PET/CT and Paraneoplastic Syndromes: A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. FDG PET/CT Basics
3. Classification of Paraneoplastic Syndromes
3.1. Neurologic Paraneoplastic Syndromes
3.1.1. Central Nervous System Syndromes
3.1.2. Peripheral Nervous System (PNS) Syndromes
3.2. Non-Neurologic Paraneoplastic Syndromes
3.2.1. Endocrinologic Syndromes
3.2.2. Dermatological Syndromes
3.2.3. Musculoskeletal Syndromes
3.2.4. Rheumatological Syndromes
3.2.5. Hematologic Syndromes
3.2.6. Gastrointestinal Syndromes
4. Role of PET/CT in Paraneoplastic Syndromes
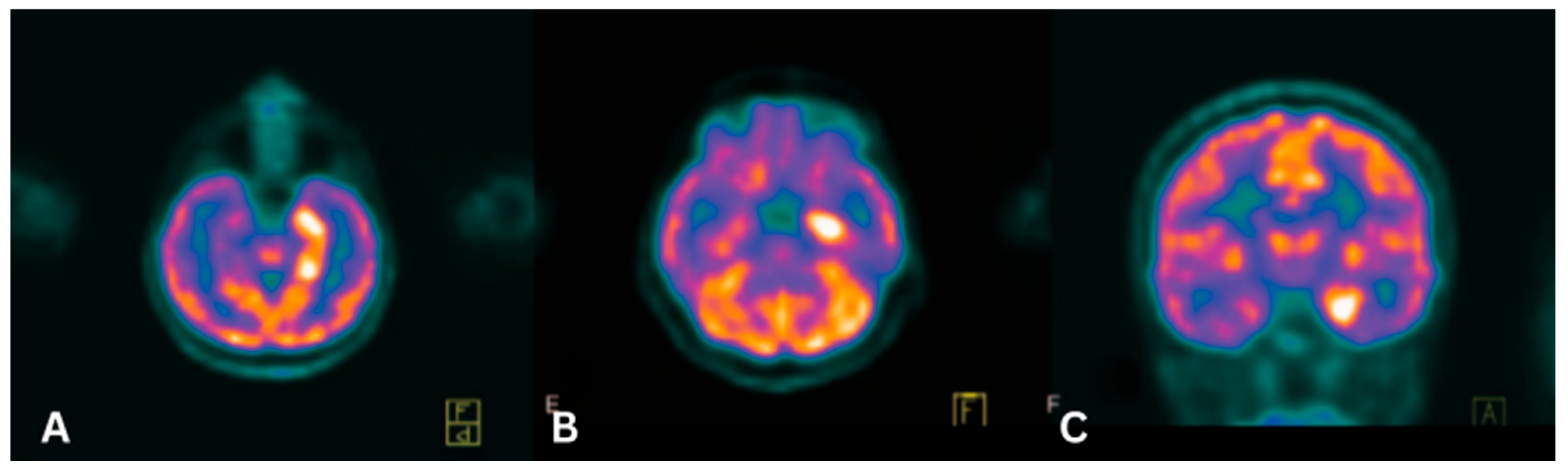
4.1. Paraneoplastic Limbic Encephalitis (PLE)
4.2. Paraneoplastic Cerebellar Degeneration (PCD)
4.3. Opsoclonus-Myoclonus Syndrome (OMS)
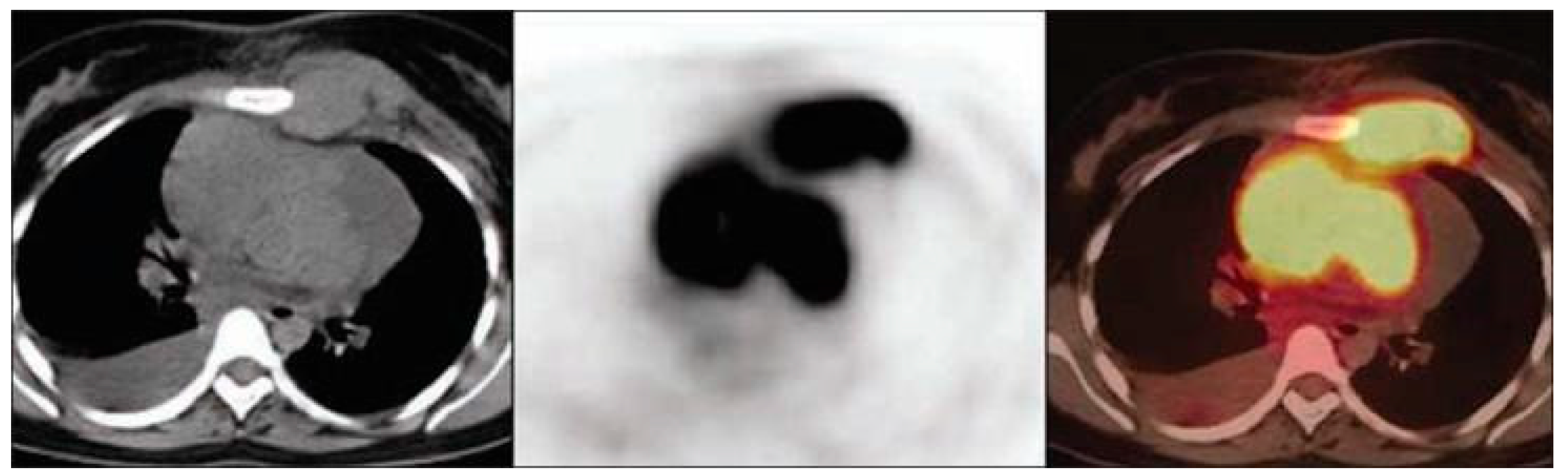
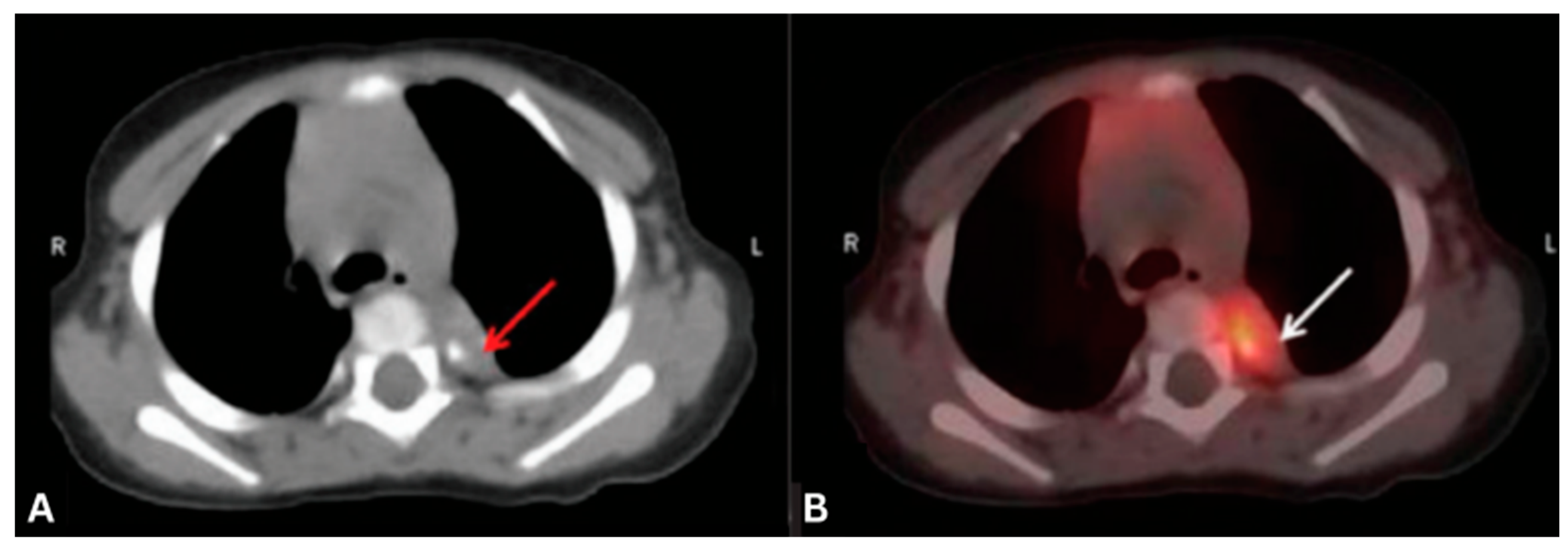
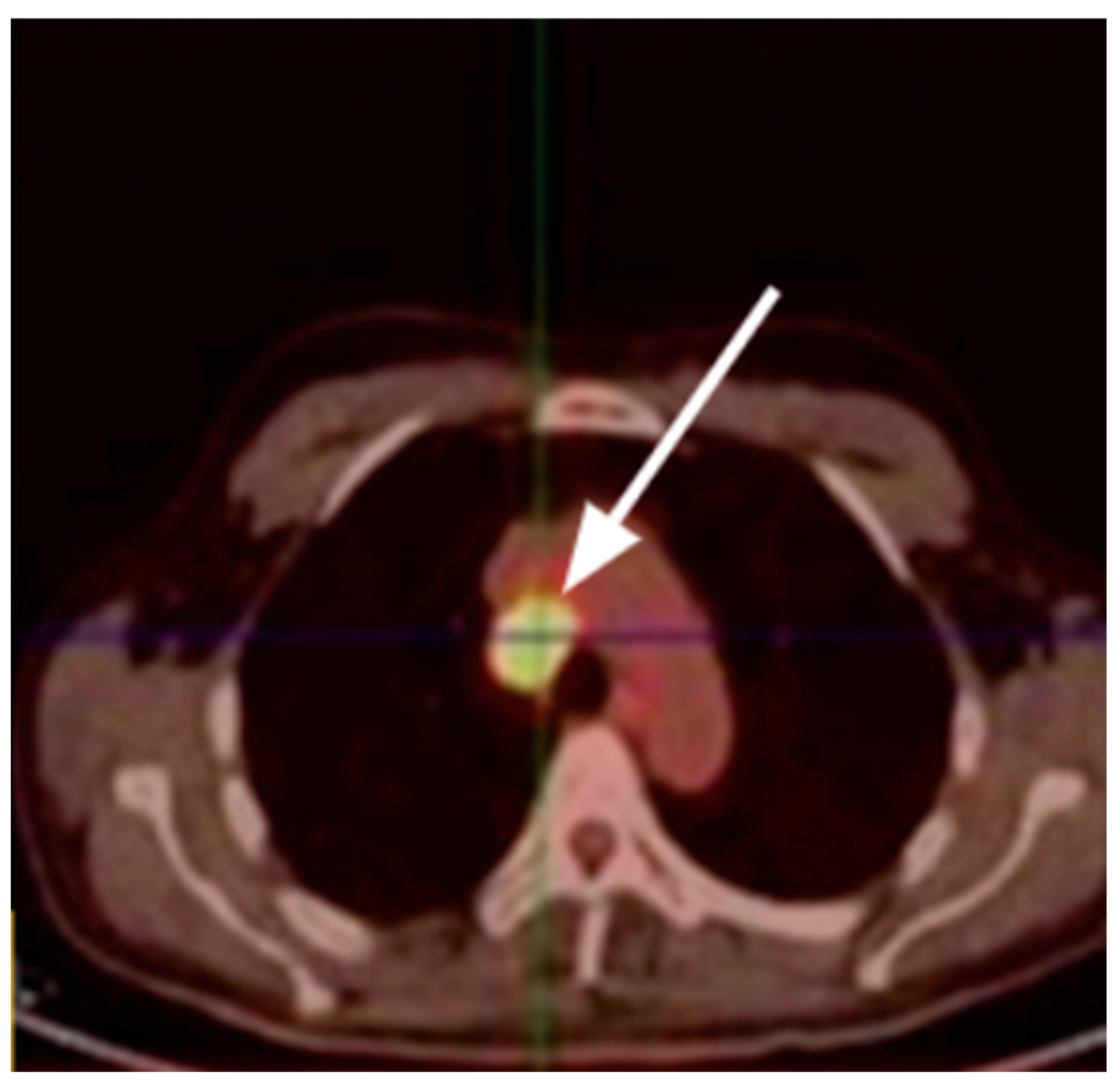
4.4. Lambert-Eaton Myasthenic Syndrome (LEMS)
4.5. Paraneoplastic Cushing’s Syndrome (PCS)
4.6. Hypercalcemia of Malignancy
4.7. Dermatomyositis (DM)
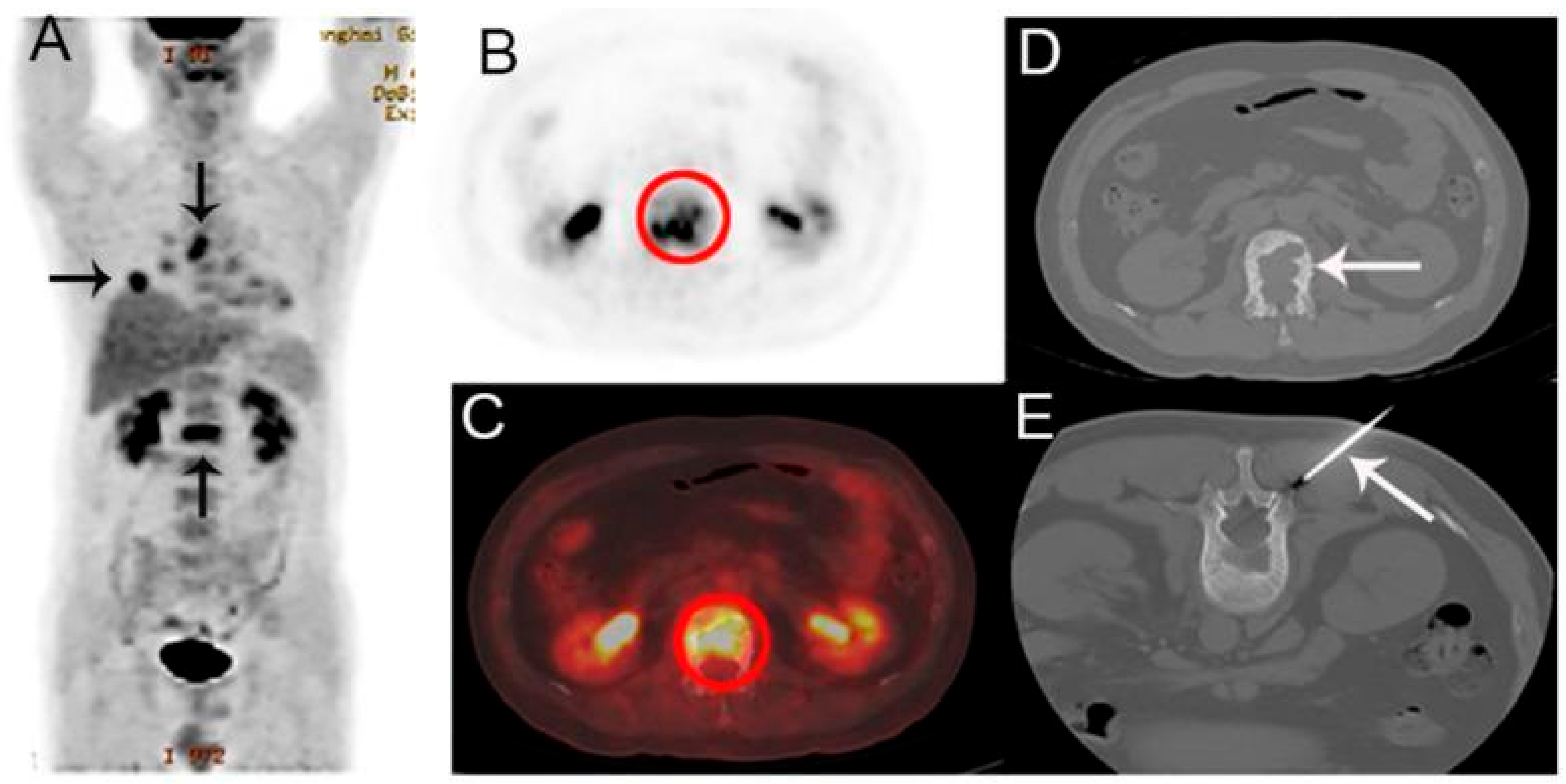
4.8. Tumor-Induced Osteomalacia (TIO)
4.9. Other Paraneoplastic Syndromes with Limited PET/CT Data
4.10. General Limitations of PET/CT and Tracer Selection Considerations
5. Summary of Diagnostic Utility of FDG PET/CT in PNSs
6. Clinical Impact on Patient Management
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic Hormone |
| ALK | Anaplastic Lymphoma Kinase |
| ALL | Acute Lymphoblastic Leukemia |
| AlF | Aluminum Fluoride |
| CLL | Chronic Lymphocytic Leukemia |
| CNS | Central Nervous System |
| CRMP5 | Collapsin Response Mediator Protein 5 |
| CSF | Cerebrospinal Fluid |
| CT | Computed Tomography |
| CV2 | Collapsin Response Mediator Protein 5 (also known as CV2) |
| DM | Dermatomyositis |
| DOTANOC | DOTA-1-Nal3-octreotide |
| DOTATATE | DOTA-DPhe1-Tyr3-Octreotate |
| EPO | Erythropoietin |
| FDG | Fluorodeoxyglucose |
| FGF23 | Fibroblast Growth Factor 23 |
| FNA | Fine Needle Aspiration |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HOA | Hypertrophic Osteoarthropathy |
| IGF | Insulin-like Growth Factor |
| IL | Interleukin |
| LEMS | Lambert-Eaton Myasthenic Syndrome |
| MIBG | Metaiodobenzylguanidine |
| MRI | Magnetic Resonance Imaging |
| NPV | Negative Predictive Value |
| NSCLC | Non-Small Cell Lung Cancer |
| OMS | Opsoclonus-Myoclonus Syndrome |
| PET | Positron Emission Tomography |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| PET/MRI | Positron Emission Tomography/Magnetic Resonance Imaging |
| PLR | Paraneoplastic Leukemoid Reaction |
| PMT | Phosphaturic Mesenchymal Tumor |
| PPV | Positive Predictive Value |
| PNS | Paraneoplastic Syndromes |
| PCD | Paraneoplastic Cerebellar Degeneration |
| PLE | Paraneoplastic Limbic Encephalitis |
| PTH | Parathyroid Hormone |
| PTHrP | Parathyroid Hormone-related Protein |
| RT | Radiotherapy |
| SCLC | Small Cell Lung Cancer |
| SPECT | Single Photon Emission Computed Tomography |
| SSR | Somatostatin Receptor |
| SSN | Subacute Sensory Neuronopathy |
| SUVmax | Maximum Standardized Uptake Value |
| TGF | Transforming Growth Factor |
| TIO | Tumor-Induced Osteomalacia |
| VEGF | Vascular Endothelial Growth Factor |
| VGCC | Voltage-Gated Calcium Channel |
| VIP | Vasoactive Intestinal Peptide |
| VIPoma | Vasoactive Intestinal Peptide-Secreting Tumor |
References
- Pelosof, L.C.; Gerber, D.E. Paraneoplastic Syndromes: An Approach to Diagnosis and Treatment. Mayo Clin. Proc. 2010, 85, 838–854. [Google Scholar] [CrossRef]
- Kanaji, N.; Watanabe, N.; Kita, N.; Bandoh, S.; Tadokoro, A.; Ishii, T.; Dobashi, H.; Matsunaga, T. Paraneoplastic Syndromes Associated with Lung Cancer. World J. Clin. Oncol. 2014, 5, 197–223. [Google Scholar] [CrossRef]
- Honnorat, J.; Antoine, J.-C. Paraneoplastic Neurological Syndromes. Orphanet J. Rare Dis. 2007, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Lourenço, A.; Ribeiro, M. Psychiatric Manifestations of Paraneoplastic Syndromes. Eur. Psychiatry 2022, 65, S661. [Google Scholar] [CrossRef]
- Dec, M.; Arasiewicz, H. Paraneoplastic Syndromes in Patients with Melanoma. Postep. Dermatol. Alergol. 2024, 41, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.H. The Role of [18F]Fluoro-2-Deoxyglucose-PET Scanning in the Diagnosis of Paraneoplastic Neurological Disorders. Brain 2001, 124, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Opalińska, M.; Sowa-Staszczak, A.; Wężyk, K.; Jagiełła, J.; Słowik, A.; Hubalewska-Dydejczyk, A. Additional Value of [18F]FDG PET/CT in Detection of Suspected Malignancy in Patients with Paraneoplastic Neurological Syndromes Having Negative Results of Conventional Radiological Imaging. J. Clin. Med. 2022, 11, 1537. [Google Scholar] [CrossRef]
- Younes-Mhenni, S.; Janier, M.F.; Cinotti, L.; Antoine, J.C.; Tronc, F.; Cottin, V.; Ternamian, P.J.; Trouillas, P.; Honnorat, J. FDG-PET Improves Tumour Detection in Patients with Paraneoplastic Neurological Syndromes. Brain 2004, 127, 2331–2338. [Google Scholar] [CrossRef]
- Bronstein, Y.; Tummala, S.; Rohren, E. F-18 FDG PET/CT for Detection of Malignant Involvement of Peripheral Nerves: Case Series and Literature Review. Clin. Nucl. Med. 2011, 36, 96–100. [Google Scholar] [CrossRef]
- Lee, J.R.; Kim, J.S.; Roh, J.-L.; Lee, J.H.; Baek, J.H.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Detection of Occult Primary Tumors in Patients with Cervical Metastases of Unknown Primary Tumors: Comparison of (18)F FDG PET/CT with Contrast-Enhanced CT or CT/MR Imaging-Prospective Study. Radiology 2015, 274, 764–771. [Google Scholar] [CrossRef]
- Altini, C.; Lavelli, V.; Ruta, R.; Ferrari, C.; Nappi, A.G.; Pisani, A.; Sardaro, A.; Rubini, G. Typical and Atypical PET/CT Findings in Non-Cancerous Conditions. Hell. J. Nucl. Med. 2020, 23, 48–59. [Google Scholar]
- Kirienko, M.; Gelardi, F.; Fiz, F.; Bauckneht, M.; Ninatti, G.; Pini, C.; Briganti, A.; Falconi, M.; Oyen, W.J.G.; van der Graaf, W.T.A.; et al. Personalised PET Imaging in Oncology: An Umbrella Review of Meta-Analyses to Guide the Appropriate Radiopharmaceutical Choice and Indication. Eur. J. Nucl. Med. Mol. Imaging 2024, 52, 208–224. [Google Scholar] [CrossRef]
- Vatankulu, B.; Yilmaz Aksoy, S.; Sager, S.; Halaç, M. Comments on Kristensen et al.: Clinical Value of FDG-PET/CT in Suspected Paraneoplastic Syndromes: A Retrospective Analysis of 137 Patients. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 580–581. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Matsuyama, T.; Ohmine, K.; Suzuki, T.; Mori, M.; Nagai, T.; Muroi, K.; Ozawa, K. “Incidental Carcinomas” Detected by PET/CT Scans in the Patients with Malignant Lymphoma: A Single-Center Experience with 363 Patients. Blood 2010, 116, 4132. [Google Scholar] [CrossRef]
- Frings, V.; van Velden, F.H.P.; Velasquez, L.M.; Hayes, W.; van de Ven, P.M.; Hoekstra, O.S.; Boellaard, R. Repeatability of Metabolically Active Tumor Volume Measurements with FDG PET/CT in Advanced Gastrointestinal Malignancies: A Multicenter Study. Radiology 2014, 273, 539–548. [Google Scholar] [CrossRef]
- Zasadny, K.R.; Kison, P.V.; Francis, I.R.; Wahl, R.L. FDG-PET Determination of Metabolically Active Tumor Volume and Comparison with CT. Clin. Positron Imaging 1998, 1, 123–129. [Google Scholar] [CrossRef]
- Kumar, A.; Dutta, R.; Kannan, U.; Kumar, R.; Khilnani, G.C.; Gupta, S.D. Evaluation of Mediastinal Lymph Nodes Using F-FDG PET-CT Scan and Its Histopathologic Correlation. Ann. Thorac. Med. 2011, 6, 11–16. [Google Scholar] [CrossRef]
- Henry, K. Paraneoplastic Syndromes: Definitions, Classification, Pathophysiology and Principles of Treatment. Semin. Diagn. Pathol. 2019, 36, 204–210. [Google Scholar] [CrossRef]
- Castagnoli, H.; Manni, C.; Marchesani, F.; Rossi, G.; Fattori, S.; Capoccetti, F. The Role of 18F-FDG PET/CT in Management of Paraneoplastic Limbic Encephalitis Combined with Small Cell Lung Cancer: A Case Report. Med. Baltim. 2019, 98, e16593. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, B.; Schröder, J.-B.; Warnecke, T.; Heindel, W.; Schäfers, M.; Weckesser, M.; Buerke, B. Imaging Workup of Suspected Classical Paraneoplastic Neurological Syndromes: A Systematic Review and Retrospective Analysis of 18F-FDG-PET-CT. Acad. Radiol. 2017, 24, 1195–1202. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Marcus, C.V.; Fragomeni, R.S.; Rowe, S.P.; Javadi, M.S.; Solnes, L.B. Whole-Body 18F-FDG PET and 18F-FDG PET/CT in Patients with Suspected Paraneoplastic Syndrome: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. J. Nucl. Med. 2017, 58, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Berner, U.; Menzel, C.; Rinne, D.; Kriener, S.; Hamscho, N.; Döbert, N.; Diehl, M.; Kaufmann, R.; Grünwald, F. Paraneoplastic Syndromes: Detection of Malignant Tumors Using [(18)F]FDG-PET. Q. J. Nucl. Med. Mol. Imaging 2003, 47, 85–89. [Google Scholar]
- Gultekin, S.H. Paraneoplastic Limbic Encephalitis: Neurological Symptoms, Immunological Findings and Tumour Association in 50 Patients. Brain 2000, 123, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Gurrieri, C.; Visentin, A.; Bussè, C.; Piazza, F.; Manara, R.; Trentin, L.; Briani, C. Limbic Encephalitis with HU-Antibodies in T-Cell Anaplastic Lymphoma. A Case Report. Appl. Sci. 2021, 11, 6548. [Google Scholar] [CrossRef]
- Venkatraman, A.; Opal, P. Paraneoplastic Cerebellar Degeneration with anti-Yo Antibodies—A Review. Ann. Clin. Transl. Neurol. 2016, 3, 655–663. [Google Scholar] [CrossRef]
- Lawn, N.D.; Westmoreland, B.F.; Kiely, M.J.; Lennon, V.A.; Vernino, S. Clinical, Magnetic Resonance Imaging, and Electroencephalographic Findings in Paraneoplastic Limbic Encephalitis. Mayo Clin. Proc. 2003, 78, 1363–1368. [Google Scholar] [CrossRef]
- Shen, K.; Xu, Y.; Guan, H.; Zhong, W.; Chen, M.; Zhao, J.; Li, L.; Wang, M. Paraneoplastic Limbic Encephalitis Associated with Lung Cancer. Sci. Rep. 2018, 8, 6792. [Google Scholar] [CrossRef]
- Inoue, T.; Kanno, R.; Moriya, A.; Nakamura, K.; Watanabe, Y.; Matsumura, Y.; Suzuki, H. A Case of Paraneoplastic Limbic Encephalitis in a Patient with Invasive Thymoma with Anti-Glutamate Receptor Antibody-Positive Cerebrospinal Fluid: A Case Report. Ann. Thorac. Cardiovasc. Surg. 2017, 24, 200–204. [Google Scholar] [CrossRef]
- Shams’ili, S.; Grefkens, J.; de Leeuw, B.; van den Bent, M.; Hooijkaas, H.; van der Holt, B.; Vecht, C.; Sillevis Smitt, P. Paraneoplastic Cerebellar Degeneration Associated with Antineuronal Antibodies: Analysis of 50 Patients. Brain 2003, 126, 1409–1418. [Google Scholar] [CrossRef]
- Whaley, J.J.J.-V.; Carrera-Muiños, A.; Hernandez-Gutierrez, K.G.; Rodriguez-Cid, J.R.; Otero-Cerdeira, M.E.; Garcia-Montes, V. Paraneoplastic Cerebellar Degeneration Associated with Anti-CV2/CRMP5 Antibodies in Ovarian Cancer: Case Report and Review of Literature. Case Rep. Oncol. 2021, 14, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Blaes, F.; Dharmalingam, B. Childhood Opsoclonus-Myoclonus Syndrome: Diagnosis and Treatment. Expert. Rev. Neurother. 2016, 16, 641–648. [Google Scholar] [CrossRef]
- Raffaghello, L.; Conte, M.; De Grandis, E.; Pistoia, V. Immunological Mechanisms in Opsoclonus-Myoclonus Associated Neuroblastoma. Eur. J. Paediatr. Neurol. 2009, 13, 219–223. [Google Scholar] [CrossRef]
- Du, H.; Cai, W. Opsoclonus-Myoclonus Syndrome Associated with Neuroblastoma: Insights into Antitumor Immunity. Pediatr. Blood Cancer 2022, 69, e29949. [Google Scholar] [CrossRef] [PubMed]
- Garner, S.; Giakas, A.; Holder, K.; Galvan, B.; Edwards, H. Opsoclonus Myoclonus and Ataxia Syndrome with Supraventricular Tachycardia. Proc. Bayl. Univ. Med. Cent. 2023, 36, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, C.; Dawit, S.; Henry, K.; Farrugia, L.; Sharma, A.; Grill, M. Ncmp-04. Opsoclonus-Myoclonus Syndrome Associated with Contactin-Associated Protein-like 2 Autoantibody in the Setting of Non-Small Cell Lung Carcinoma. Neuro Oncol. 2019, 21, vi180. [Google Scholar] [CrossRef]
- Kostoglou, A.; Vlastos, D.; Bakalis, A.; Ghosh, D. Breast Cancer-Associated Opsoclonus-Myoclonus Syndrome: A Case Report. World J. Surg. Oncol. 2021, 19, 328. [Google Scholar] [CrossRef]
- Lebeer, M.; Montagna, M.; Coito, S.; Reynders, T.; Raskin, J. A Rare Case of Opsoclonus-Myoclonus Associated with SCLC. Acta Neurol. Belg. 2020, 120, 1017–1019. [Google Scholar] [CrossRef]
- Noguchi, K.; Ikawa, Y.; Takenaka, M.; Sakai, Y.; Fujiki, T.; Kuroda, R.; Ikeda, H.; Nakada, S.; Nomura, K.; Sakai, S.; et al. Presence of Identical B-Cell Clone in Both Cerebrospinal Fluid and Tumor Tissue in a Patient with Opsoclonus-Myoclonus Syndrome Associated with Neuroblastoma. Pediatr. Hematol. Oncol. 2023, 40, 363–370. [Google Scholar] [CrossRef]
- Krug, P.; Schleiermacher, G.; Michon, J.; Valteau-Couanet, D.; Brisse, H.; Peuchmaur, M.; Sarnacki, S.; Martelli, H.; Desguerre, I.; Tardieu, M. Opsoclonus-Myoclonus in Children Associated or Not with Neuroblastoma. Eur. J. Paediatr. Neurol. 2010, 14, 400–409. [Google Scholar] [CrossRef]
- Matsuo, H. Lambert-Eaton myasthenic syndrome. Brain Nerve 2024, 76, 630–634. [Google Scholar]
- Kesner, V.G.; Oh, S.J.; Dimachkie, M.M.; Barohn, R.J. Lambert-Eaton Myasthenic Syndrome. Neurol. Clin. 2018, 36, 379–394. [Google Scholar] [CrossRef]
- Bi, W.L.; Bannykh, S.I.; Martel, M.; Baehring, J.M. Paraneoplastic Subacute Sensory Neuronopathy Secondary to a Malignant Mixed Müllerian Tumor. Obstet. Gynecol. 2006, 107, 504–506. [Google Scholar] [CrossRef]
- Camdessanché, J.-P.; Antoine, J.-C.; Honnorat, J.; Vial, C.; Petiot, P.; Convers, P.; Michel, D. Paraneoplastic Peripheral Neuropathy Associated with anti-Hu Antibodies. Brain 2002, 125, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S. Advances in the Understanding of Sensory Neuronopathies. J. Neurol. Neurosurg. Psychiatry 2021, 92, 343. [Google Scholar] [CrossRef]
- Martinez, A.R.M.; Nunes, M.B.; Nucci, A.; França, M.C., Jr. Sensory Neuronopathy and Autoimmune Diseases. Autoimmune Dis. 2012, 2012, 873587. [Google Scholar] [CrossRef]
- Malinow, K.; Yannakakis, G.D.; Glusman, S.M.; Edlow, D.W.; Griffin, J.; Pestronk, A.; Powell, D.L.; Ramsey-Goldman, R.; Eidelman, B.H.; Medsger, T.A., Jr. Subacute Sensory Neuronopathy Secondary to Dorsal Root Ganglionitis in Primary Sjögren’s Syndrome. Ann. Neurol. 1986, 20, 535–537. [Google Scholar] [CrossRef]
- Mongay-Ochoa, N.; Vogrig, A.; Muñiz-Castrillo, S.; Honnorat, J. Anti-Hu-Associated Paraneoplastic Syndromes Triggered by Immune-Checkpoint Inhibitor Treatment. J. Neurol. 2020, 267, 2154–2156. [Google Scholar] [CrossRef]
- Chalk, C.H.; Lennon, V.A.; Stevens, J.C.; Windebank, A.J. Seronegativity for Type 1 Antineuronal Nuclear Antibodies (‘anti-Hu’) in Subacute Sensory Neuronopathy Patients without Cancer. Neurology 1993, 43, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Mignotte, H.; Shestaeva, O.; Vignoud, L.; Guillem, P.; Ruckly, S.; Chabre, O.; Sakhri, L.; Duruisseaux, M.; Mousseau, M.; Timsit, J.-F.; et al. Prognostic Impact of Paraneoplastic Cushing’s Syndrome in Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Richa, C.G.; Saad, K.J.; Halabi, G.H.; Gharios, E.M.; Nasr, F.L.; Merheb, M.T. Case-Series of Paraneoplastic Cushing Syndrome in Small-Cell Lung Cancer. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Qi, X.; Yu, L.; Lin, L. Management of Small Cell Lung Cancer Complicated with Paraneoplastic Cushing’s Syndrome: A Systematic Literature Review. Front. Endocrinol. Lausanne 2023, 14, 1177125. [Google Scholar] [CrossRef]
- Noorlander, I.; Elte, J.W.; Manintveld, O.C.; Tournoy, K.G.; Praet, M.M.; van Meerbeeck, J.P.; Aerts, J.G. A Case of Recurrent Non-Small-Cell Lung Carcinoma and Paraneoplastic Cushing’s Syndrome. Lung Cancer 2006, 51, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.R.; Grossman, A.B. The Ectopic Adrenocorticotropic Hormone Syndrome: Rarely Easy, Always Challenging. Endocrinol. Metab. Clin. N. Am. 2018, 47, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Giovanella, L.; Lococo, F.; Bertagna, F. An Unusual Case of Thymic Carcinoid Causing Cushing’s Syndrome Due to Ectopic ACTH Secretion Detected by (18)F-FDG PET/CT. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 253–254. [Google Scholar] [PubMed]
- Sato, K.; Onuma, E.; Yocum, R.C.; Ogata, E. Treatment of Malignancy-Associated Hypercalcemia and Cachexia with Humanized Anti-Parathyroid Hormone-Related Protein Antibody. Semin. Oncol. 2003, 30, 167–173. [Google Scholar] [CrossRef]
- Kukreja, S.C.; Shevrin, D.H.; Wimbiscus, S.A.; Ebeling, P.R.; Danks, J.A.; Rodda, C.P.; Wood, W.I.; Martin, T.J. Antibodies to Parathyroid Hormone-Related Protein Lower Serum Calcium in Athymic Mouse Models of Malignancy-Associated Hypercalcemia Due to Human Tumors. J. Clin. Invest. 1988, 82, 1798–1802. [Google Scholar] [CrossRef]
- Pitts, S.; Mahipal, A.; Bajor, D.; Mohamed, A. Hypercalcemia of Malignancy Caused by Parathyroid Hormone-Related Peptide-Secreting Pancreatic Neuroendocrine Tumors (PTHrP-PNETs): Case Report. Front. Oncol. 2023, 13, 1197288. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J.; Moseley, J.M.; Gillespie, M.T. Parathyroid Hormone-Related Protein: Biochemistry and Molecular Biology. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 377–395. [Google Scholar] [CrossRef]
- Sternlicht, H.; Glezerman, I.G. Hypercalcemia of Malignancy and New Treatment Options. Ther. Clin. Risk Manag. 2015, 11, 1779–1788. [Google Scholar] [CrossRef]
- Anastasopoulou, C.; Mewawalla, P. Malignancy-Related Hypercalcemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/nbk482423/ (accessed on).
- Asonitis, N.; Angelousi, A.; Zafeiris, C.; Lambrou, G.I.; Dontas, I.; Kassi, E. Diagnosis, Pathophysiology and Management of Hypercalcemia in Malignancy: A Review of the Literature. Horm. Metab. Res. 2019, 51, 770–778. [Google Scholar] [CrossRef] [PubMed]
- DeWane, M.E.; Waldman, R.; Lu, J. Dermatomyositis: Clinical Features and Pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, I.; Kazandjieva, J.; Darlenski, R.; Tsankov, N. Dermatomyositis: Current Concepts. Clin. Dermatol. 2018, 36, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Wolstencroft, P.W.; Fiorentino, D.F. Dermatomyositis Clinical and Pathological Phenotypes Associated with Myositis-Specific Autoantibodies. Curr. Rheumatol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef]
- Okiyama, N. Clinical Features and Cutaneous Manifestations of Juvenile and Adult Patients of Dermatomyositis Associated with Myositis-Specific Autoantibodies. J. Clin. Med. 2021, 10, 1725. [Google Scholar] [CrossRef]
- Wilgenbus, K.; Lentner, A.; Kuckelkorn, R.; Handt, S.; Mittermayer, C. Further Evidence That Acanthosis Nigricans Maligna Is Linked to Enhanced Secretion by the Tumour of Transforming Growth Factor Alpha. Arch. Derm. Res. 1992, 284, 266–270. [Google Scholar] [CrossRef]
- Koyama, S.; Ikeda, K.; Sato, M.; Shibahara, K.; Yuhara, K.; Fukutomi, H.; Fukunaga, K.; Kanazawa, N.; Yuzawa, K.; Fukao, K.; et al. Transforming Growth Factor-Alpha (TGF?)-Producing Gastric Carcinoma with Acanthosis Nigricans: An Endocrine Effect of TGF? In the Pathogenesis of Cutaneous Paraneoplastic Syndrome and Epithelial Hyperplasia of the Esophagus. J. Gastroenterol. 1997, 32, 71–77. [Google Scholar] [CrossRef]
- Torley, D.; Bellus, G.A.; Munro, C.S. Genes, Growth Factors and Acanthosis Nigricans. Br. J. Dermatol. 2002, 147, 1096–1101. [Google Scholar] [CrossRef]
- Cruz, P.D., Jr.; Hud, J.A., Jr. Excess Insulin Binding to Insulin-like Growth Factor Receptors: Proposed Mechanism for Acanthosis Nigricans. J. Invest. Dermatol. 1992, 98, S82–S85. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Lam, J.M.; Barankin, B.; Leong, K.F.; Hon, K.L. Acanthosis Nigricans: An Updated Review. Curr. Pediatr. Rev. 2022, 19, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Salati, S.A.; Alqarawi, L.A.; Alquraishi, Y.F. Acanthosis Nigricans: An Extensive Review. J. Pak. Assoc. Dermatol. 2021, 31. [Google Scholar]
- Kutlubay, Z.; Engin, B.; Bairamov, O.; Tüzün, Y. Acanthosis Nigricans: A Fold (Intertriginous) Dermatosis. Clin. Dermatol. 2015, 33, 466–470. [Google Scholar] [CrossRef]
- Antiga, E.; Bech, R.; Maglie, R.; Genovese, G.; Borradori, L.; Bockle, B.; Caproni, M.; Caux, F.; Chandran, N.S.; Corrà, A.; et al. S2k Guidelines on the Management of Paraneoplastic Pemphigus/Paraneoplastic Autoimmune Multiorgan Syndrome Initiated by the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1118–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, B. Paraneoplastic Pemphigus. J. Dermatol. 2007, 34, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Oursler, J.R.; Labib, R.S.; Ariss-Abdo, L.; Burke, T.; O’Keefe, E.J.; Anhalt, G.J. Human Autoantibodies against Desmoplakins in Paraneoplastic Pemphigus. J. Clin. Invest. 1992, 89, 1775–1782. [Google Scholar] [CrossRef]
- Poot, A.M.; Siland, J.; Jonkman, M.F.; Pas, H.H.; Diercks, G.F.H. Direct and Indirect Immunofluorescence Staining Patterns in the Diagnosis of Paraneoplastic Pemphigus. Br. J. Dermatol. 2016, 174, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Numata, S.; Teye, K.; Tsuruta, D.; Sogame, R.; Ishii, N.; Koga, H.; Natsuaki, Y.; Tsuchisaka, A.; Hamada, T.; Karashima, T.; et al. Anti-α-2-Macroglobulin-like-1 Autoantibodies Are Detected Frequently and May Be Pathogenic in Paraneoplastic Pemphigus. J. Invest. Dermatol. 2013, 133, 1785–1793. [Google Scholar] [CrossRef]
- Kim, Y.; Stein, E.; Remotti, F.; Lee, F.Y. Tumor-Induced Osteomalacia Secondary to a Fibroblast Growth Factor 23-Secreting Phosphaturic Mesenchymal Tumor in the Foot. JBJS Case Connect. 2014, 4, e22. [Google Scholar] [CrossRef]
- Nagae, K.; Uchi, H.; Ito, T.; Moroi, Y.; Oda, Y.; Furue, M. Osteomalacia Induced by a Phosphaturic Mesenchymal Tumor Secreting Fibroblast Growth Factor 23. Eur. J. Dermatol. 2015, 25, 199–200. [Google Scholar] [CrossRef]
- Imel, E.A.; Peacock, M.; Pitukcheewanont, P.; Heller, H.J.; Ward, L.M.; Shulman, D.; Kassem, M.; Rackoff, P.; Zimering, M.; Dalkin, A.; et al. Sensitivity of Fibroblast Growth Factor 23 Measurements in Tumor-Induced Osteomalacia. J. Clin. Endocrinol. Metab. 2006, 91, 2055–2061. [Google Scholar] [CrossRef]
- Minisola, S.; Peacock, M.; Fukumoto, S.; Cipriani, C.; Pepe, J.; Tella, S.H.; Collins, M.T. Tumour-Induced Osteomalacia. Nat. Rev. Primer 2017, 3, 17044. [Google Scholar] [CrossRef]
- Hartley, I.R.; Roszko, K.L.; Li, X.; Pozo, K.; Streit, J.; Del Rivero, J.; Magone, M.T.; Smith, M.R.; Vold, R.; Dambkowski, C.L.; et al. Infigratinib Reduces Fibroblast Growth Factor 23 (FGF23) and Increases Blood Phosphate in Tumor-Induced Osteomalacia. JBMR Plus 2022, 6, e10661. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.Y.; Skalski, M.R.; Patel, D.B.; Schein, A.J.; White, E.A.; Tomasian, A.; Masih, S.; Matcuk, G.R., Jr. Hypertrophic Osteoarthropathy: Clinical and Imaging Features. Radiographics 2017, 37, 157–195. [Google Scholar] [CrossRef]
- Awan, A.B.; Javaid, F.; Moorthy, A.; Sunmboye, K. E026 Hypertrophic Pulmonary Osteoarthropathy, Rheumatoid Arthritis Mimic: Don’t Distract with Immunology. Rheumatology 2023, 62, kead104.275. [Google Scholar] [CrossRef]
- Ong, S.K.; Li, X.; Chen, T. More than Knee Pain: A Case of Hypertrophic Osteoarthropathy Secondary to Lung Cancer. J. Emerg. Med. 2020, 59, e179–e181. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Cha, J.G.; Yoon, Y.S.; Moon, A. Early Imaging Findings of Hypertrophic Osteoarthropathy Mimicking Bone Metastasis from Extrathoracic Malignancy. J. Korean Soc. Radiol. 2021, 82, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Andreu, J.L.; Muñoz, P.; Isasi, C.; López, A. Hypertrophic Osteoarthropathy Associated with Gastrointestinal Stromal Tumour. Ann. Rheum. Dis. 2006, 65, 681–682. [Google Scholar] [CrossRef]
- Tagawa, R.; Soda, H.; Dotsu, Y.; Senju, H.; Irifune, S.; Yoshida, M.; Nakashima, S.; Umemura, A.; Iwasaki, K.; Taniguchi, H.; et al. Hypertrophic Osteoarthropathy Associated with Lung Cancer: Possible Links among Hypoxia-Inducible Factor-1α, Vascular Endothelial Growth Factor, and Hypervascularization. Thorac. Cancer 2023, 14, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Itoh, M.; Tamamushi, M.; Nakamura, H.; Aoshiba, K. Hypertrophic Osteoarthropathy Secondary to Lung Cancer. J. Clin. Rheumatol. 2017, 23, 47–50. [Google Scholar] [CrossRef]
- Nozawa, K.; Kaneko, H.; Itoh, T.; Katsura, Y.; Noguchi, M.; Suzuki, F.; Takasaki, Y.; Ogawa, H.; Takamori, K.; Sekigawa, I. Synchronous Malignant B-Cell Lymphoma and Gastric Tubular Adenocarcinoma Associated with Paraneoplastic Cutaneous Vasculitis: Hypereosinophilic Syndrome with Mixed Cryoglobulinemia Is an Important Sign of Paraneoplastic Syndrome. Rare Tumors 2009, 1, e42. [Google Scholar] [CrossRef]
- Lulla, P.; Bandeali, S.; Baker, K. Fatal Paraneoplastic Systemic Leukocytoclastic Vasculitis as a Presenting Feature of Chronic Lymphocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2011, 11 (Suppl. 1), S14–S16. [Google Scholar] [CrossRef] [PubMed]
- Ismayilov, R.; Haziyev, T.; Ozdemir, D.A.; Saglam, A.; Buyukasik, Y. Leukocytoclastic Vasculitis as a Previously Unreported Paraneoplastic Manifestation of Acute Lymphoblastic Leukemia in Adults. J. Hematop. 2020, 13, 265–268. [Google Scholar] [CrossRef]
- Braman, M.; Santmyire-Rosenberger, B.; Dugan, E.; Brun, E.; Wang, Z. Leukocytoclastic Vasculitis Presenting in Association with a Spindle Cell Sarcoma of the Heart. A Paraneoplastic Syndrome? J. Cutan. Pathol. 2005, 32, 77. [Google Scholar] [CrossRef]
- Al-Tourah, A.J.; Tsang, P.W.K.; Skinnider, B.F.; Hoskins, P.J. Paraneoplastic Erythropoietin-Induced Polycythemia Associated with Small Lymphocytic Lymphoma. J. Clin. Oncol. 2006, 24, 2388–2389. [Google Scholar] [CrossRef] [PubMed]
- Josa, V.; Ferenczi, S.; Szalai, R.; Fuder, E.; Kuti, D.; Horvath, K.; Hegedus, N.; Kovacs, T.; Bagamery, G.; Juhasz, B.; et al. Thrombocytosis and Effects of IL-6 Knock-out in a Colitis-Associated Cancer Model. Int. J. Mol. Sci. 2020, 21, 6218. [Google Scholar] [CrossRef] [PubMed]
- Abukhiran, I.A.; Jasser, J.; Syrbu, S. Paraneoplastic Leukemoid Reactions Induced by Cytokine-Secreting Tumours. J. Clin. Pathol. 2020, 73, 310–313. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, A.; Zuo, C.; Fang, Y. Intense FDG Uptake in Well-Differentiated Inflammatory Liposarcoma of the Retroperitoneum. Clin. Nucl. Med. 2024, 49, 685–687. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Shehryar, A.; Imran, M.; Ch, M.B.; Baig, A. An Uncommon Presentation of Paraneoplastic Leukemoid Reaction (PLR) in a Rare Case of Adenosquamous Carcinoma (ASC) of the Gallbladder (GB): A Case Report. Cureus 2023, 15, e41040. [Google Scholar] [CrossRef]
- Tay, S.B.; Al Jajeh, I.; Lee, L.A.L.; Lee, L. Pulmonary Mucoepidermoid Carcinoma with Multiple Paraneoplastic Syndromes. Cureus 2023, 15, e44193. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, N.; Hiremath, B. Leukemoid Reaction as a Paraneoplastic Syndrome in Hypopharyngeal Squamous Cell Carcinoma with Cutaneous Metastasis: An Exceedingly Rare Occurrence. BMJ Case Rep. 2015, 2015, bcr2015211007. [Google Scholar] [CrossRef]
- Chahine, Z.; Samhouri, Y.; Jayakrishnan, T.; Monga, D. Leukemoid Reaction Causing Arterial Thrombus in a Patient with Lung Adenocarcinoma. BMJ Case Rep. 2020, 13, e235389. [Google Scholar] [CrossRef]
- de Herder, W.W.; Hofland, J. Vasoactive Intestinal Peptide-Secreting Tumor (VIPoma). In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Pratz, K.W.; Ma, C.; Aubry, M.-C.; Vrtiska, T.J.; Erlichman, C. Large Cell Carcinoma with Calcitonin and Vasoactive Intestinal Polypeptide–Associated Verner-Morrison Syndrome. Mayo Clin. Proc. 2005, 80, 116–120. [Google Scholar] [CrossRef]
- Sherry, S.J.; Tahari, A.K.; Mirpour, S.; Colucci, A.; Subramaniam, R.M. FDG-PET/CT in the Evaluation of Paraneoplastic Neurologic Syndromes. Imaging Med. 2014, 6, 117–126. [Google Scholar] [CrossRef]
- Basu, S.; Alavi, A. Role of FDG-PET in the Clinical Management of Paraneoplastic Neurological Syndrome: Detection of the Underlying Malignancy and the Brain PET-MRI Correlates. Mol. Imaging Biol. 2008, 10, 131–137. [Google Scholar] [CrossRef]
- Bordonne, M.; Chawki, M.B.; Doyen, M.; Kas, A.; Guedj, E.; Tyvaert, L.; Verger, A. Brain 18F-FDG PET for the Diagnosis of Autoimmune Encephalitis: A Systematic Review and a Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3847–3858. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, C.; Rüber, T.; Ernst, L.; Fendler, W.P.; Kirchner, J.; Mönninghoff, C.; Herrmann, K.; Quesada, C.M.; Forsting, M.; Elger, C.E.; et al. 18F-FDG-PET/MRI in the Diagnostic Work-up of Limbic Encephalitis. PLoS ONE 2020, 15, e0227906. [Google Scholar] [CrossRef]
- Scheid, R.; Lincke, T.; Voltz, R.; von Cramon, D.Y.; Sabri, O. Serial 18F-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography and Magnetic Resonance Imaging of Paraneoplastic Limbic Encephalitis. Arch. Neurol. 2004, 61, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Widman, G.; Stuff, S.; Becker, A.J.; Witt, J.-A.; Ahmadzadehfar, H.; Helmstaedter, C.; Elger, C.E. Cancer Frequency Detected by Positron Emission Tomography-Computed Tomography in Limbic Encephalitis. Epilepsy Behav. 2018, 89, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Scheid, R.; Voltz, R.; Briest, S.; Kluge, R.; von Cramon, D.Y. Clinical Insights into Paraneoplastic Cerebellar Degeneration. J. Neurol. Neurosurg. Psychiatry 2006, 77, 529–530. [Google Scholar] [CrossRef]
- Abdulaziz, A.T.A.; Yu, X.Q.; Zhang, L.; Jiang, X.Y.; Zhou, D.; Li, J.M. Paraneoplastic Cerebellar Degeneration Associated with Cerebellar Hypermetabolism. Med. Baltim. 2018, 97, e10717. [Google Scholar] [CrossRef]
- Massa, F.; Filippi, L.; Benedetti, L.; Morbelli, S.; Nobili, F. FDG PET Unveils the Course of Paraneoplastic Cerebellar Degeneration: A Semiquantitative Analysis. Clin. Nucl. Med. 2021, 46, e327–e328. [Google Scholar] [CrossRef]
- Masangkay, N.; Basu, S.; Moghbel, M.; Kwee, T.; Alavi, A. Brain 18F-FDG-PET Characteristics in Patients with Paraneoplastic Neurological Syndrome and Its Correlation with Clinical and MRI Findings. Nucl. Med. Commun. 2014, 35, 1038–1046. [Google Scholar] [CrossRef]
- Madhavan, A.A.; Carr, C.M.; Morris, P.P.; Flanagan, E.P.; Kotsenas, A.L.; Hunt, C.H.; Eckel, L.J.; Lindell, E.P.; Diehn, F.E. Imaging Review of Paraneoplastic Neurologic Syndromes. AJNR Am. J. Neuroradiol. 2020, 41, 2176–2187. [Google Scholar] [CrossRef]
- Kumar, R.; Vankadari, K.; Mittal, B.R.; Bansal, D.; Trehan, A.; Sahu, J.K.; Sankhyan, N. Diagnostic Values of 68Ga-Labelled DOTANOC PET/CT Imaging in Pediatric Patients Presenting with Paraneoplastic Opsoclonus Myoclonus Ataxia Syndrome. Eur. Radiol. 2021, 31, 4587–4594. [Google Scholar] [CrossRef]
- Joshi, P.; Lele, V. Somatostatin Receptor Positron Emission Tomography/Computed Tomography (PET/CT) in the Evaluation of Opsoclonus-Myoclonus Ataxia Syndrome. Indian. J. Nucl. Med. 2013, 28, 108–111. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lau, W.F.E.; Hicks, R.J. Somatostatin Receptor Imaging with 68Ga DOTATATE PET/CT: Clinical Utility, Normal Patterns, Pearls, and Pitfalls in Interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Boegle, R.; Eulenburg, P.Z.; Ertl, M.; Kim, J.-S.; Dieterich, M. Longitudinal Multi-Modal Neuroimaging in Opsoclonus-Myoclonus Syndrome. J. Neurol. 2017, 264, 512–519. [Google Scholar] [CrossRef]
- Nadal, E.; Bruna, J.; Ochoa de Olza, M.; Antonio, M.; Cardenal, F. Paraneoplastic Opsoclonus-Myoclonus Syndrome as a New and Single Manifestation of Relapsing Disease in a Patient with Small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 968–969. [Google Scholar] [CrossRef] [PubMed]
- Na, C.J.; Jeong, Y.J.; Lim, S.T.; Sohn, M.-H.; Jeong, H.-J. 18F-FDG PET/CT Brain Imaging on a Patient with Paraneoplastic Opsoclonus-Myoclonus Syndrome Arising out of a Mature Cystic Teratoma. Clin. Nucl. Med. 2016, 41, e104–e106. [Google Scholar] [CrossRef]
- Takamori, M.; Iwasa, K.; Komai, K. Antibodies to Synthetic Peptides of the alA Subunit of the Voltage-Gated Calcium Channel in Lambert-Eaton Myasthenic Syndrome. Neurology 1997, 48, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Ten Brinck, M.F.; Verheijen, I.W.; van de Wardt, J.; van Dijk, G.W.; Nijhuis, F.A.; Verrips, A. Dilated Fixed Pupils and Respiratory Failure: A Rare Clinical Course of Lambert-Eaton Myasthenic Syndrome. BMJ Neurol. Open 2023, 5, e000426. [Google Scholar] [CrossRef]
- Titulaer, M.J.; Wirtz, P.W.; Willems, L.N.A.; van Kralingen, K.W.; Smitt, P.A.E.S.; Verschuuren, J.J.G.M. Screening for Small-Cell Lung Cancer: A Follow-up Study of Patients with Lambert-Eaton Myasthenic Syndrome. J. Clin. Oncol. 2008, 26, 4276–4281. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Z.; Chen, X.; Li, Q. Lambert-Eaton Myasthenic Syndrome Associated with Extrapulmonary Small Cell Cancer Detected by 18F-FDG PET/CT. Clin. Nucl. Med. 2018, 43, 697–698. [Google Scholar] [CrossRef]
- Hong, B.Y.; An, H.J.; Lim, S.H. Long-Standing Lambert-Eaton Myasthenic Syndrome Caused by Undetectable Small-Cell Lung Cancer: Why We Should Follow-up LEMS. Diagnostics 2022, 12, 1542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, H.; Han, W.; Meng, Y.; Kang, L.; Chen, Y. SOX-1 Antibodies Positive Lambert-Eaton Myasthenic Syndrome with Occult Small Cell Lung Cancer: A Case Report. Clin. Respir. J. 2024, 18, e13740. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, W.; Li, Y.; Zhang, K.; Gao, X.; Wang, J. Mediastinal Small Cell Cancer Associated with Lambert-Eaton Myasthenic Syndrome: A Case Report. Exp. Ther. Med. 2015, 10, 117–120. [Google Scholar] [CrossRef][Green Version]
- Muggia, F.M. Overview of Cancer-Related Hypercalcemia: Epidemiology and Etiology. Semin Oncol. 1990, 17 (Suppl. 5), 3–9. [Google Scholar][Green Version]
- Kim, K. THU036 68Ga-DOTATOC PET/CT in Localization of ACTH-Producing Pituitary Tumors in Patients with Cushing Disease. J. Endocr. Soc. 2023, 7, bvad114.1116. [Google Scholar] [CrossRef]
- Özkan, Z.G.; Kuyumcu, S.; Balköse, D.; Ozkan, B.; Aksakal, N.; Yılmaz, E.; Sanlı, Y.; Türkmen, C.; Aral, F.; Adalet, I. The Value of Somatostatin Receptor Imaging with In-111 Octreotide and/or Ga-68 DOTATATE in Localizing Ectopic ACTH Producing Tumors. Mol. Imaging Radionucl. Ther. 2013, 22, 49–55. [Google Scholar] [CrossRef]
- Varlamov, E.; Hinojosa-Amaya, J.M.; Stack, M.; Fleseriu, M. Diagnostic Utility of Gallium-68-Somatostatin Receptor PET/CT in Ectopic ACTH-Secreting Tumors: A Systematic Literature Review and Single-Center Clinical Experience. Pituitary 2019, 22, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Maurea, S.; Imbriaco, M.; D’Angelillo, M.; Mollica, C.; Camera, L.; Salvatore, M. Diagnostic accuracy of chemical-shift MR imaging to differentiate between adrenal adenomas and non adenoma adrenal lesions. Radiol. Med. 2006, 111, 674–686. [Google Scholar] [CrossRef][Green Version]
- Boland, G.W.; Lee, M.J.; Gazelle, G.S.; Halpern, E.F.; McNicholas, M.M.; Mueller, P.R. Characterization of adrenal masses using unenhanced CT: An analysis of the CT literature. AJR Am. J. Roentgenol. 1998, 171, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Korobkin, M.; Brodeur, F.J.; Francis, I.R.; Quint, L.E.; Dunnick, N.R.; Londy, F. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am. J. Roentgenol. 1998, 170, 747–752. [Google Scholar] [CrossRef]
- Novruzov, F.; Aliyev, A.; Wan, M.Y.S.; Syed, R.; Mehdi, E.; Aliyeva, I.; Giammarile, F.; Bomanji, J.B.; Kayani, I. The Value of [68Ga]Ga-DOTA-TATE PET/CT in Diagnosis and Management of Suspected Pituitary Tumors. Eur. J. Hybrid. Imaging 2021, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Sbardella, E.; Zatelli, M.C.; Boschetti, M.; Vitale, G.; Colao, A.; Pivonello, R. ABC Study Group Conventional and Nuclear Medicine Imaging in Ectopic Cushing’s Syndrome: A Systematic Review. J. Clin. Endocrinol. Metab. 2015, 100, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Zisser, L.; Kulterer, O.C.; Itariu, B.; Fueger, B.; Weber, M.; Mazal, P.; Vraka, C.; Pichler, V.; Kautzky-Willer, A.; Hacker, M.; et al. Diagnostic Role of PET/CT Tracers in the Detection and Localization of Tumours Responsible for Ectopic Cushing’s Syndrome. Anticancer. Res. 2021, 41, 2477–2484. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, Y.; Jiang, Z.; Wang, Z.; Ruan, S. Abstract CT543: 18F-FDG PET/CT Detects Potentially Malignant Bone Metastases in Patients with Suspected Lesions: A Prospective Clinical Trial. Cancer Res. 2022, 82, CT543. [Google Scholar] [CrossRef]
- Yao, G.; Zhou, Y.; Gu, Y.; Wang, Z.; Yang, M.; Sun, J.; Luo, Q.; Zhao, H. A Retrospective Study of Predicting Risk of Metastasis among FDG-Avid Bone Lesions in 18F-FDG PET/CT. J. Cancer 2020, 11, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Liau, N.; Ooi, C.; Reid, C.; Kirkwood, I.D.; Bartholomeusz, D. F-18 FDG PET/CT Detection of Mediastinal Malignancy in a Patient with Dermatomyositis. Clin. Nucl. Med. 2007, 32, 304–305. [Google Scholar] [CrossRef]
- Mahmood, S.; Rodríguez Martínez de Llano, S. 18F-FDG PET Detection of Unknown Primary Malignancy in Dermatomyositis. Clin. Nucl. Med. 2012, 37, e204–e205. [Google Scholar] [CrossRef]
- Motegi, S.-I.; Fujiwara, C.; Sekiguchi, A.; Hara, K.; Yamaguchi, K.; Maeno, T.; Higuchi, T.; Hirasawa, H.; Kodaira, S.; Tomonaga, H.; et al. Clinical Value of 18 F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Interstitial Lung Disease and Myositis in Patients with Dermatomyositis. J. Dermatol. 2019, 46, 213–218. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Grau, J.M.; Gámez-Cenzano, C.; Vidaller-Palacín, A.; Martínez-Gómez, X.; Trallero-Araguás, E.; Andía-Navarro, E.; Vilardell-Tarrés, M. Conventional Cancer Screening versus PET/CT in Dermatomyositis/Polymyositis. Am. J. Med. 2010, 123, 558–562. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, K.P.; Wang, Y.Y.; Pei, L.; Guan, Z.W.; Jin, J.Y.; Zhu, J.; Zhang, J.L.; Huang, F. The prediction of disease progression by 18Fluorodeoxyglucose-positron emission computed tomography/CT in patients with dermatomyositis and interstitial lung disease. Zhonghua Nei Ke Za Zhi 2021, 60, 661–664. [Google Scholar] [PubMed]
- Rayamajhi, S.J.; Gorla, A.K.R.; Basher, R.K.; Sood, A.; Mittal, B.R. Unsuspected Active Ulcerative Colitis in a Patient with Dermatomyositis: A Rare Association Detected on 18F-FDG PET/CT during the Search for an Occult Malignancy. Indian. J. Nucl. Med. 2017, 32, 130–132. [Google Scholar] [PubMed]
- Yildiz, H.; D’abadie, P.; Gheysens, O. The Role of Quantitative and Semi-Quantitative [18F]FDG-PET/CT Indices for Evaluating Disease Activity and Management of Patients with Dermatomyositis and Polymyositis. Front. Med. Lausanne 2022, 9, 883727. [Google Scholar] [CrossRef]
- Kundrick, A.; Kirby, J.; Ba, D.; Leslie, D.; Olsen, N.; Foulke, G. Positron Emission Tomography Costs Less to Patients than Conventional Screening for Malignancy in Dermatomyositis. Semin. Arthritis Rheum. 2019, 49, 140–144. [Google Scholar] [CrossRef]
- Arai-Okuda, H.; Norikane, T.; Yamamoto, Y.; Mitamura, K.; Fujimoto, K.; Takami, Y.; Wakiya, R.; Nakashima, S.; Dobashi, H.; Nishiyama, Y. 18F-FDG PET/CT in Patients with Polymyositis/Dermatomyositis: Correlation with Serum Muscle Enzymes. Eur. J. Hybrid. Imaging 2020, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Zhong, D.; Dang, Y.; Xing, H.; Du, Y.; Jing, H.; Qiao, Z.; Xing, X.; Zhuang, H.; et al. 68Ga DOTATATE PET/CT Is an Accurate Imaging Modality in the Detection of Culprit Tumors Causing Osteomalacia. Clin. Nucl. Med. 2015, 40, 642–646. [Google Scholar] [CrossRef]
- Tan, T.H.; Chen, E.-J.; Chew, M.T.; Chye, P.C.; Wong, M. Extended Whole-Body Ga-68 DOTATATE PET-CT in Evaluating Tumour-Induced Osteomalacia: Case Report and Review of Literature. Nucl. Med. Mol. Imaging 2021, 55, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Breer, S.; Brunkhorst, T.; Beil, F.T.; Peldschus, K.; Heiland, M.; Klutmann, S.; Barvencik, F.; Zustin, J.; Gratz, K.-F.; Amling, M. 68Ga DOTA-TATE PET/CT Allows Tumor Localization in Patients with Tumor-Induced Osteomalacia but Negative 111In-Octreotide SPECT/CT. Bone 2014, 64, 222–227. [Google Scholar] [CrossRef]
- Kunikowska, J.; Andryszak, N.; Skowrońska-Jóźwiak, E.; Pełka, K.; Zygmunt, A.; Lewiński, A.; Ruchała, M.; Czepczyński, R. Tumour-Induced Osteomalacia—A Long Way to the Diagnosis Facilitated by [68Ga]Ga-DOTATATE PET/CT. J. Clin. Med. 2024, 13, 1817. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, B.; Zhang, L.; Chen, Z.; Shi, X.; Yi, C.; Wang, X.; Zhang, X. Diagnostic Efficiency of 68Ga-DOTANOC PET/CT in Patients with Suspected Tumour-Induced Osteomalacia. Eur. Radiol. 2021, 31, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Hou, J.; Yang, N.; Zhou, M.; Li, Y.; Li, J.; Tang, Y.; Chen, D.; Hu, S. Utility of 18F-AlF-NOTA-Octreotide PET/CT in the Localization of Tumor-Induced Osteomalacia. J. Clin. Endocrinol. Metab. 2021, 106, e4202–e4209. [Google Scholar] [CrossRef]
- Li, J. 18F-AlF-NOTA-Octreotide PET/CT in the Localization of Tumor-Induced Osteomalacia: Case Series and Literature Review. Front. Endocrinol. Lausanne 2024, 15, 1400751. [Google Scholar] [CrossRef]
- Kato, A.; Nakamoto, Y.; Ishimori, T.; Hayakawa, N.; Ueda, M.; Temma, T.; Sano, K.; Shimizu, Y.; Saga, T.; Togashi, K. Diagnostic Performance of 68Ga-DOTATOC PET/CT in Tumor-Induced Osteomalacia. Ann. Nucl. Med. 2021, 35, 397–405. [Google Scholar] [CrossRef]
- Paquet, M.; Gauthé, M.; Zhang Yin, J.; Nataf, V.; Bélissant, O.; Orcel, P.; Roux, C.; Talbot, J.-N.; Montravers, F. Diagnostic Performance and Impact on Patient Management of 68Ga-DOTA-TOC PET/CT for Detecting Osteomalacia-Associated Tumours. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1710–1720. [Google Scholar] [CrossRef]
- Hou, G.; Zhang, Y.; Liu, Y.; Wang, P.; Xia, W.; Xing, X.; Huo, L.; Li, F.; Jing, H. Head-to-Head Comparison of 68Ga-DOTA-TATE and 68Ga-DOTA-JR11 PET/CT in Patients with Tumor-Induced Osteomalacia: A Prospective Study. Front. Oncol. 2022, 12, 811209. [Google Scholar] [CrossRef]
- El-Maouche, D.; Sadowski, S.M.; Papadakis, G.Z.; Guthrie, L.; Cottle-Delisle, C.; Merkel, R.; Millo, C.; Chen, C.C.; Kebebew, E.; Collins, M.T. 68Ga-DOTATATE for Tumor Localization in Tumor-Induced Osteomalacia. J. Clin. Endocrinol. Metab. 2016, 101, 3575–3581. [Google Scholar] [CrossRef]
- Clifton-Bligh, R.J.; Hofman, M.S.; Duncan, E.; Sim, I.-W.; Darnell, D.; Clarkson, A.; Wong, T.; Walsh, J.P.; Gill, A.J.; Ebeling, P.R.; et al. Improving Diagnosis of Tumor-Induced Osteomalacia with Gallium-68 DOTATATE PET/CT. J. Clin. Endocrinol. Metab. 2013, 98, 687–694. [Google Scholar] [CrossRef]
- Aparici, C.M.; Bains, S. Hypertrophic Osteoarthropathy Seen with NaF18 PET/CT Bone Imaging. Clin. Nucl. Med. 2011, 36, 928–929. [Google Scholar] [CrossRef]
- Makis, W.; Abikhzer, G.; Rush, C. Hypertrophic Pulmonary Osteoarthropathy Diagnosed by FDG PET-CT in a Patient with Lung Adenocarcinoma. Clin. Nucl. Med. 2009, 34, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Pelletier-Galarneau, M.; Ruddy, T.D. PET/CT for Diagnosis and Management of Large-Vessel Vasculitis. Curr. Cardiol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef]
- Sherzay, R.; Witte, T.; Derlin, T.; Hoepfner, M.; Bengel, F.M. Vessel Wall Inflammatory Activity as Determined by F-18 Fluorodeoxyglucose PET in Large Vessel Vasculitis Is Attenuated by Immunomodulatory Drugs. Diagnostics 2021, 11, 1132. [Google Scholar] [CrossRef]
- van der Geest, K.S.M.; Treglia, G.; Glaudemans, A.W.J.M.; Brouwer, E.; Sandovici, M.; Jamar, F.; Gheysens, O.; Slart, R.H.J.A. Diagnostic Value of [18F]FDG-PET/CT for Treatment Monitoring in Large Vessel Vasculitis: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3886–3902. [Google Scholar] [CrossRef]
- Zubair, A.; Qureshyl, A.; Hassan, A. Insight into Paraneoplastic Vasculitis Associated with Adenocarcinoma Colon on F18-FDG PET-CT. J. Pak. Med. Assoc. 2024, 74, 1892–1893. [Google Scholar] [CrossRef]
- Ben Shimol, J.; Amital, H.; Lidar, M.; Domachevsky, L.; Shoenfeld, Y.; Davidson, T. The Utility of PET/CT in Large Vessel Vasculitis. Sci. Rep. 2020, 10, 17709. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.A.; Krogh, K.M.; Boike, J.R. Chronic Diarrhea Secondary to Newly Diagnosed VIPoma. Case Rep. Gastroenterol. 2019, 13, 225–229. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Pennington, C.; Ng, C.Y.; Poon, F.W.; Han, S. 18F-FDG PET-CT in the Evaluation of Paraneoplastic Syndromes. Nucl. Med. Commun. 2012, 33, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Bresler, R.; Schroeder, H.W., III; Chow, D.Z.; Lim, R. 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Diagnosis of Suspected Paraneoplastic Syndromes: A Retrospective Analysis. World J. Nucl. Med. 2020, 19, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.B.; Hess, S.; Petersen, H.; Høilund-Carlsen, P.F. Clinical Value of FDG-PET/CT in Suspected Paraneoplastic Syndromes: A Retrospective Analysis of 137 Patients. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- McKeon, A.; Apiwattanakul, M.; Lachance, D.H.; Lennon, V.A.; Mandrekar, J.N.; Boeve, B.F.; Mullan, B.; Mokri, B.; Britton, J.W.; Drubach, D.A.; et al. Positron Emission Tomography-Computed Tomography in Paraneoplastic Neurologic Disorders: Systematic Analysis and Review. Arch. Neurol. 2010, 67, 322–329. [Google Scholar] [CrossRef]
- Bannas, P.; Weber, C.; Derlin, T.; Lambert, J.; Leypoldt, F.; Adam, G.; Mester, J.; Klutmann, S. 18F-FDG-PET/CT in the Diagnosis of Paraneoplastic Neurological Syndromes: A Retrospective Analysis. Eur. Radiol. 2010, 20, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Schramm, N.; Rominger, A.; Schmidt, C.; Morelli, J.N.; Schmid-Tannwald, C.; Meinel, F.G.; Reiser, M.F.; Rist, C. Detection of Underlying Malignancy in Patients with Paraneoplastic Neurological Syndromes: Comparison of 18F-FDG PET/CT and Contrast-Enhanced CT. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1014–1024. [Google Scholar] [CrossRef]
- Lebech, A.-M.; Gaardsting, A.; Loft, A.; Graff, J.; Markova, E.; Bertelsen, A.K.; Madsen, J.L.; Andersen, K.F.; von Benzon, E.; Helms, M.; et al. Whole-Body 18F-FDG PET/CT Is Superior to CT as First-Line Diagnostic Imaging in Patients Referred with Serious Nonspecific Symptoms or Signs of Cancer: A Randomized Prospective Study of 200 Patients. J. Nucl. Med. 2017, 58, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- García Vicente, A.M.; Delgado-Bolton, R.C.; Amo-Salas, M.; López-Fidalgo, J.; Caresia Aróztegui, A.P.; García Garzón, J.R.; Orcajo Rincón, J.; García Velloso, M.J.; de Arcocha Torres, M.; Alvárez Ruíz, S.; et al. 18F-Fluorodeoxyglucose Positron Emission Tomography in the Diagnosis of Malignancy in Patients with Paraneoplastic Neurological Syndrome: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1575–1587. [Google Scholar] [CrossRef]
- Hildebrandt, M.G.; Naghavi-Behzad, M.; Vogsen, M. A Role FDG-PET/CT Response Eval. Metastatic Breast Cancer? Semin. Nucl. Med. 2022, 52, 520–530. [Google Scholar] [CrossRef]
- Rania, A.M.; Noha, F.E.Q.; Salama, M.; Asmaa, M. 18F-FDG PET/CT for Monitoring of Treatment Response in Breast Cancer. Med. J. Cairo Univ. 2021, 89, 473–479. [Google Scholar]
- Sheikhbahaei, S.; Trahan, T.J.; Xiao, J.; Taghipour, M.; Mena, E.; Connolly, R.M.; Subramaniam, R.M. FDG-PET/CT and MRI for Evaluation of Pathologic Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer: A Meta-Analysis of Diagnostic Accuracy Studies. Oncologist 2016, 21, 931–939. [Google Scholar] [CrossRef]

| Syndrome | Antibodies | Associated Malignancies | FDG PET/CT Findings | Recommended PET Tracer |
|---|---|---|---|---|
| Paraneoplastic Limbic Encephalitis (PLE) | Anti-Hu, Anti-Ma2 | SCLC, Thymoma, Breast cancer | Medial temporal lobe hypermetabolism | FDG |
| Paraneoplastic Cerebellar Degeneration (PCD) | Anti-Yo, Anti-Tr, Anti-CV2 | Ovarian/breast cancer | Cerebellar hypometabolism | FDG |
| Opsoclonus-Myoclonus (OMS) | Anti-Hu, Anti-Ri, Anti-CV2 | SCLC | Hypermetabolic SCLC | FDG (Neuroblastoma: also consider 123I-MIBG if available) |
| LEMS | Anti-VGCC | SCLC | Tumor hypermetabolism | FDG |
| Sensory Neuronopathy (SSN) | Anti-Hu | SCLC | Occult lung tumors | FDG |
| Syndrome | Mechanism | Associated Malignancies | FDG PET/CT Role | Recommended PET Tracer |
|---|---|---|---|---|
| Cushing’s Syndrome | Ectopic ACTH secretion | SCLC, Thymoma, pancreatic neuroendocrine tumors | Tumor localization | 68Ga-DOTATATE |
| Hypercalcemia | PTHrP secretion | Breast cancer, lung cancer, renal cell carcinoma, and multiple myeloma | Detect primary tumors, lytic lesions | FDG |
| Dermatomyositis | Anti-TIF1-γ and anti-NXP2 antibodies | Lung, ovarian, and gastrointestinal malignancies | Occult malignancy detection | FDG |
| Tumor-Induced Osteomalacia | FGF23 overproduction | Phosphaturic mesenchymal tumors (PMTs) | Localize PMTs | 68Ga-DOTATATE |
| Leukemoid Reaction | G-CSF/GM-CSF secretion | NSCLC, Liposarcoma | Identify hypermetabolic tumors | FDG |
| Study Reference | Number of Patients | Sensitivity (%) | Specificity (%) | Prevalence (%) | Condition Studied |
|---|---|---|---|---|---|
| Sheikhbahaei et al., [21] (meta-analysis) | 1293 | 81 | 88 | NA | Neurological and Non-neurological PNS |
| Kristensen et al. [171] | 137 | 75 | 83 | 8.8 | Neurological and Non-neurological PNS |
| Vaidyanathan et al. [169] | 68 | 100 | 82 | 11.8 | Neurological and Non-neurological PNS |
| McKeon et al. [172] | 41 | 100 | 97 | 17.8 | Neurological |
| Selva-O’Callaghan et al. [143] | 55 | 67 | 98 | NA | Dermatomyositis/polymyositis |
| Bannas et al. [173] | 46 | 100 | 86 | 8.7 | Neurological PNS |
| Schramm et al. [174] | 66 | 100 | 90 | 13.6 | Neurological PNS |
| Lebech et al. [175] | 95 | 83 | 96 | 18.9 | Neurological and Non-neurological PNS |
| García Vicente et al. [176] (meta-analysis) | 793 | 87 | 86 | NA | Neurological and Non-neurological PNS |
| Bresler et al. [170] | 99 | 83 | 94 | 12.1 | Neurological and Non-neurological PNS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daraghma, M.; Aswani, Y.; Jain, S.; Laudicella, R.; Gholamrezanezhad, A.; Menda, Y.; Shariftabrizi, A. PET/CT and Paraneoplastic Syndromes: A Comprehensive Review. Cancers 2025, 17, 2637. https://doi.org/10.3390/cancers17162637
Daraghma M, Aswani Y, Jain S, Laudicella R, Gholamrezanezhad A, Menda Y, Shariftabrizi A. PET/CT and Paraneoplastic Syndromes: A Comprehensive Review. Cancers. 2025; 17(16):2637. https://doi.org/10.3390/cancers17162637
Chicago/Turabian StyleDaraghma, Motaz, Yashant Aswani, Sanchay Jain, Riccardo Laudicella, Ali Gholamrezanezhad, Yusuf Menda, and Ahmad Shariftabrizi. 2025. "PET/CT and Paraneoplastic Syndromes: A Comprehensive Review" Cancers 17, no. 16: 2637. https://doi.org/10.3390/cancers17162637
APA StyleDaraghma, M., Aswani, Y., Jain, S., Laudicella, R., Gholamrezanezhad, A., Menda, Y., & Shariftabrizi, A. (2025). PET/CT and Paraneoplastic Syndromes: A Comprehensive Review. Cancers, 17(16), 2637. https://doi.org/10.3390/cancers17162637







