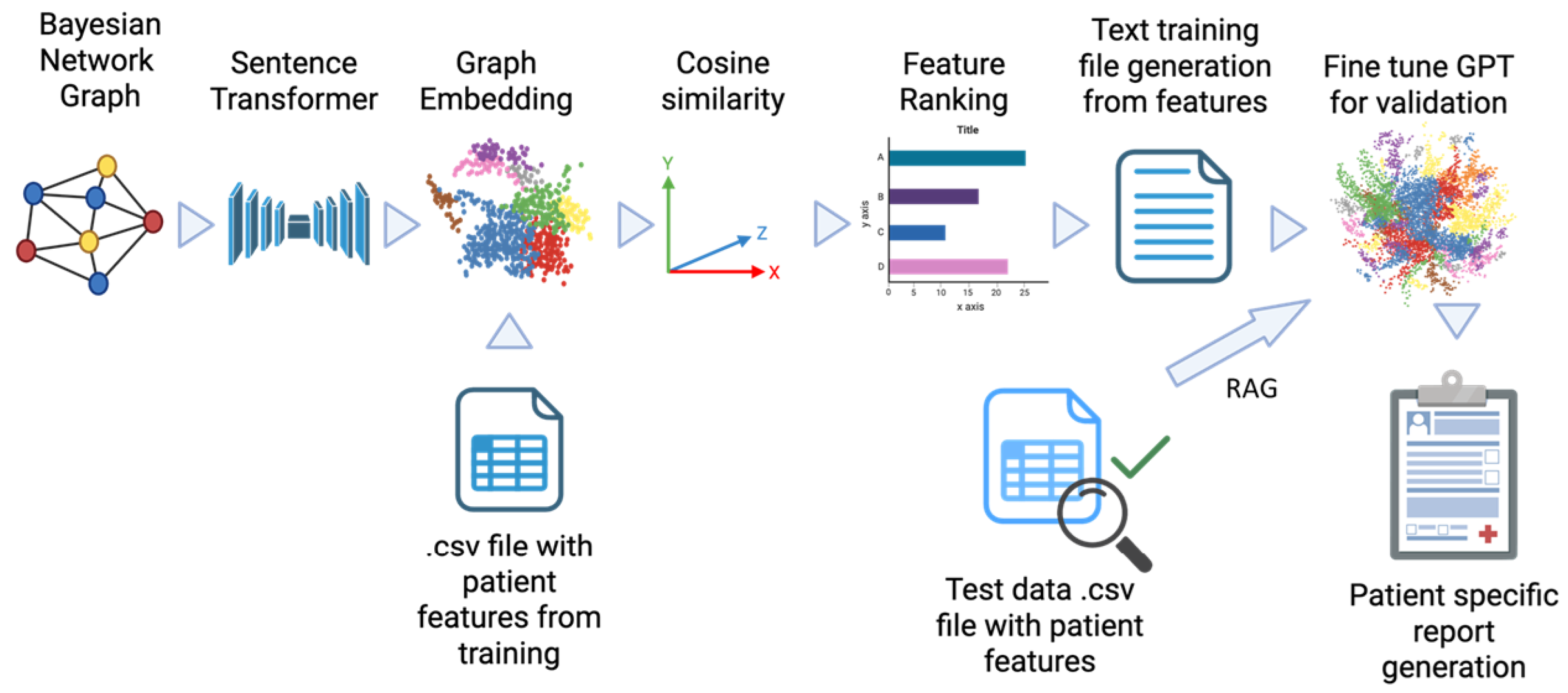
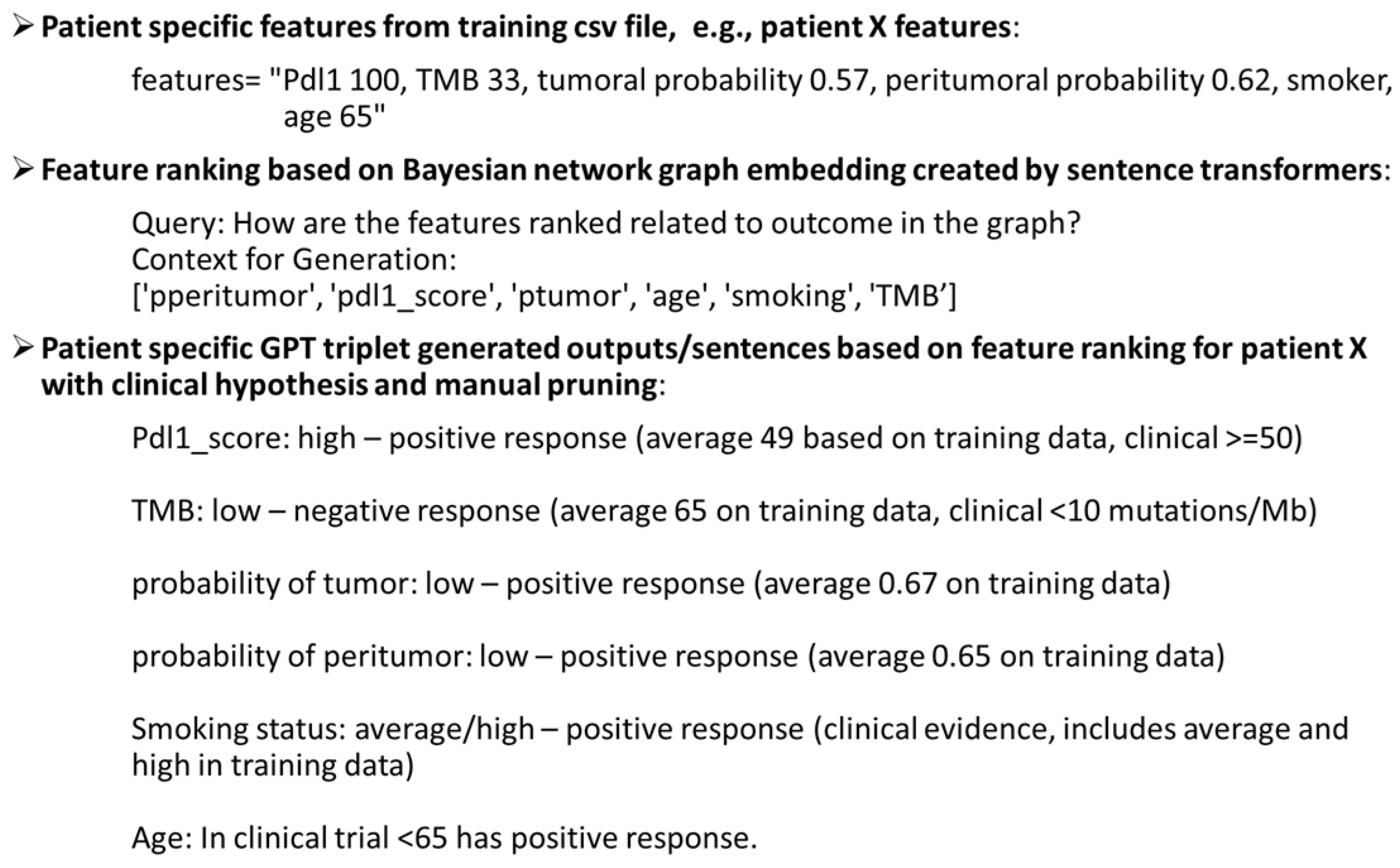
2.2.5. Bayesian Graph LLM (BgLLM) for Clinically-Explainable Response Prediction (GenAI)
3. Results
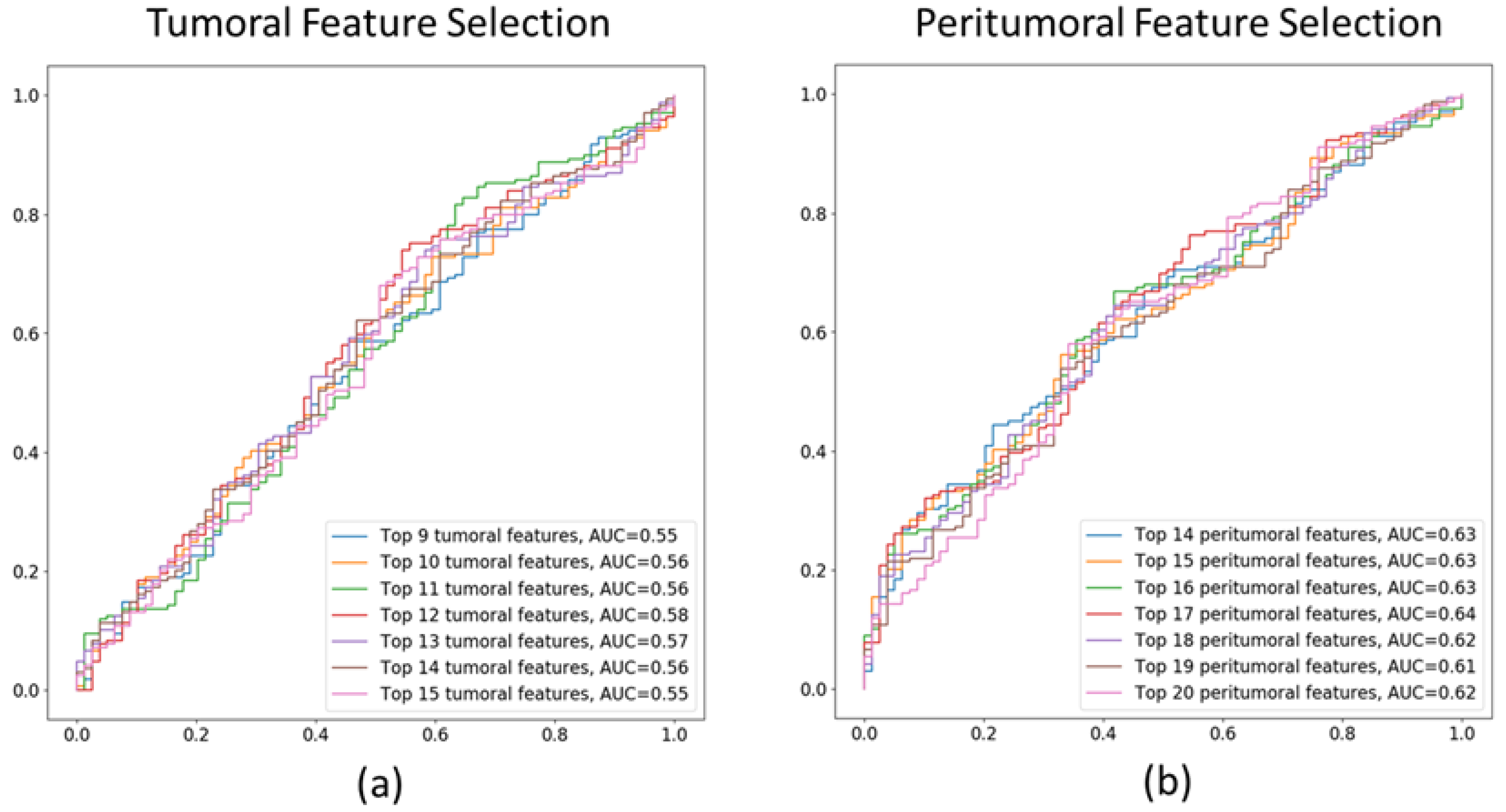
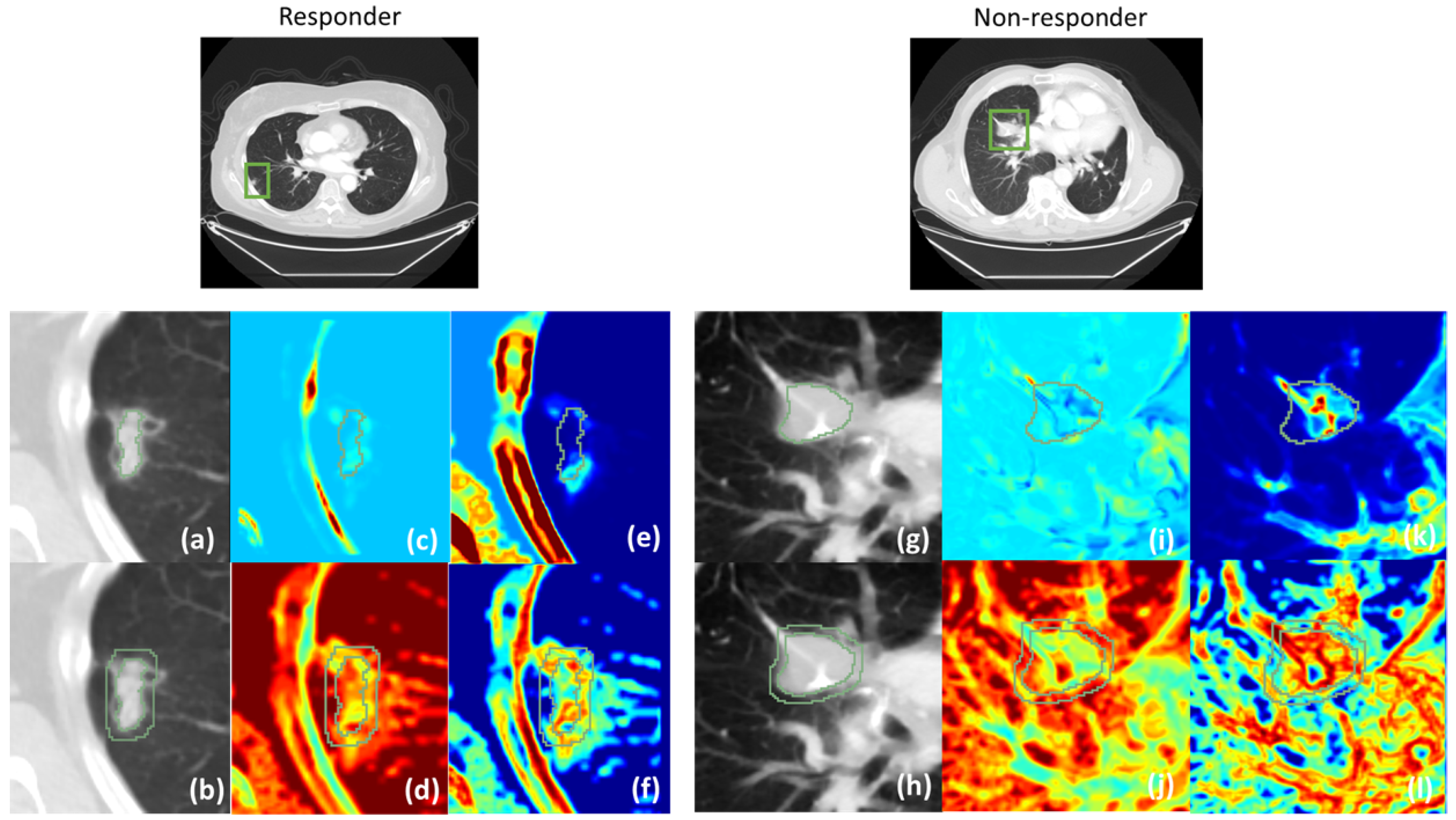
3.1. Tumoral and Peritumoral Radiomics Texture Features
3.2. Performance of ML Models in Predicting Treatment Response
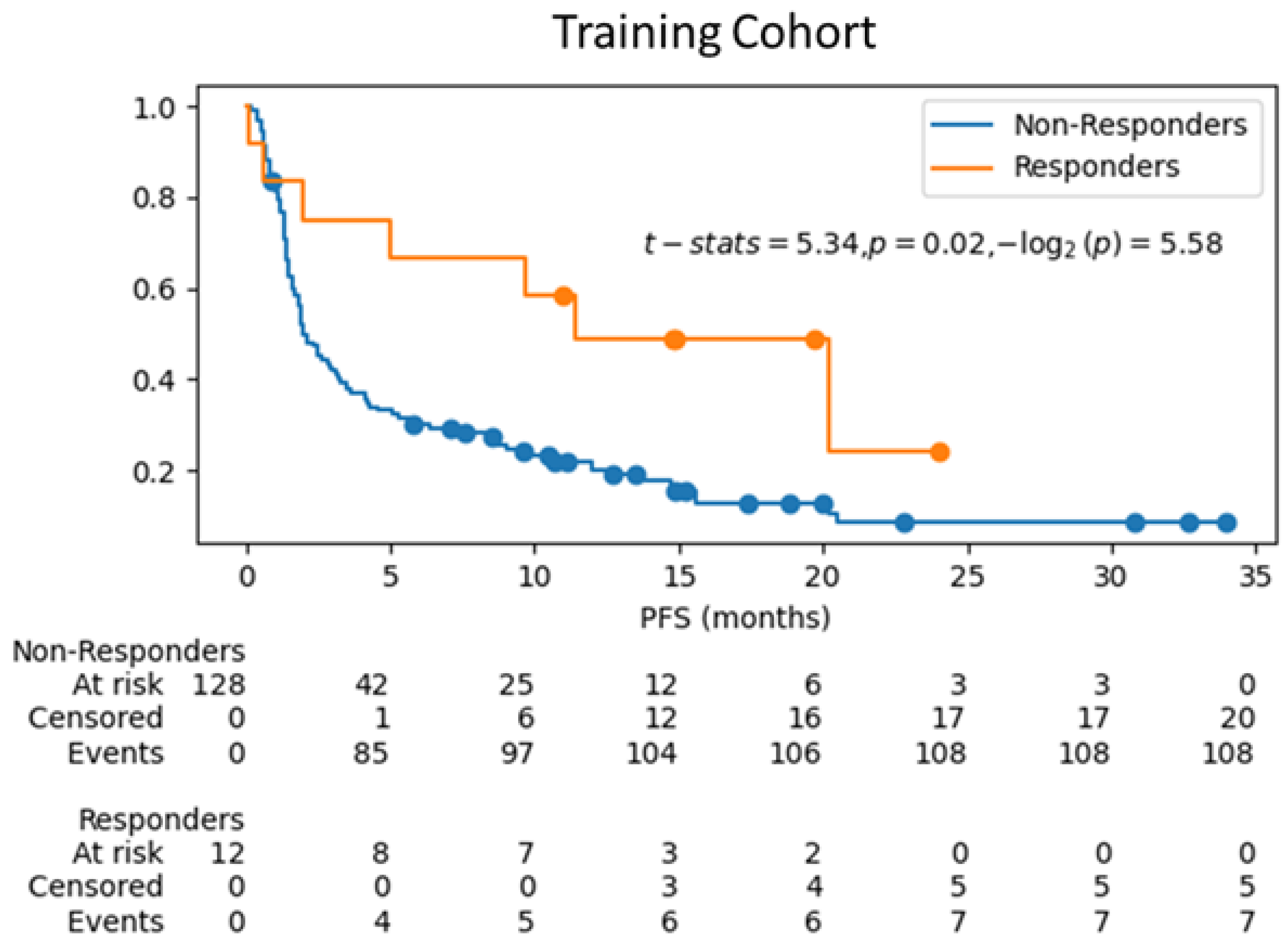
3.3. Survival Analysis on Test Cohort
3.4. LLM-Based Clinical Explanation of Treatment Response Prediction
4. Discussions
4.1. Comparison with Prior Studies
4.2. Interpretability
4.3. Limitations
4.4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mamdani, H.; Matosevic, S.; Khalid, A.; Durm, G.; Jalal, S. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Karachaliou, N.; Fernandez-Bruno, M.; Bracht, J.W.P.; Rosell, R. Challenges and unanswered questions for the next decade of immune-oncology research in NSCLC. Transl. Lung Cancer Res. 2018, 7, 691–702. [Google Scholar] [CrossRef]
- Mino-Kenudson, M.; Schalper, K.; Cooper, W.; Dacic, S.; Hirsch, F.R.; Jain, D.; Lopez-Rios, F.; Tsao, M.S.; Yatabe, Y.; Beasley, M.B.; et al. Predictive Biomarkers for Immunotherapy in Lung Cancer: Perspective From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2022, 17, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Ready, N.; Hellmann, M.D.; Awad, M.M.; Otterson, G.A.; Gutierrez, M.; Gainor, J.F.; Borghaei, H.; Jolivet, J.; Horn, L.; Mates, M.; et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): Outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J. Clin. Oncol. 2019, 37, 992–1000. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Lantuejoul, S.; Sound-Tsao, M.; Cooper, W.A.; Girard, N.; Hirsch, F.R.; Roden, A.C.; Lopez-Rios, F.; Jain, D.; Chou, T.Y.; Motoi, N.; et al. PD-L1 testing for lung cancer in 2019: Perspective from the IASLC pathology committee. J. Thorac. Oncol. 2020, 15, 499–519. [Google Scholar] [CrossRef]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: A perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L. The Potential of Radiomic-Based Phenotyping in Precision Medicine: A Review. JAMA Oncol. 2016, 2, 1636–1642. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef]
- Khorrami, M.; Bera, K.; Leo, P.; Vaidya, P.; Patil, P.; Thawani, R.; Velu, P.; Rajiah, P.; Alilou, M.; Choi, H.; et al. Stable and discriminating radiomic predictor of recurrence in early stage non-small cell lung cancer: Multi-site study. Lung Cancer 2020, 142, 90–97. [Google Scholar] [CrossRef]
- Wang, L.; Dong, T.; Xin, B.; Xu, C.; Guo, M.; Zhang, H.; Feng, D.; Wang, X.; Yu, J. Integrative nomogram of CT imaging, clinical, and hematological features for survival prediction of patients with locally advanced non-small cell lung cancer. Eur Radiol. 2019, 29, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, M.; van Amsterdam, W.A.; van Lindert, A.S.; de Jong, P.A.; Verhoeff, J.J. Prognostic factors for overall survival of stage III non-small cell lung cancer patients on computed tomography: A systematic review and meta-analysis. Radiother Oncol. 2020, 151, 152–175. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, M.; Prasanna, P.; Gupta, A.; Patil, P.; Velu, P.D.; Thawani, R.; Corredor, G.; Alilou, M.; Bera, K.; Fu, P.; et al. Changes in CT Radiomic Features Associated with Lymphocyte Distribution Predict Overall Survival and Response to Immunotherapy in Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lou, X.; Kong, N.; Xu, M.; Gao, C. Can quantitative peritumoral CT radiomics features predict the prognosis of patients with non-small cell lung cancer? A systematic review. Eur. Radiol. 2023, 33, 2105–2117. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Chen, Y.M.; Wu, Y.T.; Chao, H.S.; Chiu, H.Y.; Wang, T.W.; Chen, J.R.; Shiao, T.H.; Luo, C.F. Personalized prediction of immunotherapy response in lung cancer patients using advanced radiomics and deep learning. Cancer Imaging 2024, 24, 129. [Google Scholar] [CrossRef]
- Janzen, I.; Ho, C.; Melosky, B.; Ye, Q.; Li, J.; Wang, G.; Lam, S.; MacAulay, C.; Yuan, R. Machine Learning and Computed Tomography Radiomics to Predict Disease Progression to Upfront Pembrolizumab Monotherapy in Advanced Non-Small-Cell Lung Cancer: A Pilot Study. Cancers 2024, 17, 58. [Google Scholar] [CrossRef]
- Zhou, F.; Qiao, M.; Zhou, C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol. Immunol. 2021, 18, 279–293. [Google Scholar] [CrossRef]
- Lopez de Rodas, M.; Nagineni, V.; Ravi, A.; Datar, I.J.; Mino-Kenudson, M.; Corredor, G.; Barrera, C.; Behlman, L.; Rimm, D.L.; Herbst, R.S.; et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J. Immunother. Cancer 2022, 10, e004440. [Google Scholar] [CrossRef]
- Cadranel, J.; Sebane, L.; Ferrer, L.; Canellas, A.; Etchepare, G.; Meteye, C.; Gallinato, O.; Fallet, V.; Lacave, R.; Menu, P.; et al. Multimodal machine learning model prediction of “individual” response to immunotherapy in 1L stage IV NSCLC. J. Clin. Oncol. 2022, 40, e21151. [Google Scholar] [CrossRef]
- Ferrer, L.; Nadal, E.; Guidel, F.; Insa, A.; Menu, P.; Casal, J.; Domine, M.; Massuti, B.; Majem, M.; Martinez-Marti, A.; et al. Multimodal prediction of response to neoadjuvant nivolumab and chemotherapy for surgically resectable stage IIIA non–small cell lung cancer. J. Clin. Oncol. 2022, 40, 8542. [Google Scholar] [CrossRef]
- De Zuani, M.; Xue, H.; Park, J.S.; Dentro, S.C.; Seferbekova, Z.; Tessier, J.; Curras-Alonso, S.; Hadjipanayis, A.; Athanasiadis, E.I.; Gerstung, M.; et al. Single-cell and spatial transcriptomics analysis of non-small cell lung cancer. Nat. Commun. 2024, 15, 4388. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, T.; Shukuya, T.; Suzuki, K.; Xu, S.; Nakamura, Y.; Katayama, I.; Shirai, Y.; Matsuda, H.; Fujioka, M.; Miyashita, Y.; et al. Multimodal fully automated predictive model for therapeutic efficacy of first-line cancer immunotherapy based on clinical information and imaging modalities including brain MRI and chest CT images in advanced non-small cell lung cancer. J. Clin. Oncol. 2024, 42, 1555. [Google Scholar] [CrossRef]
- Mitra, J.; Ghose, S.; Thawani, R. A multimodal analysis of CT radiomics and clinical variables in predicting immunotherapy response for NSCLC. J. Clin. Oncol. 2025, 43, e20566. [Google Scholar] [CrossRef]
- Ye, G.; Wu, G.; Qi, Y.; Li, K.; Wang, M.; Zhang, C.; Li, F.; Wee, L.; Dekker, A.; Han, C.; et al. Non-invasive multimodal CT deep learning biomarker to predict pathological complete response of non-small cell lung cancer following neoadjuvant immunochemotherapy: A multicenter study. J. Immunother. Cancer 2024, 12, e009348. [Google Scholar] [CrossRef] [PubMed]
- Rakaee, M.; Tafavvoghi, M.; Ricciuti, B.; Alessi, J.V.; Cortellini, A.; Citarella, F.; Nibid, L.; Perrone, G.; Adib, E.; Fulgenzi, C.A.M.; et al. Deep Learning Model for Predicting Immunotherapy Response in Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2024, 11, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Vanguri, R.S.; Luo, J.; Aukerman, A.T.; Egger, J.V.; Fong, C.J.; Horvat, N.; Pagano, A.; de Arimateia Batista Araujo-Filho, J.; Geneslaw, L.; Rizvi, H.; et al. Multimodal integration of radiology, pathology and genomics for prediction of response to PD-(L)1 blockade in patients with non-small cell lung cancer. Nat. Cancer 2022, 3, 1151–1164. [Google Scholar] [CrossRef]
- Chang, R.; Qi, S.; Zuo, Y.; Yue, Y.; Zhang, X.; Guan, Y.; Qian, W. Predicting chemotherapy response in non-small-cell lung cancer via computed tomography radiomic features: Peritumoral, intratumoral, or combined? Front. Oncol. 2022, 12, 915835. [Google Scholar] [CrossRef]
- Huang, D.; Lin, C.; Jiang, Y.; Xin, E.; Xu, F.; Gan, Y.; Xu, R.; Wang, F.; Zhang, H.; Lou, K.; et al. Radiomics model based on intratumoral and peritumoral features for predicting major pathological response in non-small cell lung cancer receiving neoadjuvant immunochemotherapy. Front. Oncol. 2024, 14, 1348678. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Daly, R.; Shen, Q.; Aitken, S. Learning Bayesian Networks: Approaches and issues. Knowl. Eng. Rev. 2011, 26, 99–157. [Google Scholar] [CrossRef]
- Russell, S.J.; Norvig, P. Artificial Intelligence: A Modern Approach, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003; pp. 111–114. [Google Scholar]
- Buntine, W. Theory refinement on Bayesian networks. In Proceedings of the Uncertainty in Artificial Intelligence, Los Angeles, CA, USA, 13–15 July 1991; pp. 52–60. [Google Scholar]
- Ueno, M. Learning likelihood-equivalence Bayesian networks using an empirical Bayesian approach. Behaviormetrika 2008, 35, 115–135. [Google Scholar] [CrossRef]
- OpenAI; Achiam, J.; Adler, S.; Agarwal, S.; Ahmad, L.; Akkaya, I.; Aleman, F.L.; Almeida, D.; Altenschmidt, J.; Altman, S.; et al. GPT-4 Technical Report. 2024. Available online: http://arxiv.org/abs/2303.08774 (accessed on 12 August 2025).
- Reimers, N.; Gurevych, I. Sentence-BERT: Sentence Embeddings using Siamese BERT-Networks. In Proceedings of the 2019 Conference on Empirical Methods in Natural Language Processing, Hong Kong, China, 3–7 November 2019; Association for Computational Linguistics: Stroudsburg, PA, USA, 2019. [Google Scholar]
- Chen, H.; Zhu, J.; Wang, W.; Zhu, Y.; Xi, L. Triplet-based contrastive method enhances the reasoning ability of large language models. J. Supercomput. 2025, 81, 555. [Google Scholar] [CrossRef]
- Gao, A.K. Vec2Vec: A Compact Neural Network Approach for Transforming Text Embeddings with High Fidelity. arXiv 2023, arXiv:2306.12689. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non–Small Cell Lung Cancers With Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-line Nivolumab in stage IV or recurrent non-small-cell lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Pan, D.; Hu, A.Y.; Antonia, S.J.; Li, C.Y. A gene mutation signature predicting immunotherapy benefits in patients with NSCLC. J. Thorac. Oncol. 2021, 16, 419–427. [Google Scholar] [CrossRef]
- Popat, S.; Liu, S.V.; Scheuer, N.; Gupta, A.; Hsu, G.G.; Ramagopalan, S.V.; Griesinger, F.; Subbiah, V. Association Between Smoking History and Overall Survival in Patients Receiving Pembrolizumab for First-Line Treatment of Advanced Non–Small Cell Lung Cancer. JAMA Netw. Open 2022, 5, e2214046. [Google Scholar] [CrossRef] [PubMed]
- Corke, L.K.; Li, J.J.N.; Leighl, N.B.; Eng, L. Tobacco Use and Response to Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer. Curr. Oncol. 2022, 29, 6260–6276. [Google Scholar] [CrossRef] [PubMed]
- Kanesvaran, R.; Cordoba, R.; Maggiore, R. Immunotherapy in Older Adults With Advanced Cancers: Implications for Clinical Decision-Making and Future Research. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2018; Volume 38, pp. 400–414. [Google Scholar]
- Graves, E.E.; Maity, A.; Le, Q.T. The tumor microenvironment in non-small-cell lung cancer. Semin. Radiat. Oncol. 2010, 20, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.; Nussenbaum, B.; Wang, E.W. A practical guide to understanding Kaplan-Meier curves. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad.-Otolaryngol.-Head Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef]
- Pak, K.; Uno, H.; Kim, D.H.; Tian, L.; Kane, R.C.; Takeuchi, M.; Fu, H.; Claggett, B.; Wei, L.J. Interpretability of Cancer Clinical Trial Results Using Restricted Mean Survival Time as an Alternative to the Hazard Ratio. JAMA Oncol. 2017, 3, 1692–1696. [Google Scholar] [CrossRef]
- Lewis, P.; Perez, E.; Piktus, A.; Petroni, F.; Karpukhin, V.; Goyal, N.; Küttler, H.; Lewis, M.; Yih, W.; Rocktäschel, T.; et al. Retrieval-Augmented Generation for Knowledge-Intensive NLP Tasks. In Proceedings of the Advances in Neural Information Processing Systems, Online, 6–12 December 2020; Volume 33, pp. 9459–9474. [Google Scholar]
- Gao, Q.; Yang, L.; Lu, M.; Jin, R.; Ye, H.; Ma, T. The artificial intelligence and machine learning in lung cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 55. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Bao, X.; Han, Z.; Wang, Z.; Nie, S.; Gu, Y.; Gong, J. Short-term peri- and intra-tumoral CT radiomics to predict immunotherapy response in advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2025, 14, 785–797. [Google Scholar] [CrossRef]
- Wu, S.; Zhan, W.; Liu, L.; Xie, D.; Yao, L.; Yao, H.; Liao, G.; Huang, L.; Zhou, Y.; You, P.; et al. Pretreatment radiomic biomarker for immunotherapy responder prediction in stage IB-IV NSCLC (LCDigital-IO Study): A multicenter retrospective study. J. Immunother. Cancer 2023, 11, e007369. [Google Scholar] [CrossRef]
- Peng, J.; Zou, D.; Zhang, X.; Ma, H.; Han, L.; Yao, B. A novel sub-regional radiomics model to predict immunotherapy response in non-small cell lung carcinoma. J. Transl. Med. 2024, 22, 87. [Google Scholar] [CrossRef]
- Castellanos, E.; Snider, J.; Ali, S.M.; Backenroth, D.; Albacker, L.A.; Murugesan, K.; Li, G.; Frampton, G.M.; Alexander, B.M.; Carson, K.R. Tumor mutational burden (TMB) and PD-L1 expression as predictors of response to immunotherapy (IO) in NSCLC. J. Clin. Oncol. 2019, 37, 2630. [Google Scholar] [CrossRef]
- Kao, C.; Powers, E.; Wu, Y.; Datto, M.B.; Green, M.F.; Strickler, J.H.; Ready, N.E.; Zhang, T.; Clarke, J.M. Predictive Value of Combining Biomarkers for Clinical Outcomes in Advanced Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, 500–509. [Google Scholar] [CrossRef]
- Peisen, F.; Hänsch, A.; Hering, A.; Brendlin, A.S.; Afat, S.; Nikolaou, K.; Gatidis, S.; Eigentler, T.; Amaral, T.; Moltz, J.H.; et al. Combination of Whole-Body Baseline CT Radiomics and Clinical Parameters to Predict Response and Survival in a Stage-IV Melanoma Cohort Undergoing Immunotherapy. Cancers 2022, 14, 2992. [Google Scholar] [CrossRef] [PubMed]
- Farina, B.; Guerra, A.D.R.; Bermejo-Peláez, D.; Miras, C.P.; Peral, A.A.; Madueño, G.G.; Jaime, J.C.; Vilalta-Lacarra, A.; Pérez, J.R.; Muñoz-Barrutia, A.; et al. Integration of longitudinal deep-radiomics and clinical data improves the prediction of durable benefits to anti-PD-1/PD-L1 immunotherapy in advanced NSCLC patients. J. Transl. Med. 2023, 21, 174. [Google Scholar] [CrossRef]
- Yolchuyeva, S.; Giacomazzi, E.; Tonneau, M.; Ebrahimpour, L.; Lamaze, F.C.; Orain, M.; Coulombe, F.; Malo, J.; Belkaid, W.; Routy, B.; et al. A Radiomics-Clinical Model Predicts Overall Survival of Non-Small Cell Lung Cancer Patients Treated with Immunotherapy: A Multicenter Study. Cancers 2023, 15, 3829. [Google Scholar] [CrossRef]

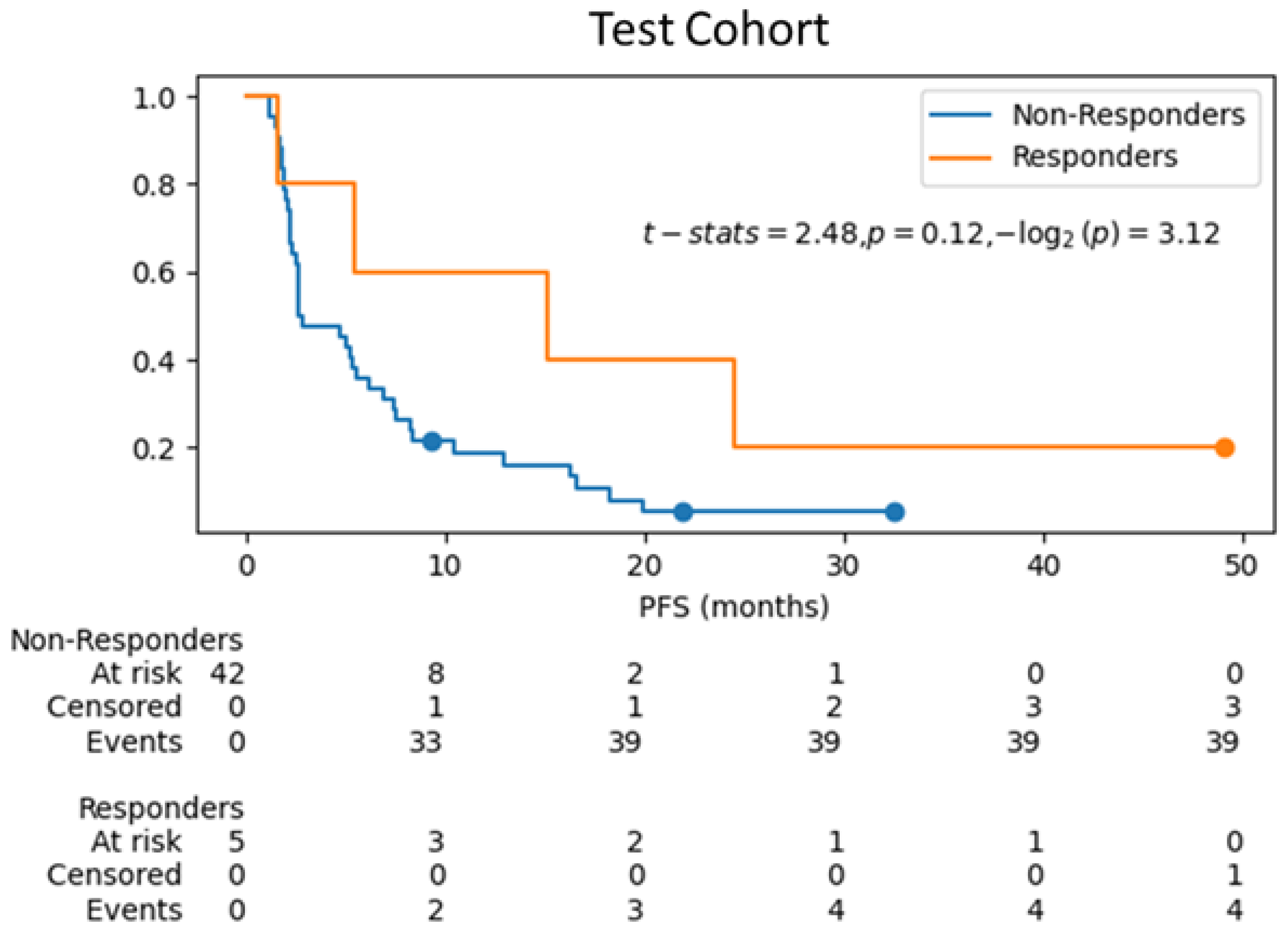
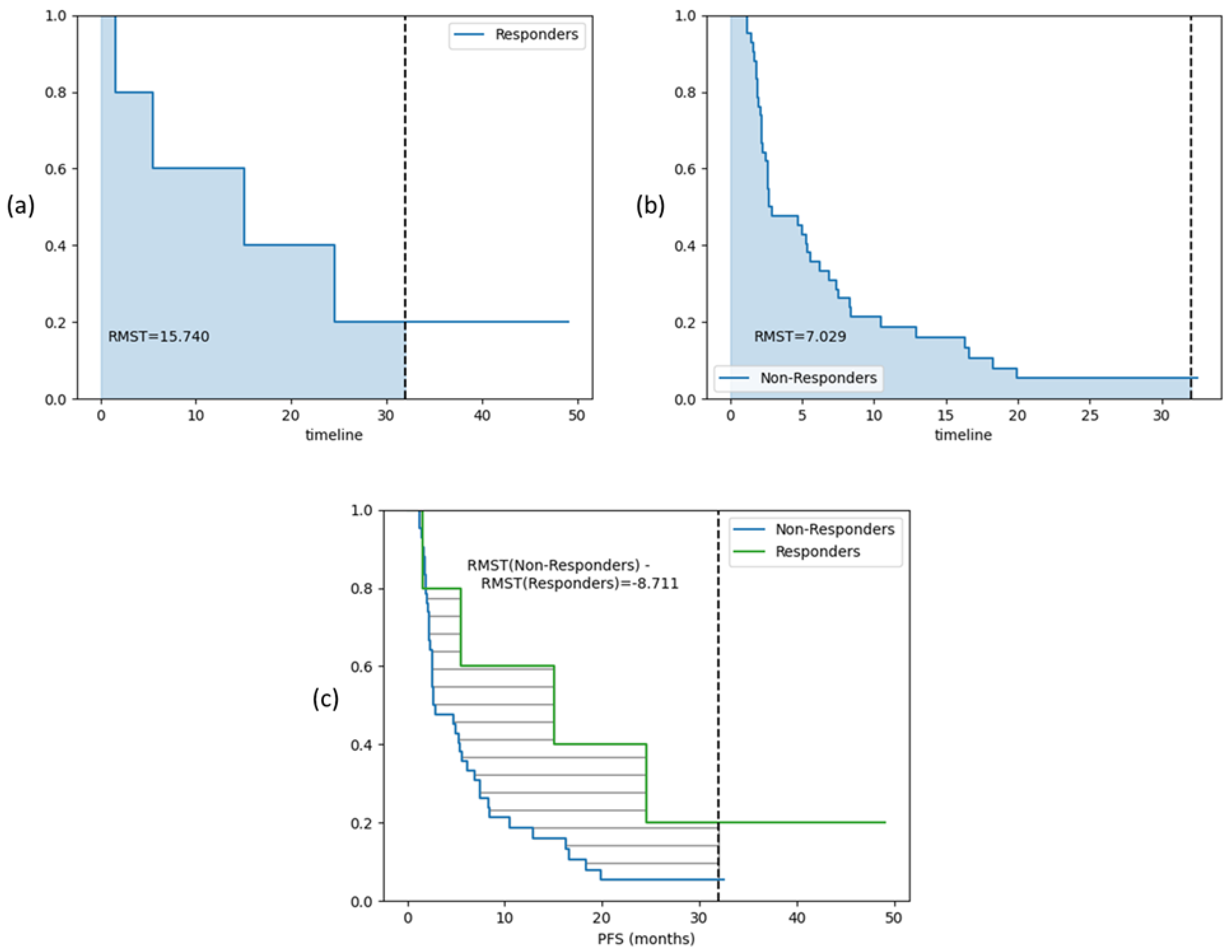
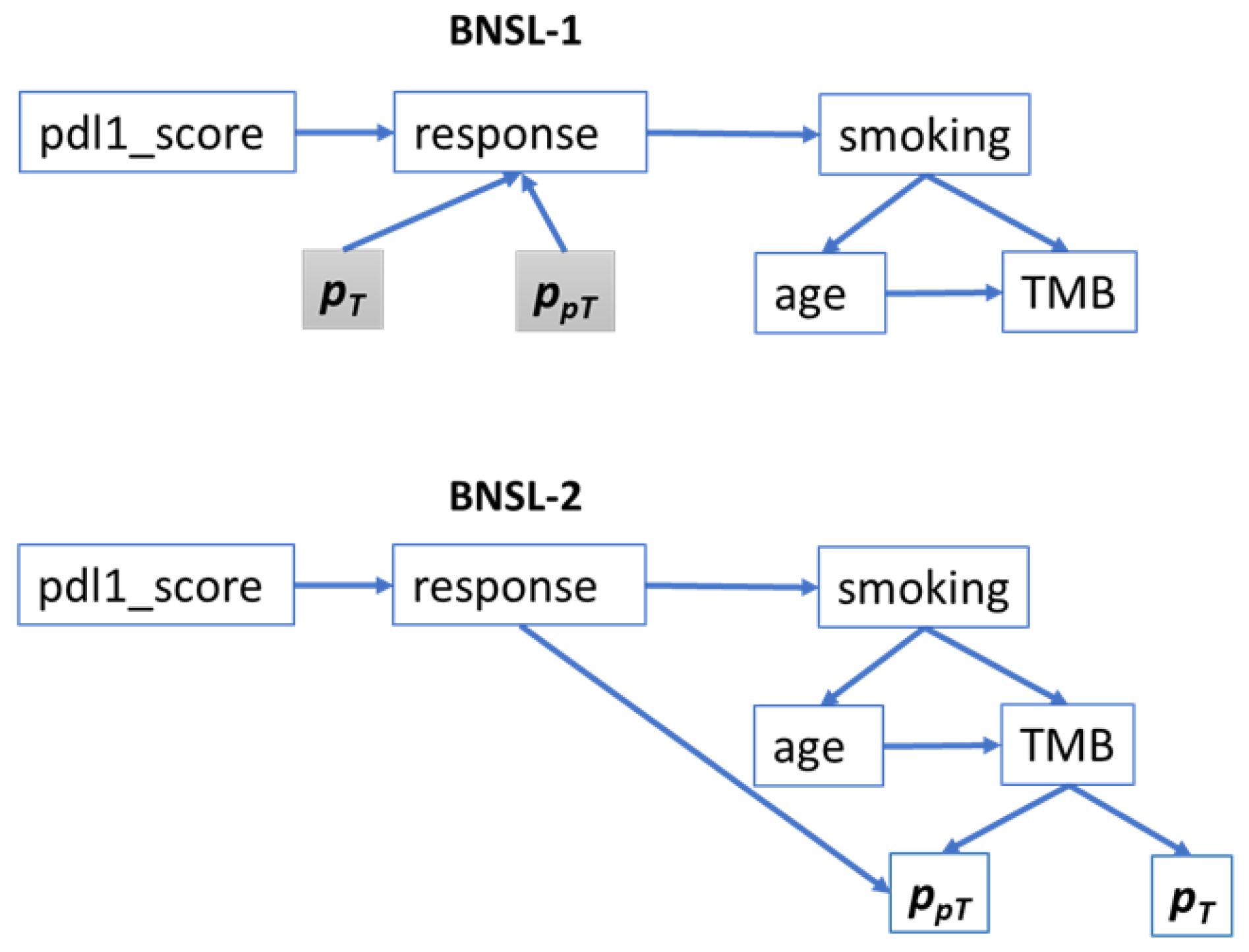
| Characteristics | Training Cohort (n = 140 Patients (%)) (n = 248 Lesions (%)) | Test Cohort (n = 47 Patients (%)) (n = 84 Lesions (%)) |
|---|---|---|
| Sex | ||
| Male | 62 (44%) | 25 (53%) |
| Female | 78 (56%) | 22 (47%) |
| Age in yrs | ||
| Median | 68 | 67 |
| Minimum | 38 | 38 |
| Maximum | 93 | 83 |
| Smoking Status | ||
| Current | 91 (65%) | 29 (62%) |
| Former | 33 (24%) | 11 (23%) |
| Never | 16 (11%) | 7 (15%) |
| PD-L1 Score (%) | ||
| 0 | 71 (51%) | 17 (36%) |
| 1–49 | 25 (18%) | 9 (19%) |
| ≥50 | 44 (31%) | 21 (45%) |
| TMB | ||
| ≥10 mutations/Mb | 139 (99%) | 47 (100%) |
| <10 mutations/Mb | 1 (1%) | 0 (0%) |
| Overall Treatment Response | ||
| Non-responders | 102 (73%) | 34 (72%) |
| Responders | 38 (27%) | 13 (28%) |
| Lesion Type 1 | ||
| Parenchymal | 152 (61%) | 51 (61%) |
| Pleural | 21 (9%) | 11 (13%) |
| Lymph node | 75 (30%) | 22 (26%) |
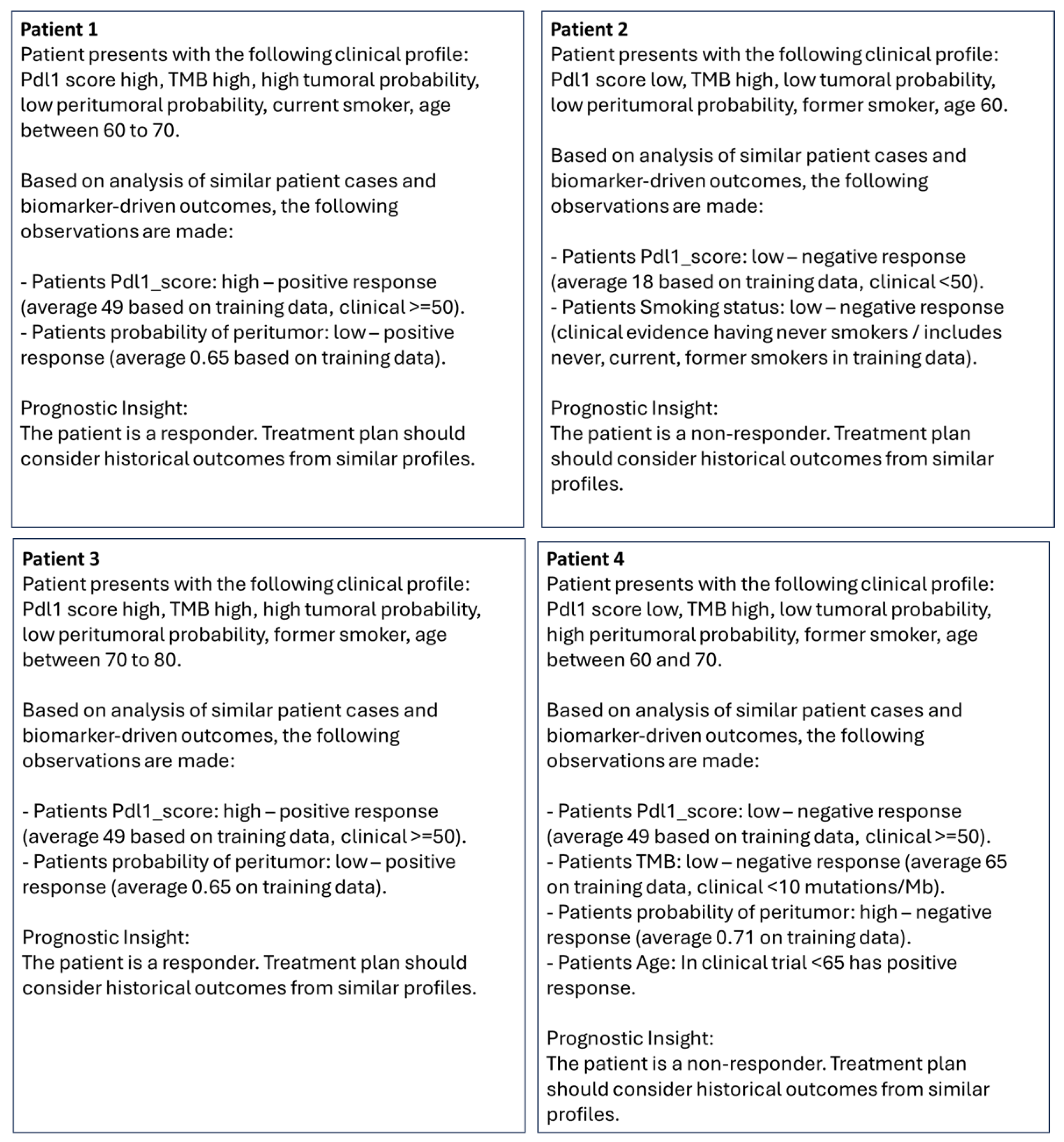
| Pdl1_score: high—positive response (average 49 based on training data, clinical ≥ 50 [41]) |
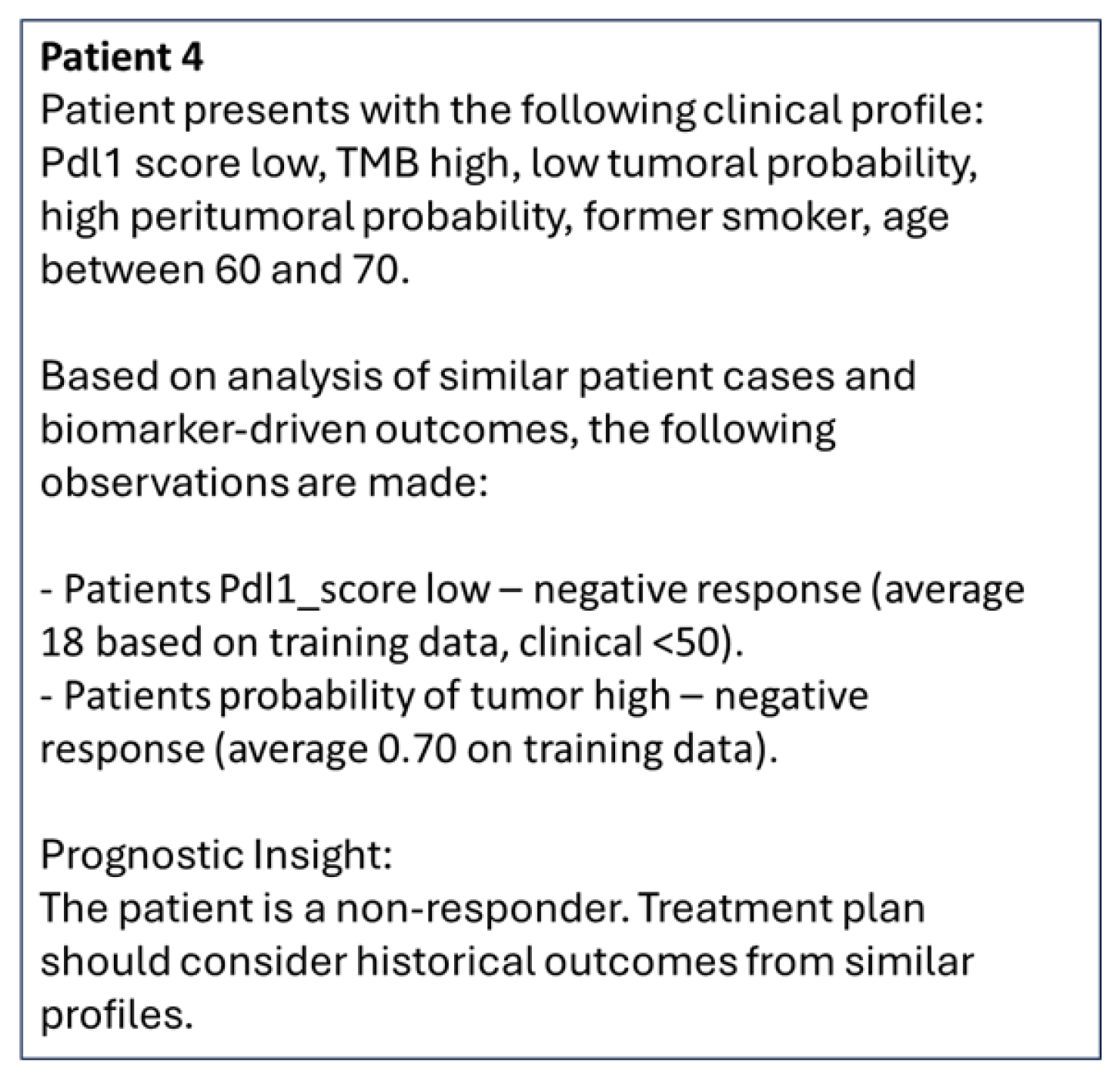
| Pdl1_score: low—negative response (average 18 based on training data, clinical < 50 [41] |
| TMB: high—positive response (average 74 on training data, clinical > 10 mutations/Mb [42,43,44]) |
| TMB: low—negative response (average 65 on training data, clinical < 10 mutations/Mb [42,43,44]) |
| p_tumor: high—negative response (average 0.70 on training data) |
| p_tumor: low—positive response (average 0.67 on training data) |
| p_peritumor: high—negative response (average 0.71 on training data) |
| p_peritumor: low—positive response (average 0.65 on training data) |
| Smoking status: former/current—positive response (clinical evidence with former and current smokers [45,46], includes former and current smokers in training data)) |
| Smoking status: never smoker—negative response (clinical evidence with never smokers [45,46]/includes never, current, former smoker in training data) |
| Age: Average same in training data between responder and non-responder (mean 67), median slightly lower at 67 in responders than non-responders at 68 but <65 has positive response than ≥65 in some clinical evidence [47]. |
| Tumoral Features | Peritumoral Features |
|---|---|
| Gradient_10thPercentile | Entropy_variance |
| Gradient_median | Correlation_median |
| Correlation_skewness | InverseDifferenceMoment_variance |
| Correlation_variance | ClusterShade_mean |
| ClusterShade_mean | Energy_variance |
| Gradient_mean | InverseDifferenceMoment_kurtosis |
| ClusterShade_skewness | HaralickCorrelation_skewness |
| ClusterProminence_90thPercentile | Entropy_kurtosis |
| Gradient_90thPercentile | Entropy_90thPercentile |
| HaralickCorrelation_skewness | Correlation_mean |
| HaralickCorrelation_variance | Gradient_90thPercentile |
| ClusterShade_variance | HaralickCorrelation_mean |
| ClusterProminence_mean | |
| Gradient_10thPercentile | |
| Entropy_median | |
| HaralickCorrelation_10thPercentile | |
| ClusterProminence_variance |
| Method | Validation | AUC | Accuracy | Recall | |
|---|---|---|---|---|---|
| 1 | RF-train-val- | Training | 0.58 | 0.66 | 0.95 |
| 2 | RF-train-val- | Training | 0.64 | 0.69 | 0.95 |
| 3 | RF-train-val-- | Training | 0.60 | 0.66 | 0.95 |
| 4 | RF-train-val-Clin-- | Training | 0.73 | 0.72 | 0.94 |
| 5 | RF-train-val-Clin---Sub | Training | 0.72 | 0.76 | 0.96 |
| 6 | RF- | Test | 0.60 | 0.73 | 1.00 |
| 7 | RF- | Test | 0.62 | 0.75 | 1.00 |
| 8 | RF-Clin | Test | 0.81 | 0.79 | 0.90 |
| 9 | RF-- | Test | 0.58 | 0.73 | 1.00 |
| 10 | RF-Clin-- | Test | 0.67 | 0.75 | 0.98 |
| 11 | RF-Clin- | Test | 0.83 | 0.79 | 0.90 |
| 12 | RF-Clin-- | Test | 0.83 | 0.80 | 0.95 |
| 13 | RF-Clin---Sub | Test | 0.80 | 0.74 | 0.94 |
| 14 | Bayes-Clin---outStruct | Test | 0.70 | 0.73 | 0.77 |
| 15 | Bayes-Clin---inStruct | Test | 0.70 | 0.73 | 0.77 |
| Patient 1 | responder | pdl1_score high | TMB high | high tumoral probability | low peritumoral probability | current smoker | age between 60 and 70 |
| Patient 2 | non responder | pdl1_score low | TMB high | low tumoral probability | low peritumoral probability | former smoker | age 60 |
| Patient 3 | responder | pdl1_score high | TMB high | high tumoral probability | low peritumoral probability | former smoker | age between 70 and 80 |
| Patient 4 | non responder | pdl1_score low | TMB high | low tumoral probability | high peritumoral probability | former smoker | age between 60 and 70 |
| Subjects | ARM ( = 0.01) | ARM ( = 0.05) |
|---|---|---|
| Test subjects (n = 47) | 0.84 ± 0.02 | 0.86 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, J.; Ghose, S.; Thawani, R. Clinically Explainable Prediction of Immunotherapy Response Integrating Radiomics and Clinico-Pathological Information in Non-Small Cell Lung Cancer. Cancers 2025, 17, 2679. https://doi.org/10.3390/cancers17162679
Mitra J, Ghose S, Thawani R. Clinically Explainable Prediction of Immunotherapy Response Integrating Radiomics and Clinico-Pathological Information in Non-Small Cell Lung Cancer. Cancers. 2025; 17(16):2679. https://doi.org/10.3390/cancers17162679
Chicago/Turabian StyleMitra, Jhimli, Soumya Ghose, and Rajat Thawani. 2025. "Clinically Explainable Prediction of Immunotherapy Response Integrating Radiomics and Clinico-Pathological Information in Non-Small Cell Lung Cancer" Cancers 17, no. 16: 2679. https://doi.org/10.3390/cancers17162679
APA StyleMitra, J., Ghose, S., & Thawani, R. (2025). Clinically Explainable Prediction of Immunotherapy Response Integrating Radiomics and Clinico-Pathological Information in Non-Small Cell Lung Cancer. Cancers, 17(16), 2679. https://doi.org/10.3390/cancers17162679







