Immune Biomarkers for Checkpoint Blockade in Solid Tumors: Transitioning from Tissue to Peripheral Blood Monitoring and Future Integrated Strategies
Simple Summary
Abstract
1. Introduction
2. Tissue-Based Immune Biomarkers
2.1. Programmed Death-Ligand 1 (PD-L1) Expression
2.2. Tumor-Infiltrating Lymphocytes (TILs)
2.3. Other Tissue-Based Immune Markers
2.4. Combination Approaches to Tissue-Based Immune Biomarkers
3. Peripheral Blood Immune Biomarkers
3.1. White Blood Cell Ratios and Composite Indices
3.2. Circulating Immune Cell Subsets and Immunophenotyping
3.3. Cytokines
3.4. Soluble Checkpoint Proteins
3.5. Autoantibodies
3.6. Emerging Tumor-Derived Circulating Biomarkers
4. Composite Approaches and Future Perspectives
4.1. Integrated Immune Profiling and Multi-Modal Biomarkers
4.2. Application of Artificial Intelligence and Machine Learning
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jamalinia, M.; Weiskirchen, R. Advances in personalized medicine: Translating genomic insights into targeted therapies for cancer treatment. Ann. Transl. Med. 2025, 13, 18. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Scolyer, R.A.; Rumcheva, P.; Moncrieff, M.; Murali, R.; McCarthy, S.W.; Saw, R.P.; Thompson, J.F. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 2012, 30, 2678–2683. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Muller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hsu, J.M.; Sun, L.; Wang, S.C.; Hung, M.C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep. Med. 2024, 5, 101621. [Google Scholar] [CrossRef]
- Ilie, M.; Long-Mira, E.; Bence, C.; Butori, C.; Lassalle, S.; Bouhlel, L.; Fazzalari, L.; Zahaf, K.; Lalvee, S.; Washetine, K.; et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti-PD-L1 therapeutic strategies. Ann. Oncol. 2016, 27, 147–153. [Google Scholar] [CrossRef]
- Goswami, M.; Toney, N.J.; Pitts, S.C.; Celades, C.; Schlom, J.; Donahue, R.N. Peripheral immune biomarkers for immune checkpoint inhibition of solid tumours. Clin. Transl. Med. 2024, 14, e1814. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.B.; Schalper, K.A.; Jacobs, I.; Potluri, S.; Wang, I.M.; Fleener, C. Peripheral immune-based biomarkers in cancer immunotherapy: Can we realize their predictive potential? J. Immunother. Cancer 2019, 7, 325. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Li, L.; Chai, Y.; Huang, J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS ONE 2015, 10, e0131403. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Byrne, D.J.; Wright, G.M.; Young, R.J.; Sturrock, S.; Cooper, W.A.; Fox, S.B. Comparison of Four PD-L1 Immunohistochemical Assays in Lung Cancer. J. Thorac. Oncol. 2018, 13, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.A.; Cha, Y.J.; Kim, J.H.; Pyo, K.H.; Hong, M.H.; Park, S.Y.; Shim, H.S.; Jung, I.; Lee, C.Y.; Cho, B.C.; et al. Dynamic changes in PD-L1 expression and CD8+ T cell infiltration in non-small cell lung cancer following chemoradiation therapy. Lung Cancer 2019, 136, 30–36. [Google Scholar] [CrossRef] [PubMed]
- van Eekelen, L.; Spronck, J.; Looijen-Salamon, M.; Vos, S.; Munari, E.; Girolami, I.; Eccher, A.; Acs, B.; Boyaci, C.; de Souza, G.S.; et al. Comparing deep learning and pathologist quantification of cell-level PD-L1 expression in non-small cell lung cancer whole-slide images. Sci. Rep. 2024, 14, 7136. [Google Scholar] [CrossRef]
- Baxi, V.; Lee, G.; Duan, C.; Pandya, D.; Cohen, D.N.; Edwards, R.; Chang, H.; Li, J.; Elliott, H.; Pokkalla, H.; et al. Association of artificial intelligence-powered and manual quantification of programmed death-ligand 1 (PD-L1) expression with outcomes in patients treated with nivolumab +/− ipilimumab. Mod. Pathol. 2022, 35, 1529–1539. [Google Scholar] [CrossRef]
- McGenity, C.; Clarke, E.L.; Jennings, C.; Matthews, G.; Cartlidge, C.; Freduah-Agyemang, H.; Stocken, D.D.; Treanor, D. Artificial intelligence in digital pathology: A systematic review and meta-analysis of diagnostic test accuracy. NPJ Digit. Med. 2024, 7, 114. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Yan, Q.; Li, S.; He, L.; Chen, N. Prognostic implications of tumor-infiltrating lymphocytes in non-small cell lung cancer: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1476365. [Google Scholar] [CrossRef]
- Jiang, P.; Gu, S.; Pan, D.; Fu, J.; Sahu, A.; Hu, X.; Li, Z.; Traugh, N.; Bu, X.; Li, B.; et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 2018, 24, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Wei, C.; Ma, Y.; Wang, M.; Wang, S.; Yu, W.; Dong, S.; Deng, W.; Bie, L.; Zhang, C.; Shen, W.; et al. Tumor-associated macrophage clusters linked to immunotherapy in a pan-cancer census. NPJ Precis. Oncol. 2024, 8, 176. [Google Scholar] [CrossRef]
- Peyraud, F.; Guegan, J.P.; Rey, C.; Lara, O.; Odin, O.; Del Castillo, M.; Vanhersecke, L.; Coindre, J.M.; Clot, E.; Brunet, M.; et al. Spatially resolved transcriptomics reveal the determinants of primary resistance to immunotherapy in NSCLC with mature tertiary lymphoid structures. Cell Rep. Med. 2025, 6, 101934. [Google Scholar] [CrossRef]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.D.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, Y.; Shu, P.; Lei, Y.; Li, X.; Wang, Y. Targeting tumor-infiltrating tregs for improved antitumor responses. Front. Immunol. 2024, 15, 1325946. [Google Scholar] [CrossRef] [PubMed]
- Sautes-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Bessede, A.; Pulido, M.; Bompas, E.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Bertucci, F.; Toulmonde, M.; Bellera, C.; et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: A phase 2 PEMBROSARC trial cohort. Nat. Med. 2022, 28, 1199–1206. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Anderson, A.C. Tim-3, a negative regulator of anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 213–216. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012, 4, 127ra137. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef]
- Yamagami, W.; Susumu, N.; Tanaka, H.; Hirasawa, A.; Banno, K.; Suzuki, N.; Tsuda, H.; Tsukazaki, K.; Aoki, D. Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int. J. Gynecol. Cancer 2011, 21, 1628–1634. [Google Scholar] [CrossRef]
- Furgiuele, S.; Descamps, G.; Lechien, J.R.; Dequanter, D.; Journe, F.; Saussez, S. Immunoscore Combining CD8, FoxP3, and CD68-Positive Cells Density and Distribution Predicts the Prognosis of Head and Neck Cancer Patients. Cells 2022, 11, 2050. [Google Scholar] [CrossRef]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef]
- Wu, X.R.; Peng, H.X.; He, M.; Zhong, R.; Liu, J.; Wen, Y.K.; Li, C.C.; Li, J.F.; Xiong, S.; Yu, T.; et al. Macrophages-based immune-related risk score model for relapse prediction in stage I-III non-small cell lung cancer assessed by multiplex immunofluorescence. Transl. Lung Cancer Res. 2022, 11, 523–542. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, Y.; Tan, S.; Cheng, T.; Luo, Y.; Zhang, L. Pretreatment neutrophil-to-lymphocyte ratio is associated with immunotherapy efficacy in patients with advanced cancer: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 446. [Google Scholar] [CrossRef]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Fruh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, J.; Tang, S.; Sun, G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Int. Immunopharmacol. 2020, 85, 106677. [Google Scholar] [CrossRef]
- Wan, L.; Wu, C.; Luo, S.; Xie, X. Prognostic Value of Lymphocyte-to-Monocyte Ratio (LMR) in Cancer Patients Undergoing Immune Checkpoint Inhibitors. Dis. Markers 2022, 2022, 3610038. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer 2018, 9, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Guo, C.; Li, S.; Huang, C. Systemic immune-inflammation index as a predictor of survival in non-small cell lung cancer patients undergoing immune checkpoint inhibition: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2025, 210, 104669. [Google Scholar] [CrossRef]
- Dai, M.; Wu, W. Prognostic role of C-reactive protein to albumin ratio in cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Front. Oncol. 2023, 13, 1148786. [Google Scholar] [CrossRef]
- Tong, W.; Xu, H.; Tang, J.; Zhao, N.; Zhou, D.; Chen, C.; Cao, D. Inflammatory factors are associated with prognosis of non-small cell lung cancer patients receiving immunotherapy: A meta-analysis. Sci. Rep. 2024, 14, 26102. [Google Scholar] [CrossRef] [PubMed]
- Krizova, L.; Benesova, I.; Zemanova, P.; Spacek, J.; Strizova, Z.; Humlova, Z.; Mikulova, V.; Petruzelka, L.; Vocka, M. Immunophenotyping of peripheral blood in NSCLC patients discriminates responders to immune checkpoint inhibitors. J. Cancer Res. Clin. Oncol. 2024, 150, 99. [Google Scholar] [CrossRef]
- Miao, K.; Zhang, X.; Wang, H.; Si, X.; Ni, J.; Zhong, W.; Zhao, J.; Xu, Y.; Chen, M.; Pan, R.; et al. Peripheral Blood Lymphocyte Subsets Predict the Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 912180. [Google Scholar] [CrossRef]
- Lao, J.; Xu, H.; Liang, Z.; Luo, C.; Shu, L.; Xie, Y.; Wu, Y.; Hao, Y.; Yuan, Y. Peripheral changes in T cells predict efficacy of anti-PD-1 immunotherapy in non-small cell lung cancer. Immunobiology 2023, 228, 152391. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, Q.; Wu, L.; Wang, Y.; Wang, J.; Zhu, L.; Zheng, S.; Hang, J. Association of the CD4+/CD8+ ratio with response to PD-1 inhibitor-based combination therapy and dermatological toxicities in patients with advanced gastric and esophageal cancer. Int. Immunopharmacol. 2023, 123, 110642. [Google Scholar] [CrossRef]
- Li, P.; Qin, P.; Fu, X.; Zhang, G.; Yan, X.; Zhang, M.; Zhang, X.; Yang, J.; Wang, H.; Ma, Z. Associations between peripheral blood lymphocyte subsets and clinical outcomes in patients with lung cancer treated with immune checkpoint inhibitor. Ann. Palliat. Med. 2021, 10, 3039–3049. [Google Scholar] [CrossRef]
- Huang, S.W.; Jiang, W.; Xu, S.; Zhang, Y.; Du, J.; Wang, Y.Q.; Yang, K.Y.; Zhang, N.; Liu, F.; Zou, G.R.; et al. Systemic longitudinal immune profiling identifies proliferating Treg cells as predictors of immunotherapy benefit: Biomarker analysis from the phase 3 CONTINUUM and DIPPER trials. Signal Transduct. Target. Ther. 2024, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Chung, C.; Sun, P.; Lee, D.H.; Lee, S.I.; Park, D.; Koh, J.S.; Kim, Y.; Yi, H.S.; Lee, J.E. Circulating regulatory T cells predict efficacy and atypical responses in lung cancer patients treated with PD-1/PD-L1 inhibitors. Cancer Immunol. Immunother. 2022, 71, 579–588. [Google Scholar] [CrossRef]
- Moller, M.; Orth, V.; Umansky, V.; Hetjens, S.; Braun, V.; Reissfelder, C.; Hardt, J.; Seyfried, S. Myeloid-derived suppressor cells in peripheral blood as predictive biomarkers in patients with solid tumors undergoing immune checkpoint therapy: Systematic review and meta-analysis. Front. Immunol. 2024, 15, 1403771. [Google Scholar] [CrossRef]
- Gaissler, A.; Bochem, J.; Spreuer, J.; Ottmann, S.; Martens, A.; Amaral, T.; Wagner, N.B.; Claassen, M.; Meier, F.; Terheyden, P.; et al. Early decrease of blood myeloid-derived suppressor cells during checkpoint inhibition is a favorable biomarker in metastatic melanoma. J. Immunother. Cancer 2023, 11, e006802. [Google Scholar] [CrossRef] [PubMed]
- Dyikanov, D.; Zaitsev, A.; Vasileva, T.; Wang, I.; Sokolov, A.A.; Bolshakov, E.S.; Frank, A.; Turova, P.; Golubeva, O.; Gantseva, A.; et al. Comprehensive peripheral blood immunoprofiling reveals five immunotypes with immunotherapy response characteristics in patients with cancer. Cancer Cell 2024, 42, 759–779.e12. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Y.; Till, B.; Song, Y.; Wang, Z. Pretreatment Peripheral B Cells Are Associated With Tumor Response to Anti-PD-1-Based Immunotherapy. Front. Immunol. 2020, 11, 563653. [Google Scholar] [CrossRef]
- Barth, D.A.; Stanzer, S.; Spiegelberg, J.A.; Bauernhofer, T.; Absenger, G.; Szkandera, J.; Gerger, A.; Smolle, M.A.; Hutterer, G.C.; Ahyai, S.A.; et al. Patterns of Peripheral Blood B-Cell Subtypes Are Associated With Treatment Response in Patients Treated With Immune Checkpoint Inhibitors: A Prospective Longitudinal Pan-Cancer Study. Front. Immunol. 2022, 13, 840207. [Google Scholar] [CrossRef] [PubMed]
- Willsmore, Z.N.; Booth, L.; Patel, A.; Di Meo, A.; Prassas, I.; Chauhan, J.; Wu, Y.; Fitzpartick, A.; Stoker, K.; Kapiris, M.; et al. Circulating immunoregulatory B cell and autoreactive antibody profiles predict lack of toxicity to anti-PD-1 checkpoint inhibitor treatment in advanced melanoma. J. Immunother. Cancer 2025, 13, e011682. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Cristiani, C.M.; Capone, M.; Garofalo, C.; Madonna, G.; Mallardo, D.; Tuffanelli, M.; Vanella, V.; Greco, M.; Foti, D.P.; Viglietto, G.; et al. Altered frequencies and functions of innate lymphoid cells in melanoma patients are modulated by immune checkpoints inhibitors. Front. Immunol. 2022, 13, 811131. [Google Scholar] [CrossRef]
- Zhao, Q.; Bi, Y.; Sun, H.; Xiao, M. Serum IL-5 and IFN-gamma Are Novel Predictive Biomarkers for Anti-PD-1 Treatment in NSCLC and GC Patients. Dis. Markers 2021, 2021, 5526885. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, J.H.; Gide, T.N.; Menzies, A.M.; Guminski, A.; Carlino, M.S.; Breen, E.J.; Yang, J.Y.H.; Ghazanfar, S.; Kefford, R.F.; et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. 2019, 25, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Hardy-Werbin, M.; Rocha, P.; Arpi, O.; Taus, A.; Nonell, L.; Duran, X.; Villanueva, X.; Joseph-Pietras, D.; Nolan, L.; Danson, S.; et al. Serum cytokine levels as predictive biomarkers of benefit from ipilimumab in small cell lung cancer. Oncoimmunology 2019, 8, e1593810. [Google Scholar] [CrossRef]
- Mao, X.C.; Yang, C.C.; Yang, Y.F.; Yan, L.J.; Ding, Z.N.; Liu, H.; Yan, Y.C.; Dong, Z.R.; Wang, D.X.; Li, T. Peripheral cytokine levels as novel predictors of survival in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 884592. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Chen, H.; Yang, K.; Zhang, G.; Mao, B.; Hu, Y.; Zhang, H.; Xu, J. Peripheral cytokine levels as predictive biomarkers of benefit from immune checkpoint inhibitors in cancer therapy. Biomed. Pharmacother. 2020, 129, 110457. [Google Scholar] [CrossRef]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef]
- Murakami, S.; Shibaki, R.; Matsumoto, Y.; Yoshida, T.; Goto, Y.; Kanda, S.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; Ohe, Y. Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody. Thorac. Cancer 2020, 11, 3585–3595. [Google Scholar] [CrossRef]
- Brun, S.S.; Hansen, T.F.; Wen, S.W.C.; Nyhus, C.H.; Bertelsen, L.; Jakobsen, A.; Hansen, T.S.; Nederby, L. Soluble programmed death ligand 1 as prognostic biomarker in non-small cell lung cancer patients receiving nivolumab, pembrolizumab or atezolizumab therapy. Sci. Rep. 2024, 14, 8993. [Google Scholar] [CrossRef]
- Oya, K.; Nakamura, Y.; Shen, L.T.; Ishizuki, S.; Matsusaka, S.; Fujisawa, Y. Soluble PD-L1 predicts tumor response and immune-related adverse events in patients with advanced melanoma treated with anti-PD-1 antibodies. J. Dermatol. 2024, 51, 807–815. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Zhang, C.; Meng, X.; Sun, B.; Zhang, G.; Fan, Y.; Kang, X. The Clinical Significance of Soluble Programmed Cell Death-Ligand 1 (sPD-L1) in Patients With Gliomas. Front. Oncol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Himuro, H.; Nakahara, Y.; Igarashi, Y.; Kouro, T.; Higashijima, N.; Matsuo, N.; Murakami, S.; Wei, F.; Horaguchi, S.; Tsuji, K.; et al. Clinical roles of soluble PD-1 and PD-L1 in plasma of NSCLC patients treated with immune checkpoint inhibitors. Cancer Immunol. Immunother. 2023, 72, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, R.; Ieguchi, K.; Watanabe, M.; Takayanagi, D.; Goshima, T.; Onoue, R.; Hamada, K.; Kubota, Y.; Horiike, A.; Ishiguro, T.; et al. Increased Plasma Soluble PD-1 Concentration Correlates with Disease Progression in Patients with Cancer Treated with Anti-PD-1 Antibodies. Biomedicines 2021, 9, 1929. [Google Scholar] [CrossRef] [PubMed]
- Pistillo, M.P.; Fontana, V.; Morabito, A.; Dozin, B.; Laurent, S.; Carosio, R.; Banelli, B.; Ferrero, F.; Spano, L.; Tanda, E.; et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: An Italian melanoma intergroup study. Cancer Immunol. Immunother. 2019, 68, 97–107. [Google Scholar] [CrossRef]
- Daban, A.; Gonnin, C.; Phan, L.; Saldmann, A.; Granier, C.; Lillo-Lelouet, A.; Le Beller, C.; Pouchot, J.; Weiss, L.; Tartour, E.; et al. Preexisting autoantibodies as predictor of immune related adverse events (irAEs) for advanced solid tumors treated with immune checkpoint inhibitors (ICIs). Oncoimmunology 2023, 12, 2204754. [Google Scholar] [CrossRef]
- Genta, S.; Lajkosz, K.; Yee, N.R.; Spiliopoulou, P.; Heirali, A.; Hansen, A.R.; Siu, L.L.; Saibil, S.; Stayner, L.A.; Yanekina, M.; et al. Autoimmune PaneLs as PrEdictors of Toxicity in Patients TReated with Immune Checkpoint InhibiTors (ALERT). J. Exp. Clin. Cancer Res. 2023, 42, 276. [Google Scholar] [CrossRef] [PubMed]
- de Moel, E.C.; Rozeman, E.A.; Kapiteijn, E.H.; Verdegaal, E.M.E.; Grummels, A.; Bakker, J.A.; Huizinga, T.W.J.; Haanen, J.B.; Toes, R.E.M.; van der Woude, D. Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2019, 7, 6–11. [Google Scholar] [CrossRef]
- Giannicola, R.; D’Arrigo, G.; Botta, C.; Agostino, R.; Del Medico, P.; Falzea, A.C.; Barbieri, V.; Staropoli, N.; Del Giudice, T.; Pastina, P.; et al. Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol. Clin. Oncol. 2019, 11, 81–90. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Kallergi, G.; Vetsika, E.K.; Aggouraki, D.; Lagoudaki, E.; Koutsopoulos, A.; Koinis, F.; Katsarlinos, P.; Trypaki, M.; Messaritakis, I.; Stournaras, C.; et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758834017750121. [Google Scholar] [CrossRef]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef]
- Harel, M.; Lahav, C.; Jacob, E.; Dahan, N.; Sela, I.; Elon, Y.; Raveh Shoval, S.; Yahalom, G.; Kamer, I.; Zer, A.; et al. Longitudinal plasma proteomic profiling of patients with non-small cell lung cancer undergoing immune checkpoint blockade. J. Immunother. Cancer 2022, 10, e004582. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Xiao, H.; Xiong, Y.; Lu, X.; Yang, X.; Luo, W.; Luo, J.; Zhang, S.; Cheng, Y.; et al. Distinct circulating cytokine/chemokine profiles correlate with clinical benefit of immune checkpoint inhibitor monotherapy and combination therapy in advanced non-small cell lung cancer. Cancer Med. 2023, 12, 12234–12252. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reynies, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougouin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Coleman, K.; Schroeder, A.; Ma, N.; Irwin, D.J.; Lee, E.B.; Shinohara, R.T.; Li, M. SpaGCN: Integrating gene expression, spatial location and histology to identify spatial domains and spatially variable genes by graph convolutional network. Nat. Methods 2021, 18, 1342–1351. [Google Scholar] [CrossRef]
- Ren, H.; Walker, B.L.; Cang, Z.; Nie, Q. Identifying multicellular spatiotemporal organization of cells with SpaceFlow. Nat. Commun. 2022, 13, 4076. [Google Scholar] [CrossRef]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 2018, 23, 181–193.e7. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Wang, M.; Zhang, J.; Guo, C.; Han, G.; Wang, L. Integrated peripheral blood multi-omics profiling identifies immune signatures predictive of neoadjuvant PD-1 blockade efficacy in head and neck squamous cell carcinoma. J. Transl. Med. 2025, 23, 693. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, M.; Gao, Q.; Yuan, S.; Deng, W.; Bie, L.; Ma, Y.; Zhang, C.; Li, S.; Luo, S.; et al. Dynamic peripheral blood immune cell markers for predicting the response of patients with metastatic cancer to immune checkpoint inhibitors. Cancer Immunol. Immunother. 2023, 72, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lutes, L.; Barnoud, C.; Scheiermann, C. The circadian immune system. Sci. Immunol. 2022, 7, eabm2465. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Ludon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef] [PubMed]
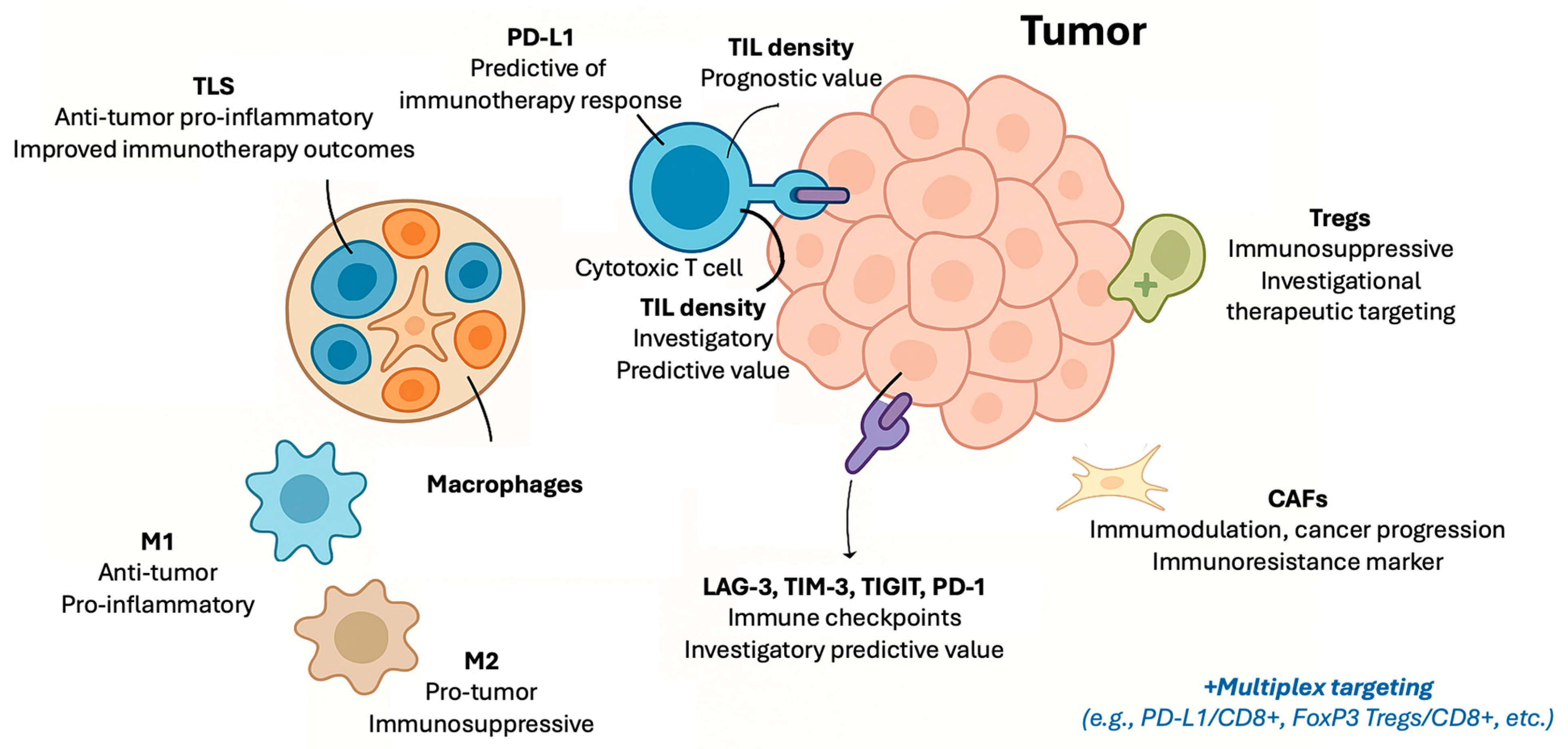
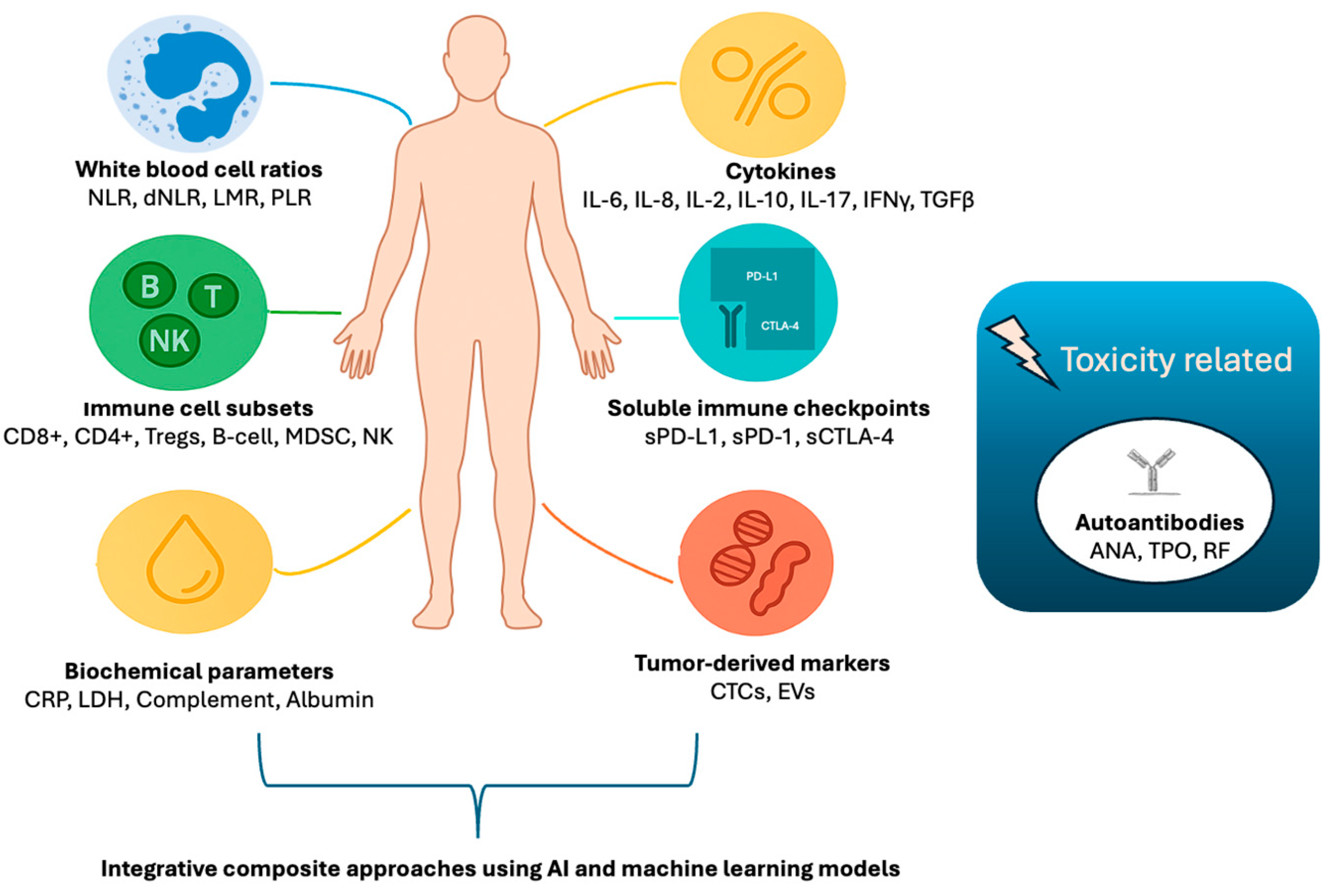
| Biomarker | Type of Marker | Prognostic Value | Predictive Value for Immunotherapy | Clinical Use | Assessment Method | Limitations | Key Tumor Types |
|---|---|---|---|---|---|---|---|
| PD-L1 [2,6,9,10,11,12,13,14,15,16,17,18,19,20] | Immune checkpoint protein | Prognostic value varies by context | Validated predictive biomarker for multiple tumor types | Routine clinical use | IHC and digital pathology | Heterogeneous expression, assay variability, and dynamic changes | NSCLC, urothelial, HNSCC, TNBC, gastric, and cervical |
| TILs [3,4,21,22,23,24,25] | Immune cell infiltration | Generally supported by evidence, with tumor-type variability | Emerging predictive marker; standardization ongoing | Recommended (breast cancer) and investigational (others) | IHC and digital pathology | Lack of standardized scoring and spatial heterogeneity | Melanoma, breast cancer, and NSCLC |
| Macrophages (M1/M2) [26,27] | Immune cell infiltration | Potential prognostic relevance; evidence evolving | Preliminary evidence suggests possible predictive role | Experimental | IHC and flow cytometry | Phenotypic plasticity and lack of standardized markers | Various solid tumors |
| CAFs [28] | Stromal cell component | Emerging evidence of association with poor prognosis | Investigational; may influence immunotherapy resistance | Experimental | IHC and multiplex assays | Heterogeneity and lack of standardized markers | NSCLC, skin, and other solid tumors |
| Tregs [29,30,31,32] | Immune cell infiltration | Associated with immune suppression; prognostic impact varies | Investigational predictive role; therapeutic targeting under study | Experimental | IHC and flow cytometry | Heterogeneity and complex roles in tumor immunity | Various solid tumors |
| TLS [33,34] | Organized immune structures | Supported by growing evidence; standardization pending | Emerging predictive marker; clinical validation ongoing | Investigational | IHC and digital pathology | Lack of standardized quantification | Various solid tumors and sarcoma |
| LAG-3 [35,36,37] | Immune checkpoint protein | Investigational | Investigational | Experimental | IHC and flow cytometry | Limited assay validation | Various solid tumors |
| TIM-3 [35,36,37] | Immune checkpoint protein | Investigational | Investigational | Experimental | IHC and flow cytometry | Limited assay validation | Various solid tumors |
| TIGIT [35,36,37] | Immune checkpoint protein | Investigational | Investigational | Experimental | IHC and flow cytometry | Limited assay validation | Various solid tumors |
| Multiplex Immune Markers [35,36,37,38,39,40,41,42,43,44] | PD-L1/CD8+, FoxP3-CD8+, etc. | Investigational | Predictive, not validated in clinical trails | Experimental | Multiplex techniques (e.g., IF) | Variability in studies and not validated in trials | Various solid tumors |
| Biomarker Category | Specific Markers/Indices | Biological Role | Clinical Relevance and Evidence | Limitations |
|---|---|---|---|---|
| White Blood Cell Ratios [45,46,47,48,49] | NLR, LMR, PLR, and dNLR | Reflect systemic inflammation and immune balance | Prognostic and predictive value across multiple tumors; composite scores improve stratification | Affected by infection, medications, comorbidities; lack of standardized cutoffs and timing |
| Biochemical Parameters [50,51,52,53,54,55] | CRP, LDH, complement components (C3, C4), and albumin | Markers of systemic inflammation, nutritional status, and immune activation | Included in composite scores; complement proteins emerging as immune modulators; albumin reflects nutritional/immune status | Influenced by non-cancer factors (infection, nutrition); need for further validation |
| Immune Cell Subsets [56,57,58,59,60,61,62,63,64,65,66,67,68,69] | CD8+ T-cells, CD4+ T-cells, Tregs, MDSCs, NK cells, B-cells, and hILCs | Effector, regulatory, suppressive, and innate immunity | Predictive and prognostic significance; dynamic changes during therapy correlate with response and toxicity | Complex analysis; need for assay standardization and prospective validation |
| Cytokines [70,71,72,73,74] | IFN-γ, IL-2, IL-6, IL-8, TNF-α, TGF-β, IL-10, and IL-17 | Immune activation or suppression through signaling | Baseline and dynamic levels predict response, survival, and irAEs; composite cytokine signatures promising | Biological variability; assay standardization needed; pleiotropic effects |
| Soluble Checkpoint Proteins [75,76,77,78,79,80,81,82] | sPD-L1, sPD-1, and sCTLA-4 | Modulate immune checkpoint pathways systemically | Elevated sPD-L1 linked to poor prognosis and resistance; dynamic changes correlate with therapy response | Assay variability; unclear biological functions of soluble vs. membrane forms |
| Autoantibodies [83,84,85,86,87] | ANA, anti-TPO, rheumatoid factor, and others | Reflect autoimmunity and immune activation | Associated with immune-related adverse events; possible link to treatment efficacy | Variability in assays; heterogeneity of targets; clinical utility still investigational |
| Tumor-Derived Circulating Biomarkers [88,89,90,91] | CTCs and EVs | Reflect tumor burden, immune evasion via checkpoint expression | CTC PD-L1 expression and PD-L1+ EVs correlate with resistance and prognosis; promising for monitoring | Technical challenges in isolation, characterization, and standardization |
| Integrative Composite Approaches [40,64,68,92,93,94] | Multi-modal biomarker panels combining tissue, peripheral blood, soluble factors, and tumor-derived markers | Capture complex immune landscapes and tumor heterogeneity | Composite immunoscores improve prediction of immunotherapy response; integration of AI/ML enhances biomarker discovery | Data harmonization, standardization, and clinical validation remain significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trontzas, I.P.; Syrigos, K.N. Immune Biomarkers for Checkpoint Blockade in Solid Tumors: Transitioning from Tissue to Peripheral Blood Monitoring and Future Integrated Strategies. Cancers 2025, 17, 2639. https://doi.org/10.3390/cancers17162639
Trontzas IP, Syrigos KN. Immune Biomarkers for Checkpoint Blockade in Solid Tumors: Transitioning from Tissue to Peripheral Blood Monitoring and Future Integrated Strategies. Cancers. 2025; 17(16):2639. https://doi.org/10.3390/cancers17162639
Chicago/Turabian StyleTrontzas, Ioannis P., and Konstantinos N. Syrigos. 2025. "Immune Biomarkers for Checkpoint Blockade in Solid Tumors: Transitioning from Tissue to Peripheral Blood Monitoring and Future Integrated Strategies" Cancers 17, no. 16: 2639. https://doi.org/10.3390/cancers17162639
APA StyleTrontzas, I. P., & Syrigos, K. N. (2025). Immune Biomarkers for Checkpoint Blockade in Solid Tumors: Transitioning from Tissue to Peripheral Blood Monitoring and Future Integrated Strategies. Cancers, 17(16), 2639. https://doi.org/10.3390/cancers17162639






