Clinical and Molecular Differences Suggest Different Responses to Immune Checkpoint Inhibitors in Microsatellite-Stable Solid Tumors with High Tumor Mutational Burden †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcome Evaluation
2.3. Safety
2.4. Next-Generation Sequencing (NGS)
2.5. Ethics Approval
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Gene Data
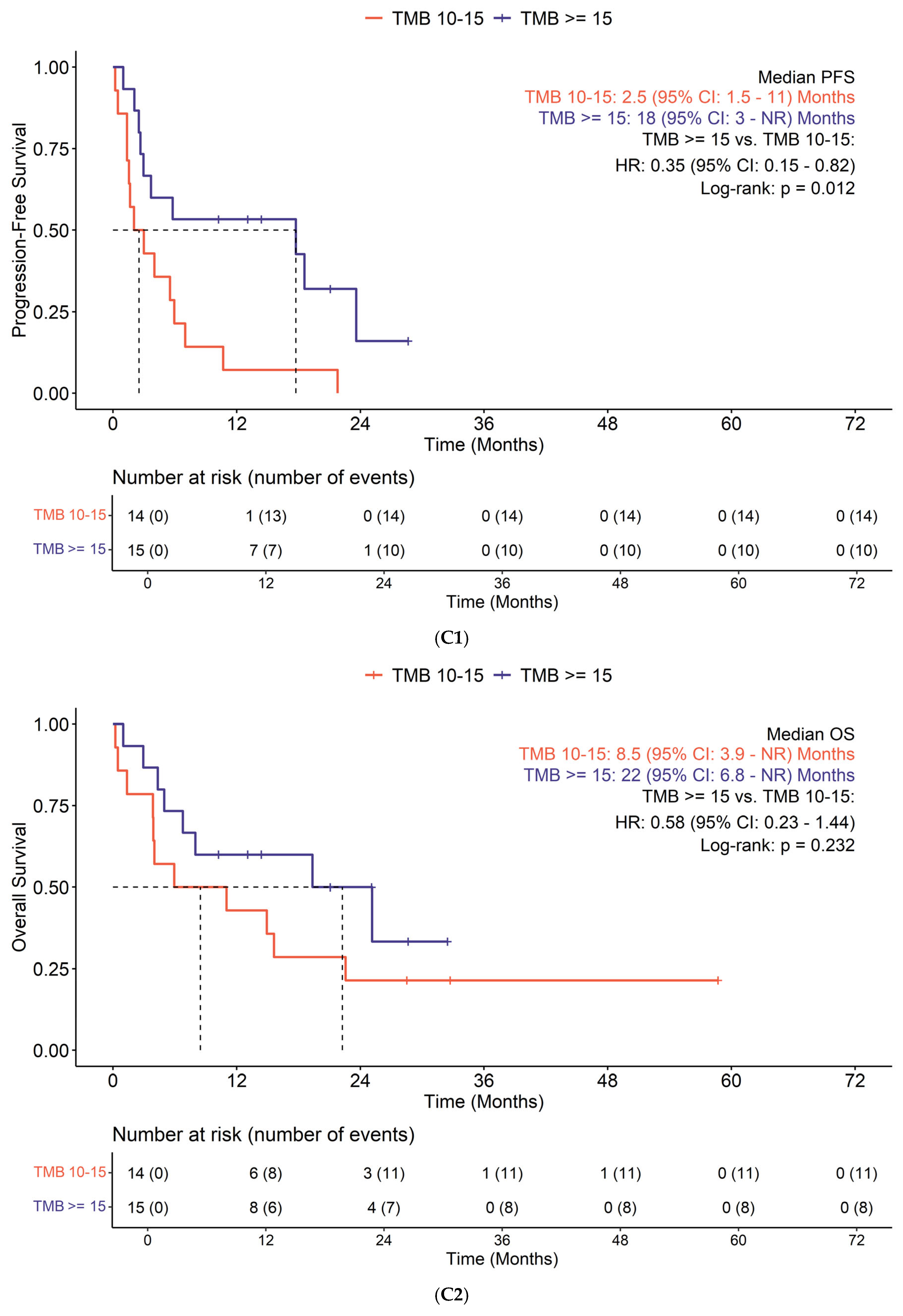
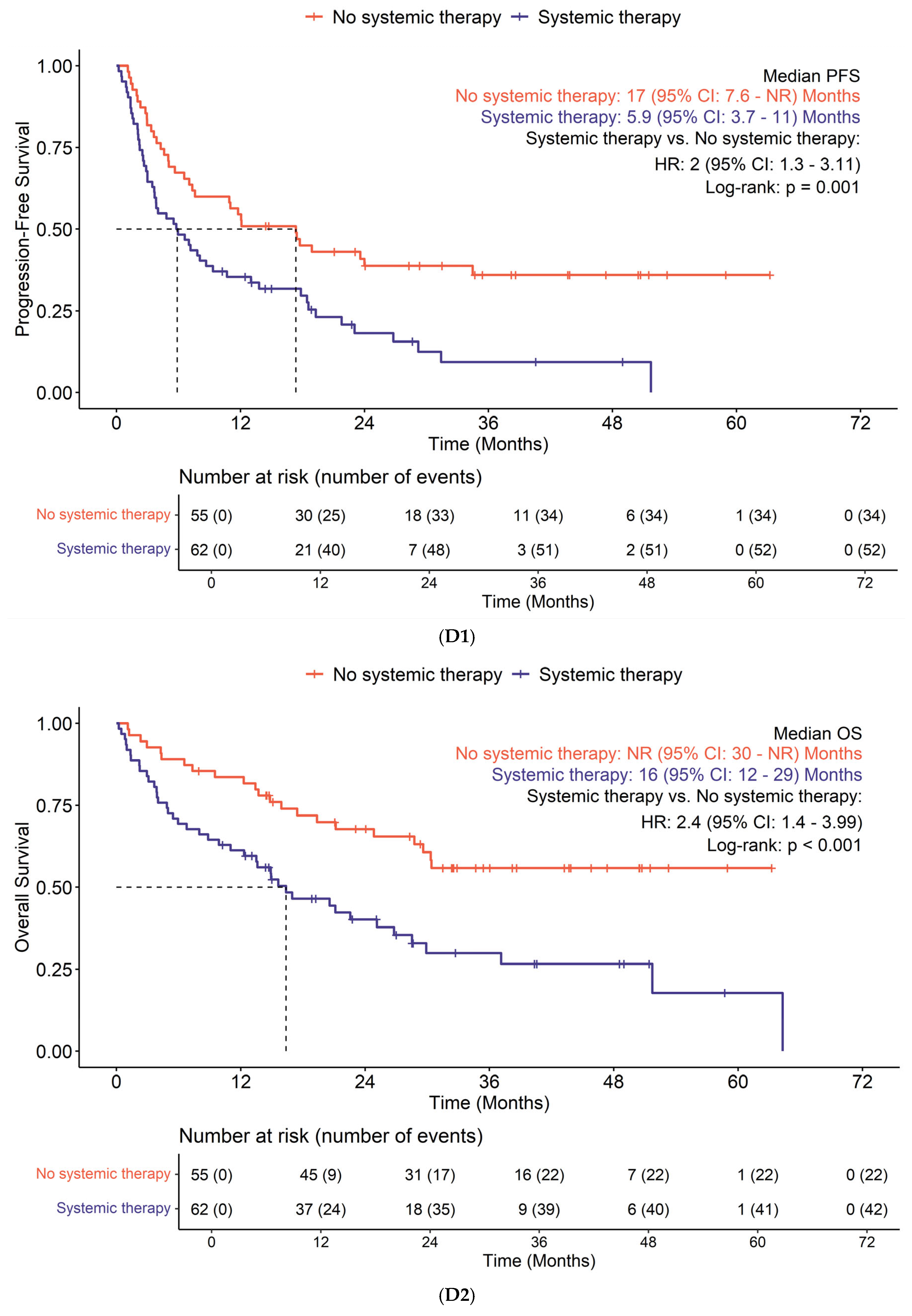
3.3. Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Adrenal corticoid carcinoma |
| BC | Breast cancer |
| CR | Complete response |
| CRC | Colorectal cancer |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DM | Diabetes mellitus |
| dMMR | Deficient mismatch repair |
| FDA | Food and drug administration |
| GC | Gynecologic cancers |
| HR | Hazard ratios |
| ICI | Immune checkpoint inhibitor |
| IQR | Interquartile range |
| irAEs | Immune-related adverse events |
| MSI-H | High microsatellite instability |
| mut/Mb | Mutations per megabase |
| N | Number |
| N/A | Not assessed |
| NE | Not evaluable |
| NGS | Next-generation sequencing |
| NSCLC | Non-small cell lung cancer |
| ORR | Overall response rate |
| OS | Overall survival |
| PBC | Pancreaticobiliary cancers |
| PD | Progression of disease |
| PD-L1 | Programmed cell death ligand 1 |
| PFS | Progression-free survival |
| PR P | Artial response |
| SCC | Squamous cell carcinoma |
| SCLC | Small cell lung cancer |
| SD | Stable disease |
| TMB | Tumor mutational burden |
| UGC | Upper gastrointestinal cancers |
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef]
- Prasad, V.; Haslam, A.; Olivier, T. Updated Estimates of Eligibility and Response: Immune Checkpoint Inhibitors. J. Clin. Oncol. 2024, 42, e14613. [Google Scholar] [CrossRef]
- Mohamed, Y.I.; Lee, S.S.; Demir, T.; Chamseddine, S.; Hu, Z.I.; Xiao, L.; Elsayes, K.; Morris, J.S.; Wolff, R.A.; Hiatia, R.; et al. Circulating Tumor DNA (CtDNA) as a Biomarker of Response to Therapy in Advanced Hepatocellular Carcinoma Treated with Nivolumab. Cancer Biomark. 2024, 41, 83–91. [Google Scholar] [CrossRef]
- Akula, V.; Chen, L.; Acikgoz, Y.; Klein, K.; Yavuz, B.G.; Cevik, L.; Demir, T.; Manne, A.; Sahin, I.; Kaseb, A.; et al. Neoadjuvant Immune Checkpoint Inhibitors for Hepatocellular Carcinoma. NPJ Precis. Oncol. 2025, 9, 60. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Kaestner, V.; Mailankody, S. Cancer Drugs Approved Based on Biomarkers and Not Tumor Type—FDA Approval of Pembrolizumab for Mismatch Repair-Deficient Solid Cancers. JAMA Oncol. 2018, 4, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB Measurement in Clinical Practice: Considerations on Assay Requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Artyomov, M.N.; Mardis, E.R.; Schreiber, R.D. Tumor Neoantigens: Building a Framework for Personalized Cancer Immunotherapy. J. Clin. Investig. 2015, 125, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Richard, C.; Fumet, J.D.; Chevrier, S.; Derangere, V.; Ledys, F.; Lagrange, A.; Favier, L.; Coudert, B.; Arnould, L.; Truntzer, C.; et al. Exome Analysis Reveals Genomic Markers Associated with Better Efficacy of Nivolumab in Lung Cancer Patients. Clin. Cancer Res. 2019, 25, 957–966. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kronbichler, A.; Eisenhut, M.; Hong, S.H.; van der Vliet, H.J.; Kang, J.; Shin, J., II.; Gamerith, G. Tumor Mutational Burden and Efficacy of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1798. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Demir, T.; Moloney, C.; Mahalingam, D. Threading the Needle: Navigating Novel Immunotherapeutics in Pancreatic Ductal Adenocarcinoma. Cancers 2025, 17, 715. [Google Scholar] [CrossRef]
- Demir, T.; Moloney, C.; Mahalingam, D. Emerging Targeted Therapies and Strategies to Overcome Resistance in Biliary Tract Cancers. Crit. Rev. Oncol. Hematol. 2024, 199, 104388. [Google Scholar] [CrossRef]
- Subbiah, V.; Solit, D.B.; Chan, T.A.; Kurzrock, R. The FDA Approval of Pembrolizumab for Adult and Pediatric Patients with Tumor Mutational Burden (TMB) ≥10: A Decision Centered on Empowering Patients and Their Physicians. Ann. Oncol. 2020, 31, 1115–1118. [Google Scholar] [CrossRef]
- Prasad, V.; Addeo, A. The FDA Approval of Pembrolizumab for Patients with TMB >10 Mut/Mb: Was It a Wise Decision? No. Ann. Oncol. 2020, 31, 1112–1114. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Rousseau, B.; Foote, M.B.; Maron, S.B.; Diplas, B.H.; Lu, S.; Argilés, G.; Cercek, A.; Diaz, L.A. The Spectrum of Benefit from Checkpoint Blockade in Hypermutated Tumors. N. Engl. J. Med. 2021, 384, 1168–1170. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. IRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health and Human Services: Bethesda, MD, USA, 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 14 August 2025).
- Patricia Grambsch, B.M.; Therneau, T.M. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e10. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, W.; Feng, F.; Jiang, X.; Chen, H.; Zhang, D.; Zhang, T. Comut-Viz: Efficiently Creating and Browsing Comutation Plots Online. BMC Bioinform. 2023, 24, 226. [Google Scholar] [CrossRef]
- Nizamuddin, I.; Doukas, P.; de Viveiros, P.A.H.; Mi, X.; Katam, N.; Chae, Y.K.; Behdad, A.; Wehbe, F.H.; Ma-halingam, D. Microsatellite-stable tumors with high tumor mutational burden in association with tumor re-sponse to immune checkpoint inhibitor therapy across solid tumors and correlation with specific oncogenic alterations. In Proceedings of the Poster Presentation at ASCO 2022 Annual Meeting, Online, 3–7 June 2022. [Google Scholar]
- Budczies, J.; Kazdal, D.; Menzel, M.; Beck, S.; Kluck, K.; Altbürger, C.; Schwab, C.; Allgäuer, M.; Ahadova, A.; Kloor, M.; et al. Tumour Mutational Burden: Clinical Utility, Challenges and Emerging Improvements. Nat. Rev. Clin. Oncol. 2024, 21, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Xu, H.; Xu, X.; Guo, T.; Ge, W. High Tumor Mutation Burden Predicts Better Efficacy of Immunotherapy: A Pooled Analysis of 103078 Cancer Patients. Oncoimmunology 2019, 8, e1629258. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Bibeau, F.; Greillier, L.; Fumet, J.D.; Ilie, A.; Monville, F.; Laugé, C.; Catteau, A.; Boquet, I.; Majdi, A.; et al. Immunoscore Immune Checkpoint Using Spatial Quantitative Analysis of CD8 and PD-L1 Markers Is Predictive of the Efficacy of Anti- PD1/PD-L1 Immunotherapy in Non-Small Cell Lung Cancer. EBioMedicine 2023, 92, 104633. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver Metastasis Restrains Immunotherapy Efficacy via Macrophage-Mediated T Cell Elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Cohen, R.; Raeisi, M.; Chibaudel, B.; Shi, Q.; Yoshino, T.; Zalcberg, J.R.; Adams, R.; Cremolini, C.; Van Cutsem, E.; Heinemann, V.; et al. Prognostic Value of Liver Metastases in Colorectal Cancer Treated by Systemic Therapy: An ARCAD Pooled Analysis. Eur. J. Cancer 2024, 207, 114160. [Google Scholar] [CrossRef]
- Wang, C.; Sandhu, J.; Ouyang, C.; Ye, J.; Lee, P.P.; Fakih, M. Clinical Response to Immunotherapy Targeting Programmed Cell Death Receptor 1/Programmed Cell Death Ligand 1 in Patients With Treatment-Resistant Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. JAMA Netw. Open 2021, 4, e2118416. [Google Scholar] [CrossRef]
- Bullock, A.J.; Schlechter, B.L.; Fakih, M.G.; Tsimberidou, A.M.; Grossman, J.E.; Gordon, M.S.; Wilky, B.A.; Pimentel, A.; Mahadevan, D.; Balmanoukian, A.S.; et al. Botensilimab plus Balstilimab in Relapsed/Refractory Microsatellite Stable Metastatic Colorectal Cancer: A Phase 1 Trial. Nat. Med. 2024, 30, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Newell, F.; Pires da Silva, I.; Johansson, P.A.; Menzies, A.M.; Wilmott, J.S.; Addala, V.; Carlino, M.S.; Rizos, H.; Nones, K.; Edwards, J.J.; et al. Multiomic Profiling of Checkpoint Inhibitor-Treated Melanoma: Identifying Predictors of Response and Resistance, and Markers of Biological Discordance. Cancer Cell 2022, 40, 88–102.e7. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Niknafs, N.; Marrone, K.; Bruhm, D.C.; White, J.R.; Naidoo, J.; Hummelink, K.; Monkhorst, K.; Lalezari, F.; Lanis, M. Multimodal genomic features predict outcome of immune checkpoint blockade in non-small-cell lung cancer. Nat. Cancer 2020, 1, 99–111. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018, 33, 853–861.e4. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.; Lee, B.; Lee, S.H.; Sun, J.M.; Park, W.Y.; Ahn, J.S.; Ahn, M.J.; Park, K. DNA Damage Response and Repair Pathway Alteration and Its Association With Tumor Mutation Burden and Platinum-Based Chemotherapy in SCLC. J. Thorac. Oncol. 2019, 14, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Lee, J.V.; Housley, F.; Yau, C.; Nakagawa, R.; Winkler, J.; Anttila, J.M.; Munne, P.M.; Savelius, M.; Houlahan, K.E.; Van de Mark, D.; et al. Combinatorial Immunotherapies Overcome MYC-Driven Immune Evasion in Triple Negative Breast Cancer. Nat. Commun. 2022, 13, 3671. [Google Scholar] [CrossRef]
- Li, J.; Dong, T.; Wu, Z.; Zhu, D.; Gu, H. The Effects of MYC on Tumor Immunity and Immunotherapy. Cell Death Discov. 2023, 9, 103. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC Oncogene—The Grand Orchestrator of Cancer Growth and Immune Evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef]
- Casey, S.C.; Baylot, V.; Felsher, D.W. The MYC Oncogene Is a Global Regulator of the Immune Response. Blood 2018, 131, 2007–2015. [Google Scholar] [CrossRef]
- Wang, H.; Helin, K. Roles of H3K4 Methylation in Biology and Disease. Trends Cell Biol. 2025, 35, 115–128. [Google Scholar] [CrossRef]
- Lee, J.J.; Sholl, L.M.; Lindeman, N.I.; Granter, S.R.; Laga, A.C.; Shivdasani, P.; Chin, G.; Luke, J.J.; Ott, P.A.; Hodi, F.S.; et al. Targeted Next-Generation Sequencing Reveals High Frequency of Mutations in Epigenetic Regulators across Treatment-Naïve Patient Melanomas. Clin. Epigenetics 2015, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Lu, L.; Ge, B.; Gao, S.; Ma, Y.; Liang, B.; Yu, K.; Yang, K. MLL2 protein is a prognostic marker for gastrointestinal diffuse large B-cell lymphoma. Int. J. Clin. Exp. Pathol. 2015, 8, 13043–13050. [Google Scholar]
- Ge, S.; Li, B.; Li, Y.; Li, Z.; Liu, Z.; Chen, Z.; Wu, J.; Gao, J.; Shen, L. Genomic Alterations in Advanced Gastric Cancer Endoscopic Biopsy Samples Using Targeted Next-Generation Sequencing. Am. J. Cancer Res. 2017, 7, 1540–1553. [Google Scholar] [PubMed]
- Choi, M.; Kadara, H.; Zhang, J.; Parra, E.R.; Rodriguez-Canales, J.; Gaffney, S.G.; Zhao, Z.; Behrens, C.; Fujimoto, J.; Chow, C.; et al. Mutation Profiles in Early-Stage Lung Squamous Cell Carcinoma with Clinical Follow-up and Correlation with Markers of Immune Function. Ann. Oncol. 2017, 28, 83–89. [Google Scholar] [CrossRef]
- Li, X. Emerging Role of Mutations in Epigenetic Regulators Including MLL2 Derived from The Cancer Genome Atlas for Cervical Cancer. BMC Cancer 2017, 17, 252. [Google Scholar] [CrossRef]
- Liang, N.; Niu, Y.; Zhang, X.; Ma, T. The predictive values of loss-of-function variants in histone methyltransferases for response to immune checkpoint inhibitors in solid tumors. J. Clin. Oncol. 2021, 39, 2586. [Google Scholar]
- Jiang, Y.; Xie, J.; Cheng, Q.; Cai, Z.; Xu, K.; Lu, W.; Wang, F.; Wu, X.; Song, Y.; Lv, T.; et al. Comprehensive Genomic and Spatial Immune Infiltration Analysis of Survival Outliers in Extensive-Stage Small Cell Lung Cancer Receiving First-Line Chemoimmunotherapy. Int. Immunopharmacol. 2024, 141, 112901. [Google Scholar] [CrossRef]
- Li, J.; Chin, C.R.; Ying, H.Y.; Meydan, C.; Teater, M.R.; Xia, M.; Farinha, P.; Takata, K.; Chu, C.S.; Jiang, Y.; et al. Loss of CREBBP and KMT2D Cooperate to Accelerate Lymphomagenesis and Shape the Lymphoma Immune Microenvironment. Nat. Commun. 2024, 15, 2879. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X.; Zhu, Y.; Yi, H.M.; Shen, Y.G.; Wang, L.; Cheng, S.; Xu, P.P.; Xu, H.M.; Zhou, L.T.; Huang, Y.H.; et al. KMT2D Mutations Promoted Tumor Progression in Diffuse Large B-Cell Lymphoma through Altering Tumor-Induced Regulatory T Cell Trafficking via FBXW7-NOTCH-MYC/TGF-Β1 Axis. Int. J. Biol. Sci. 2024, 20, 3972–3985. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Zhang, C.; Zhang, C.; Wang, H. TERT Mutations Correlate with Higher TMB Value and Unique Tumor Microenvironment and May Be a Potential Biomarker for Anti-CTLA4 Treatment. Cancer Med. 2020, 9, 7151–7160. [Google Scholar] [CrossRef]
- Du, J.; Zhang, E.; Huang, Z. The Predictive Value of next Generation Sequencing for Matching Advanced Hepatocellular Carcinoma Patients to Targeted and Immunotherapy. Front. Immunol. 2024, 15, 1358306. [Google Scholar] [CrossRef]
- Hwang, I.; Kang, S.Y.; Kim, D.G.; Jang, K.T.; Kim, K.M. Clinicopathologic and Genomic Characteristics of Biliary Tract Carcinomas with TERT Promoter Mutations among East Asian Population. Pathol. Res. Pract. 2025, 266, 155806. [Google Scholar] [CrossRef]
- Ou, Q.; Yu, Y.; Li, A.; Chen, J.; Yu, T.; Xu, X.; Xie, X.; Chen, Y.; Lin, D.; Zeng, Q.; et al. Association of Survival and Genomic Mutation Signature with Immunotherapy in Patients with Hepatocellular Carcinoma. Ann. Transl. Med. 2020, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
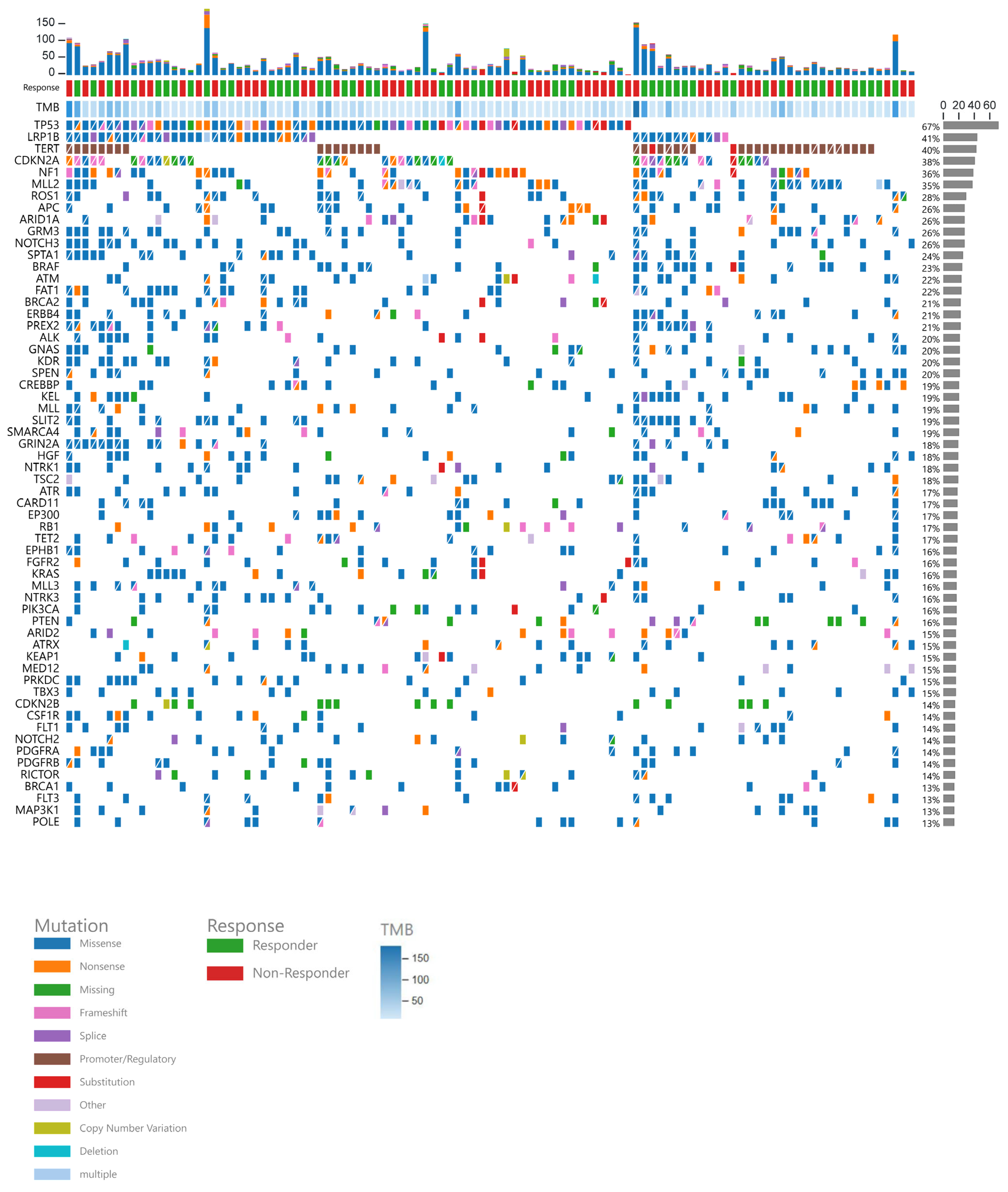
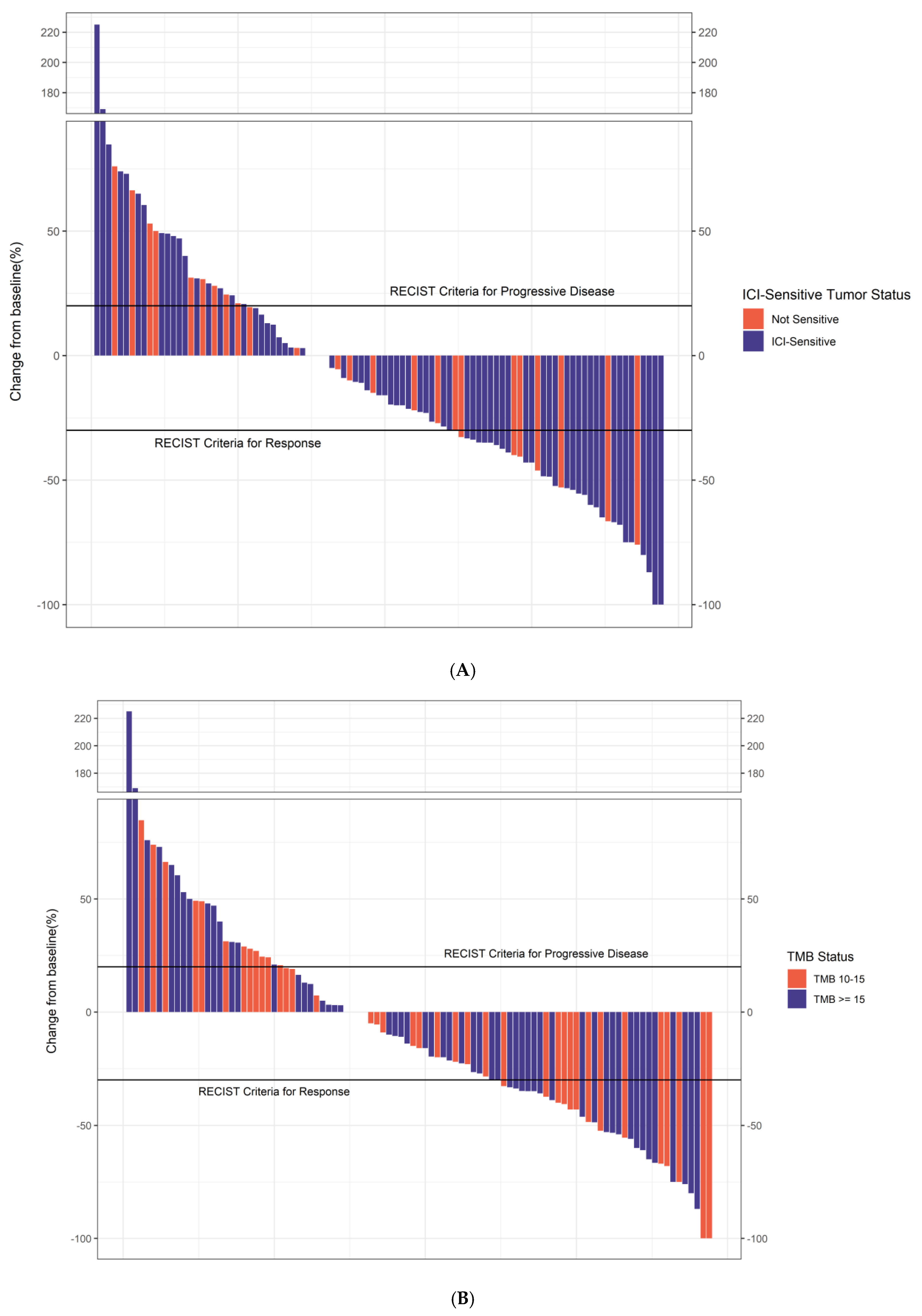
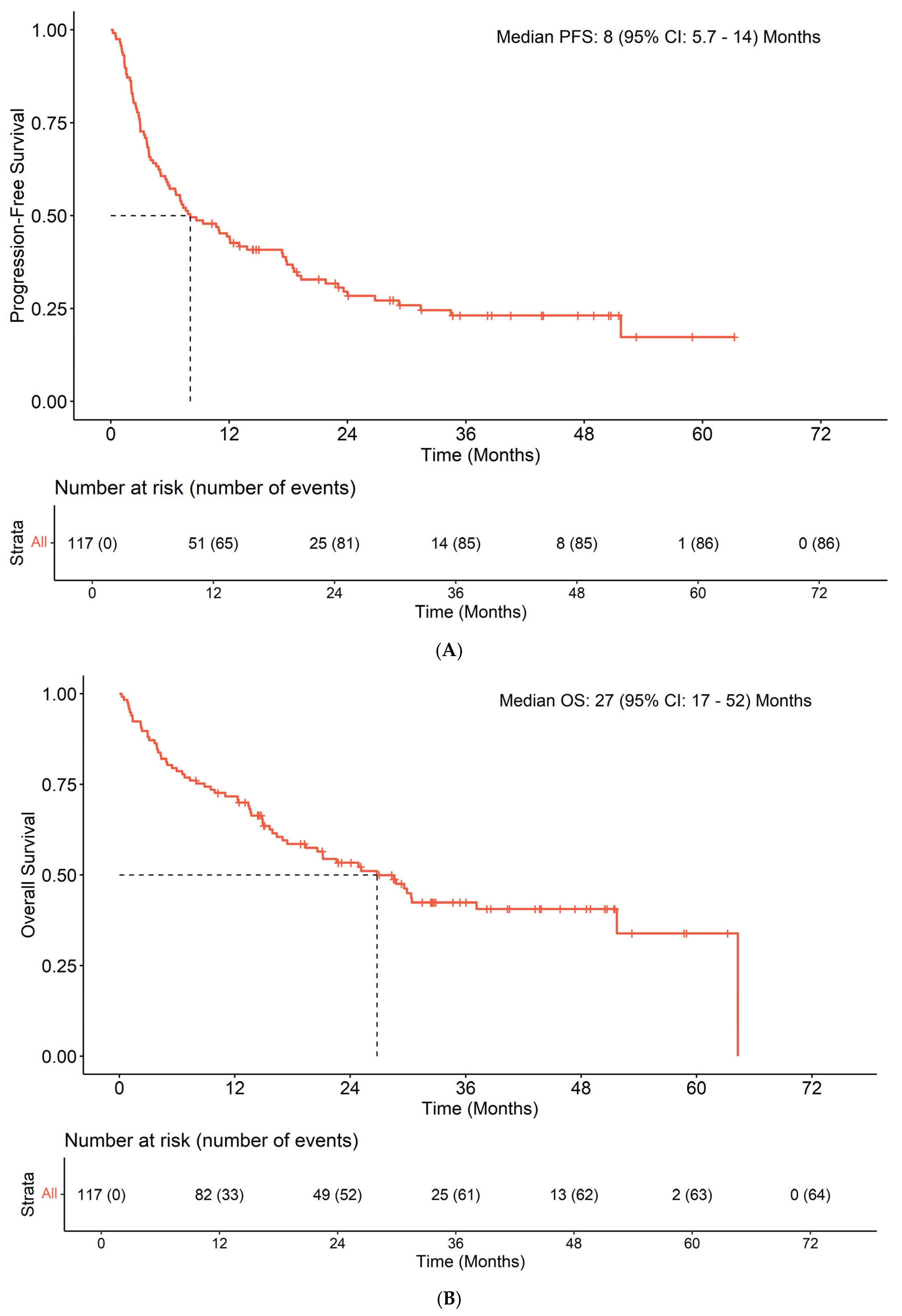
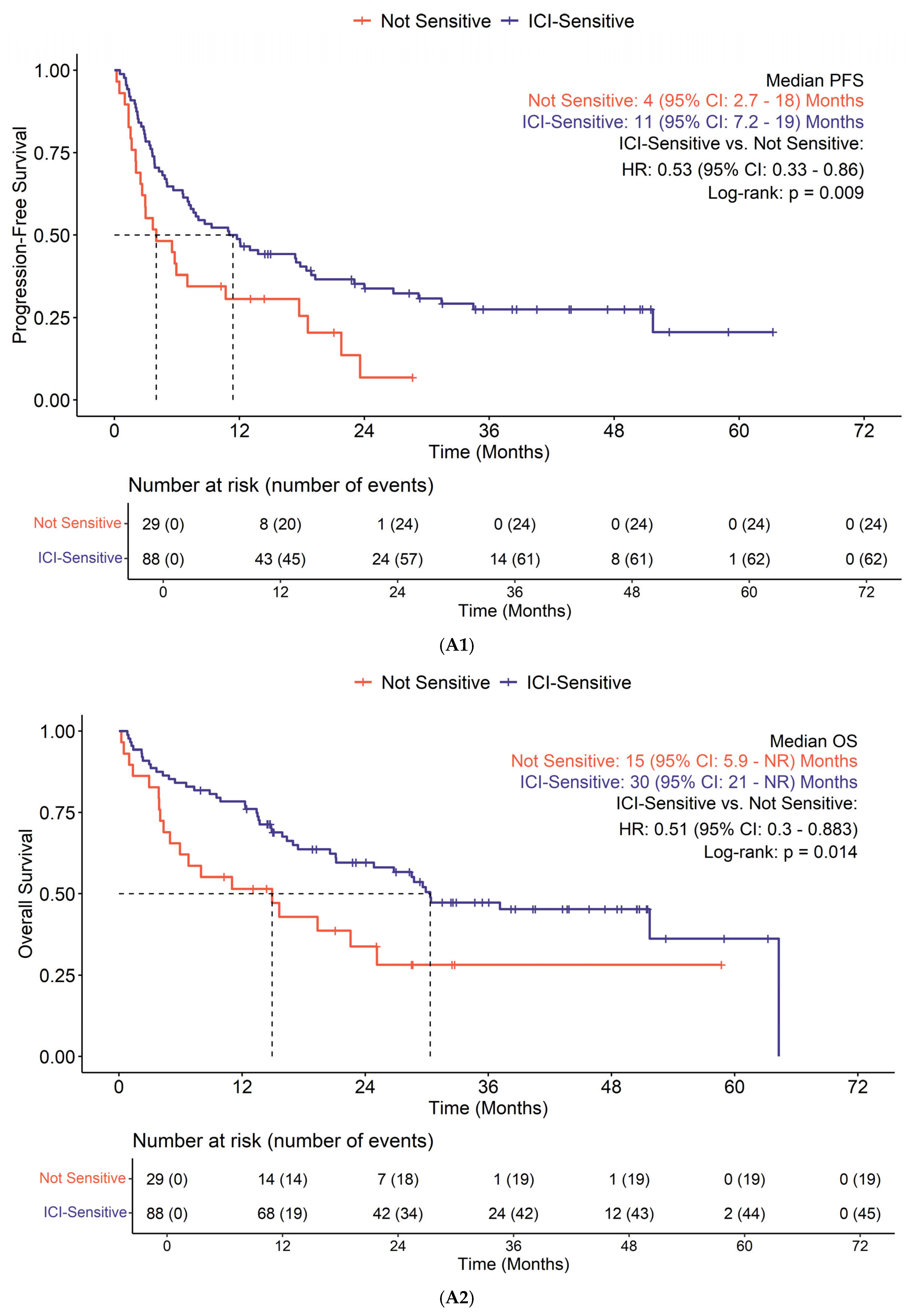

| Characteristic | Overall, n = 117 1 | [SD, <6 m]/PD, n = 51 1 | CR/PR/[SD, >6 m], n = 54 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 68 (62, 76) | 68 (62, 77) | 69 (62, 76) | 0.8 |
| Sex | 0.9 | |||
| Female | 46 (39%) | 20 (39%) | 22 (41%) | |
| Male | 71 (61%) | 31 (61%) | 32 (59%) | |
| Race | 0.2 | |||
| Asian | 4 (3.4%) | 3 (5.9%) | 1 (1.9%) | |
| Black/African American | 7 (6.0%) | 1 (2.0%) | 4 (7.4%) | |
| Unknown | 3 (2.6%) | 2 (3.9%) | 0 (0%) | |
| White | 103 (88%) | 45 (88%) | 49 (91%) | |
| PD-L1 status | >0.9 | |||
| Positive | 36 (46%) | 16 (46%) | 18 (49%) | |
| Negative | 31 (39%) | 13 (37%) | 13 (35%) | |
| NE | 12 (15%) | 6 (17%) | 6 (16%) | |
| Unknown | 38 | 16 | 17 | |
| Previous therapies | 0.039 | |||
| No systemic therapy | 55 (47%) | 19 (37%) | 31 (57%) | |
| Adjuvant/neoadjuvant/definitive therapy | 62 (53%) | 32 (63%) | 23 (43%) | |
| Previous therapy lines | 0.10 | |||
| One line | 29 (48%) | 11 (35%) | 14 (61%) | |
| Two lines | 17 (28%) | 10 (32%) | 5 (22%) | |
| Three lines | 5 (8.2%) | 5 (16%) | 0 (0%) | |
| Four or more lines | 10 (16%) | 5 (16%) | 4 (17%) | |
| Unknown | 56 | 20 | 31 | |
| All responses | <0.001 | |||
| Complete response | 15 (13%) | 0 (0%) | 15 (28%) | |
| Partial response | 21 (18%) | 0 (0%) | 21 (39%) | |
| Stable disease | 22 (19%) | 4 (7.8%) | 18 (33%) | |
| Progressive disease | 47 (40%) | 47 (92%) | 0 (0%) | |
| NE | 8 (6.8%) | 0 (0%) | 0 (0%) | |
| N/A | 4 (3.4%) | 0 (0%) | 0 (0%) | |
| First line immunotherapy | 0.048 | |||
| Ipilimumab | 2 (1.7%) | 2 (3.9%) | 0 (0%) | |
| Nivolumab | 32 (27%) | 15 (29%) | 14 (26%) | |
| Ipilimumab/Nivolumab | 19 (16%) | 5 (9.8%) | 11 (20%) | |
| Pembrolizumab | 43 (37%) | 16 (31%) | 23 (43%) | |
| Atezolizumab | 13 (11%) | 10 (20%) | 2 (3.7%) | |
| Durvalumab | 1 (0.9%) | 0 (0%) | 1 (1.9%) | |
| Other | 7 (6.0%) | 3 (5.9%) | 3 (5.6%) | |
| Known ICI-sensitive tumor | 0.13 | |||
| Non-ICI-sensitive | 29 (25%) | 16 (31%) | 10 (19%) | |
| ICI-sensitive | 88 (75%) | 35 (69%) | 44 (81%) | |
| Tumor type | 0.2 | |||
| Adrenocortical carcinoma | 2 (1.7%) | 1 (2.0%) | 1 (1.9%) | |
| Anal squamous cell carcinoma | 2 (1.7%) | 1 (2.0%) | 0 (0%) | |
| Breast cancer | 2 (1.7%) | 0 (0%) | 1 (1.9%) | |
| Colorectal carcinoma | 6 (5.1%) | 4 (7.8%) | 2 (3.7%) | |
| Gynecologic carcinoma | 4 (3.4%) | 2 (3.9%) | 2 (3.7%) | |
| Melanoma | 39 (33%) | 12 (24%) | 24 (44%) | |
| Non-small cell lung carcinoma | 33 (28%) | 18 (35%) | 12 (22%) | |
| Other skin | 6 (5.1%) | 1 (2.0%) | 4 (7.4%) | |
| Pancreatobiliary | 5 (4.3%) | 2 (3.9%) | 2 (3.7%) | |
| Small cell lung carcinoma | 7 (6.0%) | 3 (5.9%) | 3 (5.6%) | |
| Unknown primary | 4 (3.4%) | 4 (7.8%) | 0 (0%) | |
| Upper GI | 2 (1.7%) | 1 (2.0%) | 1 (1.9%) | |
| Urothelial carcinoma | 4 (3.4%) | 2 (3.9%) | 2 (3.7%) | |
| Head and neck SCC | 1 (0.9%) | 0(0%) | 1(1.9%) | |
| MSI status | 0.5 | |||
| MSI stable | 113 (96.6%) | 49 (96%) | 52 (96.3%) | |
| N/A | 4 (3.4%) | 2 (4%) | 2 (3.7%) | |
| TMB status | 0.5 | |||
| TMB 10–15 | 47 (40%) | 22 (43%) | 20 (37%) | |
| TMB ≥ 15 | 70 (60%) | 29 (57%) | 34 (63%) |
| Group | Tumor Type | Median TMB Level (mut/Mb) | IQR (mut/Mb) |
|---|---|---|---|
| ICI-sensitive | Unknown primary (SCC) | 27.8 | 21.1, 49.5 |
| Melanoma | 26.5 | 15.1, 56.7 | |
| Non-small cell lung cancer | 14.9 | 13.5, 26.5 | |
| Adrenal corticoid carcinoma | 35.8 | 29.8, 41.9 | |
| Anal SCC | 10.1 | 10.1, 10.1 | |
| Head and neck SCC | 16.7 | 16.7, 16.7 | |
| Genitourinary carcinoma ¥ | 15.8 | 13.9, 19.5 | |
| Other skin cancers * | 27.7 | 13.9, 48.2 | |
| Non-ICI-sensitive | Breast cancer | 11.3 | 10.9, 11.7 |
| Colorectal cancer | 15.2 | 12.0, 27.8 | |
| Gynecologic cancers (non-SCC) | 11.3 | 10.3, 24.1 | |
| Pancreaticobiliary cancers | 15.1 | 15.0, 19.3 | |
| Small cell lung cancer | 14.9 | 11.0, 17.1 | |
| Upper gastrointestinal cancers Ɨ (non-SCC) | 15.3 | 13.3, 17.3 |
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total (n = 48/117) (41%) |
|---|---|---|---|---|---|---|
| Thyroid disease | 3 (2.5%) | 2 (1.7%) | 0 | 0 | 0 | 5 (4.2%) |
| Adrenal insufficiency | 1 (0.8%) | 1 (0.8%) | 0 | 0 | 0 | 2 (1.7%) |
| Colitis | 2 (1.7%) | 1 (0.8%) | 4 (3.4%) | 2 (1.7%) | 0 | 9 (7.6%) |
| Pneumonitis | 0 | 1 (0.8%) | 3 (2.5%) | 1 (0.8%) | 1 (0.8%) | 6 (5%) |
| Infusion reactions | 0 | 1 (0.8%) | 0 | 0 | 0 | 1 (0.8%) |
| Severe skin reactions | 5 (4.2%) | 1 (0.8%) | 1 (0.8%) | 0 | 0 | 7 (5.9%) |
| Hepatitis | 2 (1.7%) | 1 (0.8%) | 3 (2.5%) | 3 (2.5%) | 0 | 9 (7.6%) |
| Nephritis | 0 | 1 (0.8%) | 1 (0.8%) | 0 | 0 | 2 (1.7%) |
| Hypophysitis | 0 | 5 (4.2%) | 0 | 0 | 0 | 5 (4.2%) |
| Myocarditis | 0 | 0 | 2 (1.7%) | 0 | 0 | 2 (1.7%) |
| Type 1 DM | 0 | 0 | 0 | 0 | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizamuddin, I.; Demir, T.; Dobinda, K.; Chen, R.; Kocherginsky, M.; Doukas, P.; Katam, N.; Moloney, C.; Mahalingam, D. Clinical and Molecular Differences Suggest Different Responses to Immune Checkpoint Inhibitors in Microsatellite-Stable Solid Tumors with High Tumor Mutational Burden. Cancers 2025, 17, 2673. https://doi.org/10.3390/cancers17162673
Nizamuddin I, Demir T, Dobinda K, Chen R, Kocherginsky M, Doukas P, Katam N, Moloney C, Mahalingam D. Clinical and Molecular Differences Suggest Different Responses to Immune Checkpoint Inhibitors in Microsatellite-Stable Solid Tumors with High Tumor Mutational Burden. Cancers. 2025; 17(16):2673. https://doi.org/10.3390/cancers17162673
Chicago/Turabian StyleNizamuddin, Imran, Tarik Demir, Katrina Dobinda, Ruohui Chen, Masha Kocherginsky, Peter Doukas, Neelima Katam, Carolyn Moloney, and Devalingam Mahalingam. 2025. "Clinical and Molecular Differences Suggest Different Responses to Immune Checkpoint Inhibitors in Microsatellite-Stable Solid Tumors with High Tumor Mutational Burden" Cancers 17, no. 16: 2673. https://doi.org/10.3390/cancers17162673
APA StyleNizamuddin, I., Demir, T., Dobinda, K., Chen, R., Kocherginsky, M., Doukas, P., Katam, N., Moloney, C., & Mahalingam, D. (2025). Clinical and Molecular Differences Suggest Different Responses to Immune Checkpoint Inhibitors in Microsatellite-Stable Solid Tumors with High Tumor Mutational Burden. Cancers, 17(16), 2673. https://doi.org/10.3390/cancers17162673






