Maintenance Treatment with 5-Azacitidine in Patients with Acute Myeloblastic Leukemia Ineligible for Intensive Treatment and with Response After Induction Chemotherapy: A Phase II Clinical Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
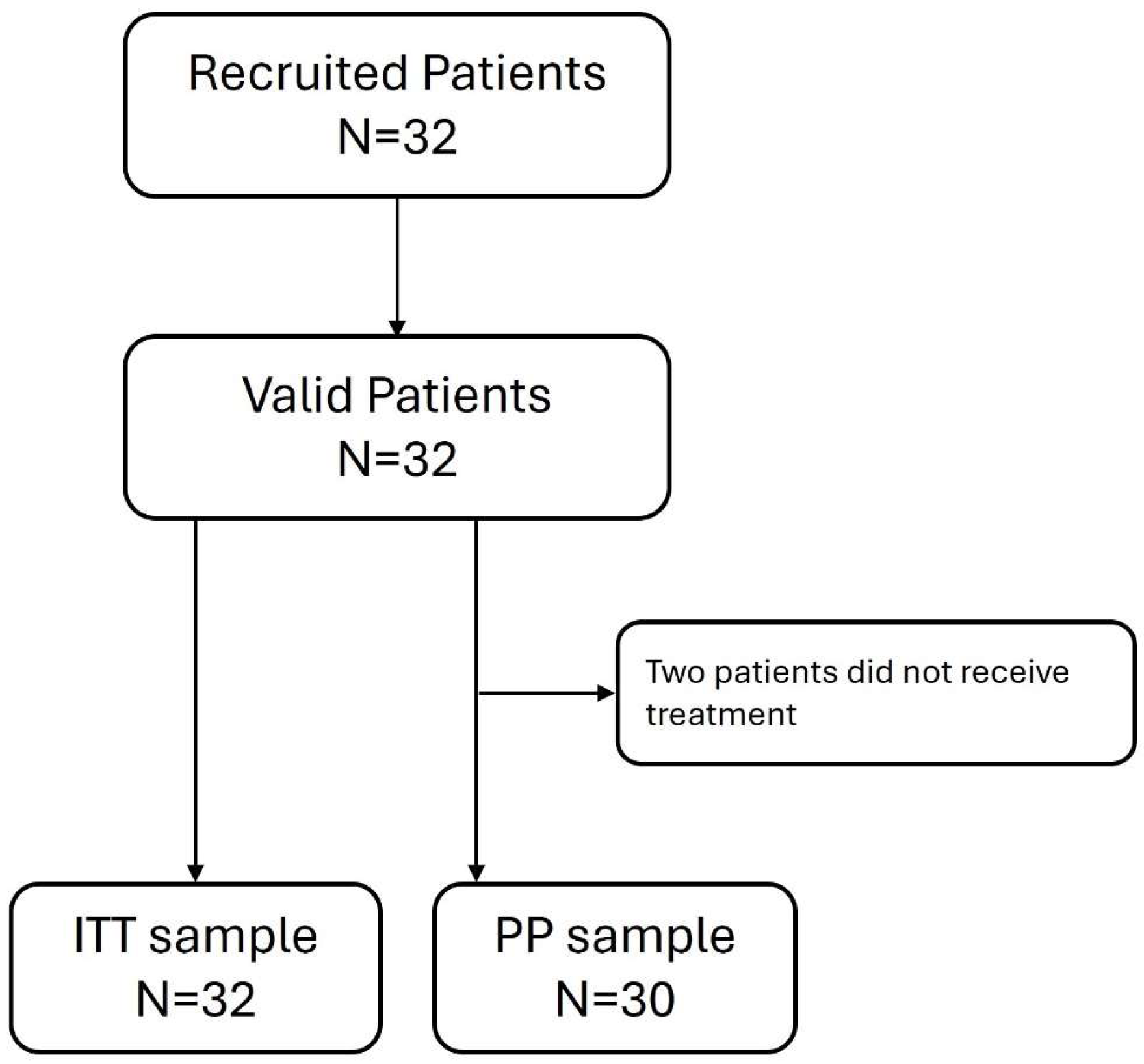
2.1. Study Design and Participants
2.2. Selection Criteria
2.3. Treatment
2.4. Efficacy Endpoints and Variables
2.5. Safety Assessments
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
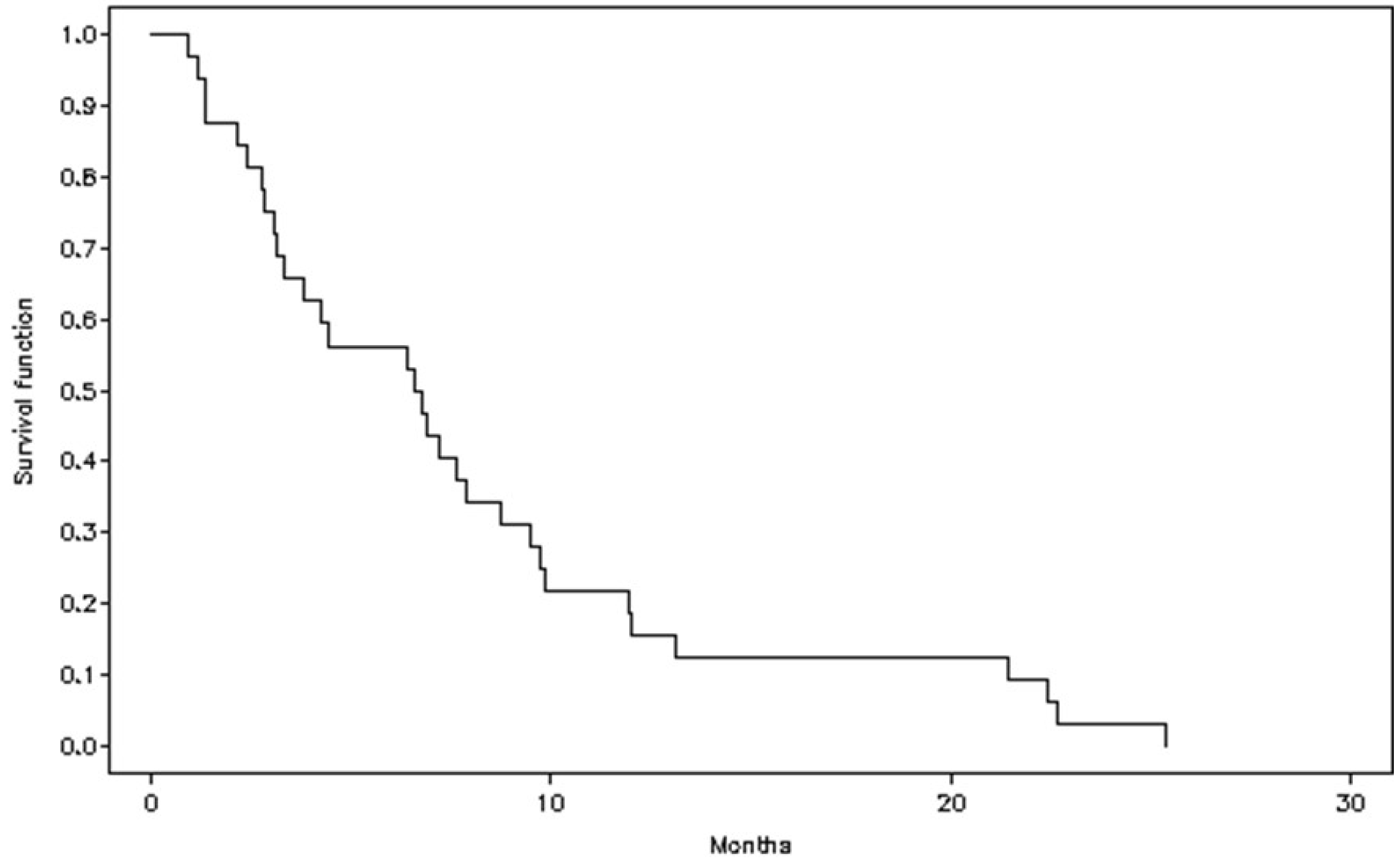
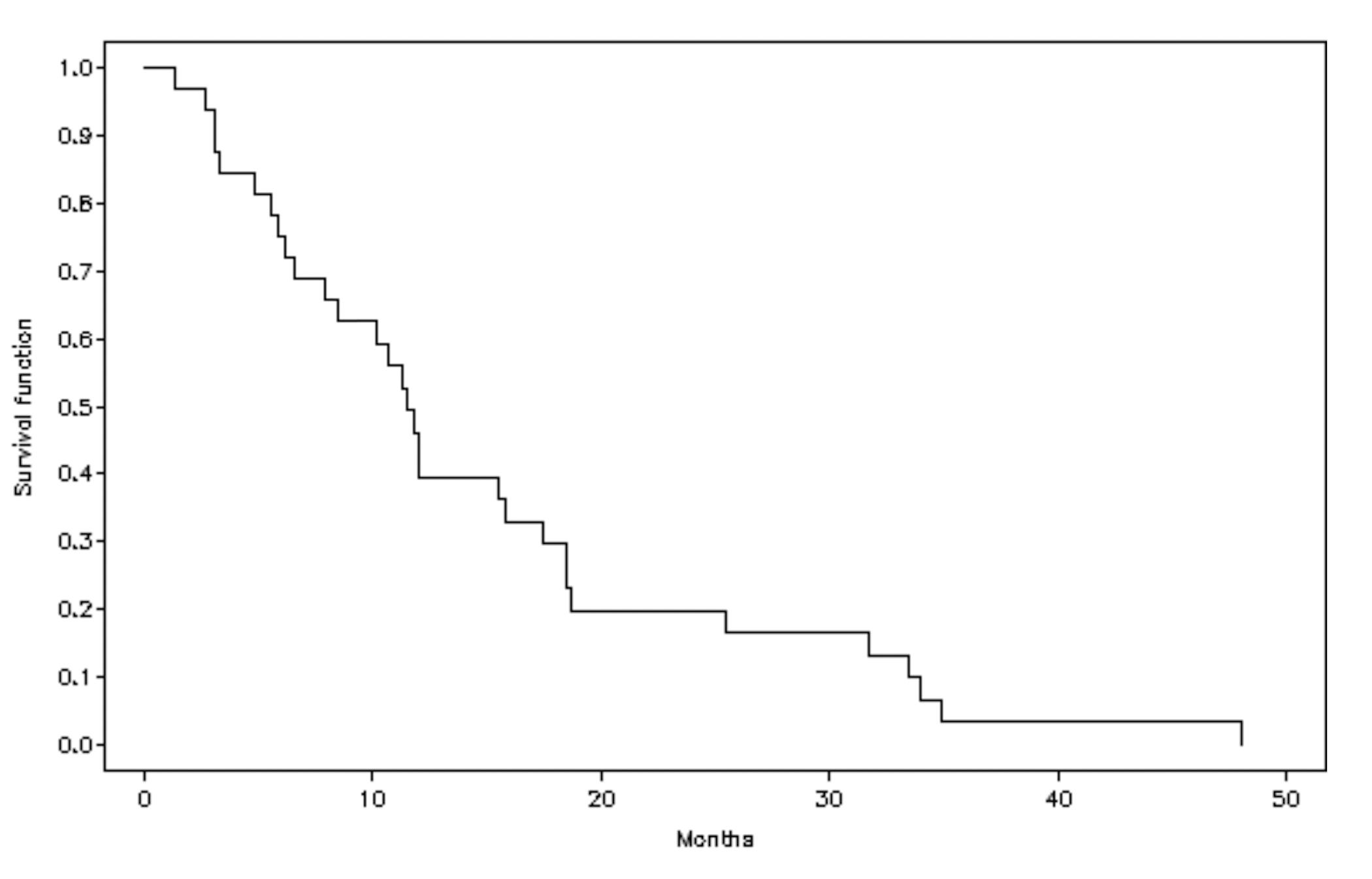
3.2. Primary Efficacy Outcome: Treatment Response
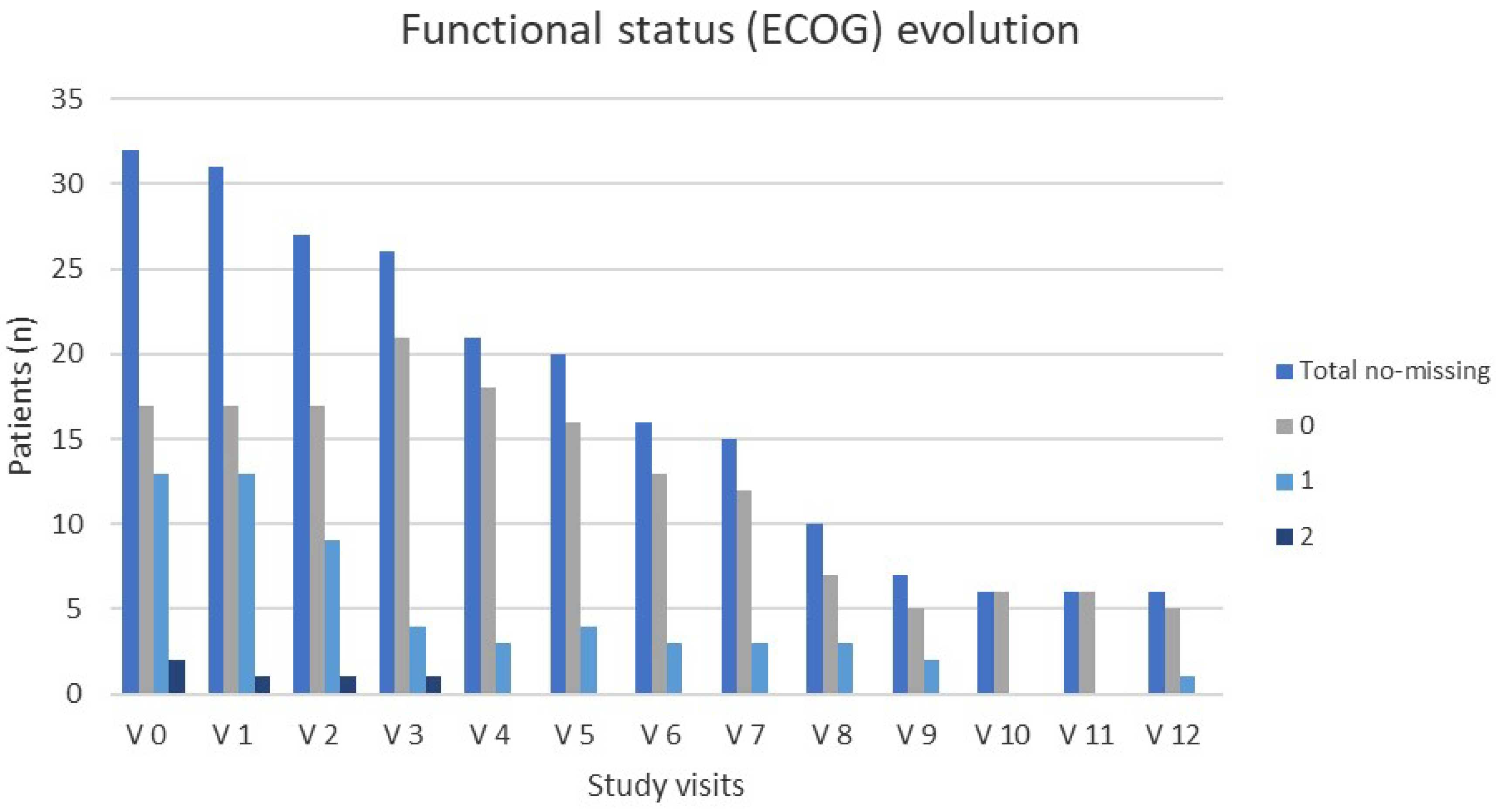
3.3. Impact of AZA Treatment on Quality of Life (QoL)
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| MRD | Minimal residual disease |
| AZA | Azacitidine |
| CR | Complete remission |
| PR | Partial remission |
| DP | Disease progression |
| ECOG | Eastern Cooperative Oncologic Group |
| ULN | Upper limit of normal |
| PFS | Progression-free survival |
| OS | Overall survival |
References
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of Acute Myeloid Leukemia: Recent Progress and Enduring Challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Saif, A.; Kazmi, S.F.A.; Naseem, R.; Shah, H.; Butt, M.O. Acute Myeloid Leukemia: Is That All There Is? Cureus 2018, 10, e3198. [Google Scholar] [CrossRef]
- Yamashita, M.; Dellorusso, P.V.; Olson, O.C.; Passegué, E. Dysregulated Haematopoietic Stem Cell Behaviour in Myeloid Leukaemogenesis. Nat. Rev. Cancer 2020, 20, 365–382. [Google Scholar] [CrossRef]
- Tebbi, C.K. Etiology of Acute Leukemia: A Review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Nachtkamp, K.; Kobbe, G.; Gattermann, N.; Germing, U. Myelodysplastic Syndromes: New Methods of Diagnosis, Prognostication, and Treatment. Dtsch. Ärzteblatt Int. 2023, 120, 203. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.; DiNardo, C.; Borthakur, G.; Short, N.; Jain, N.; Daver, N.; Jabbour, E.; Garcia-Manero, G.; et al. Prediction of Survival with Intensive Chemotherapy in Acute Myeloid Leukemia. Am. J. Hematol. 2022, 97, 865–876. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Boscaro, E.; Urbino, I.; Catania, F.M.; Arrigo, G.; Secreto, C.; Olivi, M.; D’Ardia, S.; Frairia, C.; Giai, V.; Freilone, R.; et al. Modern Risk Stratification of Acute Myeloid Leukemia in 2023: Integrating Established and Emerging Prognostic Factors. Cancers 2023, 15, 3512. [Google Scholar] [CrossRef]
- Alsouqi, A.; Geramita, E.; Im, A. Treatment of Acute Myeloid Leukemia in Older Adults. Cancers 2023, 15, 5409. [Google Scholar] [CrossRef]
- Urbino, I.; Secreto, C.; Olivi, M.; Apolito, V.; D’Ardia, S.; Frairia, C.; Giai, V.; Aydin, S.; Freilone, R.; Dellacasa, C.; et al. Evolving Therapeutic Approaches for Older Patients with Acute Myeloid Leukemia in 2021. Cancers 2021, 13, 5075. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.J.; Estey, E.; Kantarjian, H.M. Adult Acute Myeloid Leukemia. Mayo Clin. Proc. 2006, 81, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Burnett, A.K.; Agura, E.D.; Kantarjian, H.M. Progress in the Treatment of Acute Myeloid Leukemia. Cancer 2007, 110, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Kantarjian, H. When Less Is More: Reevaluating the Role of Intensive Chemotherapy for Older Adults With Acute Myeloid Leukemia in the Modern Era. J. Clin. Oncol. 2021, 39, 3104–3108. [Google Scholar] [CrossRef]
- Senapati, J.; Kadia, T.M.; Ravandi, F. Maintenance Therapy in Acute Myeloid Leukemia: Advances and Controversies. Haematologica 2023, 108, 2289. [Google Scholar] [CrossRef]
- Reville, P.K.; Kadia, T.M. Maintenance Therapy in AML. Front Oncol. 2021, 10, 619085. [Google Scholar] [CrossRef]
- Pleyer, L.; Döhner, H.; Dombret, H.; Seymour, J.F.; Schuh, A.C.; Beach, C.L.; Swern, A.S.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; et al. Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group. Int. J. Mol. Sci. 2017, 18, 415. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef]
- Komrokji, R.S.; Al Ali, N.H.; Sallman, D.; Padron, E.; DeZern, A.E.; Barnard, J.; Roboz, G.J.; Garcia-Manero, G.; List, A.; Steensma, D.P.; et al. Validation of International Working Group Response Criteria in Higher-Risk Myelodysplastic Syndromes: A Report on Behalf of the MDS Clinical Research Consortium. Cancer Med. 2021, 10, 447–453. [Google Scholar] [CrossRef]
- Bjordal, K.; de Graeff, A.; Fayers, P.M.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.J.; Meyza, J.W.; Brédart, A.; et al. A 12 Country Field Study of the EORTC QLQ-C30 (Version 3.0) and the Head and Neck Cancer Specific Module (EORTC QLQ-H&N35) in Head and Neck Patients. EORTC Quality of Life Group. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar] [CrossRef]
- National Caner Institute (NIH). Division of Cancer Treatment & Diagnosis Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 2 August 2025).
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of Azacitidine Compared with That of Conventional Care Regimens in the Treatment of Higher-Risk Myelodysplastic Syndromes: A Randomised, Open-Label, Phase III Study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- EU/1/08/488/001; EMA Vidaza 25 Mg/ML Powder for Suspension for Injection (Annex I-Summary of Product Characteristics). The European Medicines Agency: Amsterdam, The Netherlands, 2013.
- Röllig, C. Improving Long-Term Outcomes with Intensive Induction Chemotherapy for Patients with AML. Hematology 2023, 2023, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Timilshina, N.; Breunis, H.; Brandwein, J.M.; Minden, M.D.; Gupta, V.; O’Neill, S.; Tomlinson, G.A.; Buckstein, R.; Li, M.; Alibhai, S.M.H. Do Quality of Life or Physical Function at Diagnosis Predict Short-Term Outcomes during Intensive Chemotherapy in AML? Ann. Oncol. 2014, 25, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Molica, M.; Breccia, M.; Foa, R.; Jabbour, E.; Kadia, T.M. Maintenance Therapy in AML: The Past, the Present and the Future. Am. J. Hematol. 2019, 94, 1254–1265. [Google Scholar] [CrossRef]
- Huls, G.; Chitu, D.A.; Havelange, V.; Jongen-Lavrencic, M.; van de Loosdrecht, A.A.; Biemond, B.J.; Sinnige, H.; Hodossy, B.; Graux, C.; Kooy, R.v.M.; et al. Azacitidine Maintenance after Intensive Chemotherapy Improves DFS in Older AML Patients. Blood 2019, 133, 1457–1464. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Freeman, S.D.; Kjeldsen, L.; Milligan, D.W.; Pocock, C.F.E.; Cahalin, P.; Kell, J.; Dennis, M.; Hills, R.K. A Comparison of Limited Consolidation Chemotherapy Therapy Or Not, and Demethylation Maintenance Or Not in Older Patients with Aml and High Risk Mds: Long Term Results of the Uk Ncri Aml16 Trial. 2015. Available online: https://library.ehaweb.org/eha/2015/20th/103225/alan.burnett.a.comparison.of.limited.consolidation.chemotherapy.therapy.or.not.html?f=m1 (accessed on 2 August 2025).
- Grövdal, M.; Karimi, M.; Khan, R.; Aggerholm, A.; Antunovic, P.; Astermark, J.; Bernell, P.; Engström, L.-M.; Kjeldsen, L.; Linder, O.; et al. Maintenance Treatment with Azacytidine for Patients with High-Risk Myelodysplastic Syndromes (MDS) or Acute Myeloid Leukaemia Following MDS in Complete Remission after Induction Chemotherapy. Br. J. Haematol. 2010, 150, 293–302. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.H.; Porkka, K.; et al. The QUAZAR AML-001 Maintenance Trial: Results of a Phase III International, Randomized, Double-Blind, Placebo-Controlled Study of CC-486 (Oral Formulation of Azacitidine) in Patients with Acute Myeloid Leukemia (AML) in First Remission. Blood 2019, 134, LBA-3. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients with Newly Diagnosed AML with >30% Blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Bazinet, A.; Kantarjian, H.; Bataller, A.; Pemmaraju, N.; Borthakur, G.; Chien, K.; Alvarado, Y.; Bose, P.; Jabbour, E.; Yilmaz, M.; et al. Reduced Dose Azacitidine plus Venetoclax as Maintenance Therapy in Acute Myeloid Leukaemia Following Intensive or Low-Intensity Induction: A Single-Centre, Single-Arm, Phase 2 Trial. Lancet Haematol. 2024, 11, e287–e298. [Google Scholar] [CrossRef]
| Demographics (n = 32) | |
|---|---|
| Sex, n (%) | |
| Male | 17 (53.1) |
| Female | 15 (46.9) |
| Age (years), mean (SD) | 73.3 (3.8) |
| Clinical characteristics (n = 32) | |
| ECOG scale, n (%) | |
| 0 | 17 (53.1) |
| 1 | 13 (40.6) |
| 2 | 2 (6.3) |
| Flow cytometry (bone marrow), mean (SD) | |
| Blasts | 51.4 (31.7) |
| Bone marrow morphology, n (%) | |
| Hypercellular | 16 (50.0) |
| Hypocellular | 9 (28.1) |
| Normocellular | 7 (21.9) |
| Cytopenia at baseline, n (%) | |
| Anemia | 6 (18.8) |
| Leucopenia | 4 (12.5) |
| Neutropenia | 4 (12.5) |
| Thrombocytopenia | 2 (6.3) |
| Response to induction chemotherapy, n (%) | |
| Complete remission | 19 (61.3) |
| Partial remission | 12 (38.7) |
| N | General | PF | DF (Role) | ES | CF | SF | |
|---|---|---|---|---|---|---|---|
| V 0 | 26 | 67.9 (23.8) | 70.4 (27.8) | 62.2 (37.6) | 74.7 (29.6) | 80.1 (22.6) | 73.7 (25.9) |
| V 1 | 29 | 73.9 (22.2) | 78.2 (23.3) | 75.3 (32.6) | 79.3 (22.9) | 88.1 (18.6) | 77.6 (30.3) |
| V 4 | 20 | 67.1 (26.0) | 78.0 (18.7) | 71.7 (31.6) | 79.4 (20.5) | 88.3 (14.4) | 79.2 (29.1) |
| V 6 | 14 | 83.9 (13.7) | 83.8 (19.9) | 86.9 (24.6) | 89.9 (13.9) | 97.6 (6.1) | 98.8 (4.5) |
| V 12 | 4 | 91.7 (9.6) | 85.0 (3.3) | 91.7 (16.7) | 81.3 (12.5) | 95.8 (8.3) | 100.0 (0.0) |
| p-value | 0.0712 | 0.1200 | 0.0296 | 0.1969 | 0.0412 | 0.0275 |
| AEs | SAEs | ||
|---|---|---|---|
| N-AEs N (%)-Patients | 308 31 (96.9) | 6 5 (15.6) | |
| Severity * | G1 | 134 | - |
| G2 | 94 | - | |
| G3 | 34 | 5 | |
| G4 | 12 | - | |
| Mild | 19 | - | |
| Moderate | 11 | 1 | |
| Severe | 4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Fernández, A.; García Fortes, M.; Tormo Díaz, M.; Juan Marco, M.L.; Cuello García, R.; de La Fuente, A.; Serrano López, J.; Medina Pérez, M.Á.; Sánchez Chaparro, M.Á.; García Delgado, R. Maintenance Treatment with 5-Azacitidine in Patients with Acute Myeloblastic Leukemia Ineligible for Intensive Treatment and with Response After Induction Chemotherapy: A Phase II Clinical Trial. Cancers 2025, 17, 2678. https://doi.org/10.3390/cancers17162678
Fernández Fernández A, García Fortes M, Tormo Díaz M, Juan Marco ML, Cuello García R, de La Fuente A, Serrano López J, Medina Pérez MÁ, Sánchez Chaparro MÁ, García Delgado R. Maintenance Treatment with 5-Azacitidine in Patients with Acute Myeloblastic Leukemia Ineligible for Intensive Treatment and with Response After Induction Chemotherapy: A Phase II Clinical Trial. Cancers. 2025; 17(16):2678. https://doi.org/10.3390/cancers17162678
Chicago/Turabian StyleFernández Fernández, Alfonso, María García Fortes, Mar Tormo Díaz, María Luz Juan Marco, Rebeca Cuello García, Adolfo de La Fuente, Josefina Serrano López, Mª Ángeles Medina Pérez, Miguel Ángel Sánchez Chaparro, and Regina García Delgado. 2025. "Maintenance Treatment with 5-Azacitidine in Patients with Acute Myeloblastic Leukemia Ineligible for Intensive Treatment and with Response After Induction Chemotherapy: A Phase II Clinical Trial" Cancers 17, no. 16: 2678. https://doi.org/10.3390/cancers17162678
APA StyleFernández Fernández, A., García Fortes, M., Tormo Díaz, M., Juan Marco, M. L., Cuello García, R., de La Fuente, A., Serrano López, J., Medina Pérez, M. Á., Sánchez Chaparro, M. Á., & García Delgado, R. (2025). Maintenance Treatment with 5-Azacitidine in Patients with Acute Myeloblastic Leukemia Ineligible for Intensive Treatment and with Response After Induction Chemotherapy: A Phase II Clinical Trial. Cancers, 17(16), 2678. https://doi.org/10.3390/cancers17162678





