IGSF11-Mediated Immune Modulation: Unlocking a Novel Pathway in Emerging Cancer Immunotherapies
Simple Summary
Abstract
1. Introduction
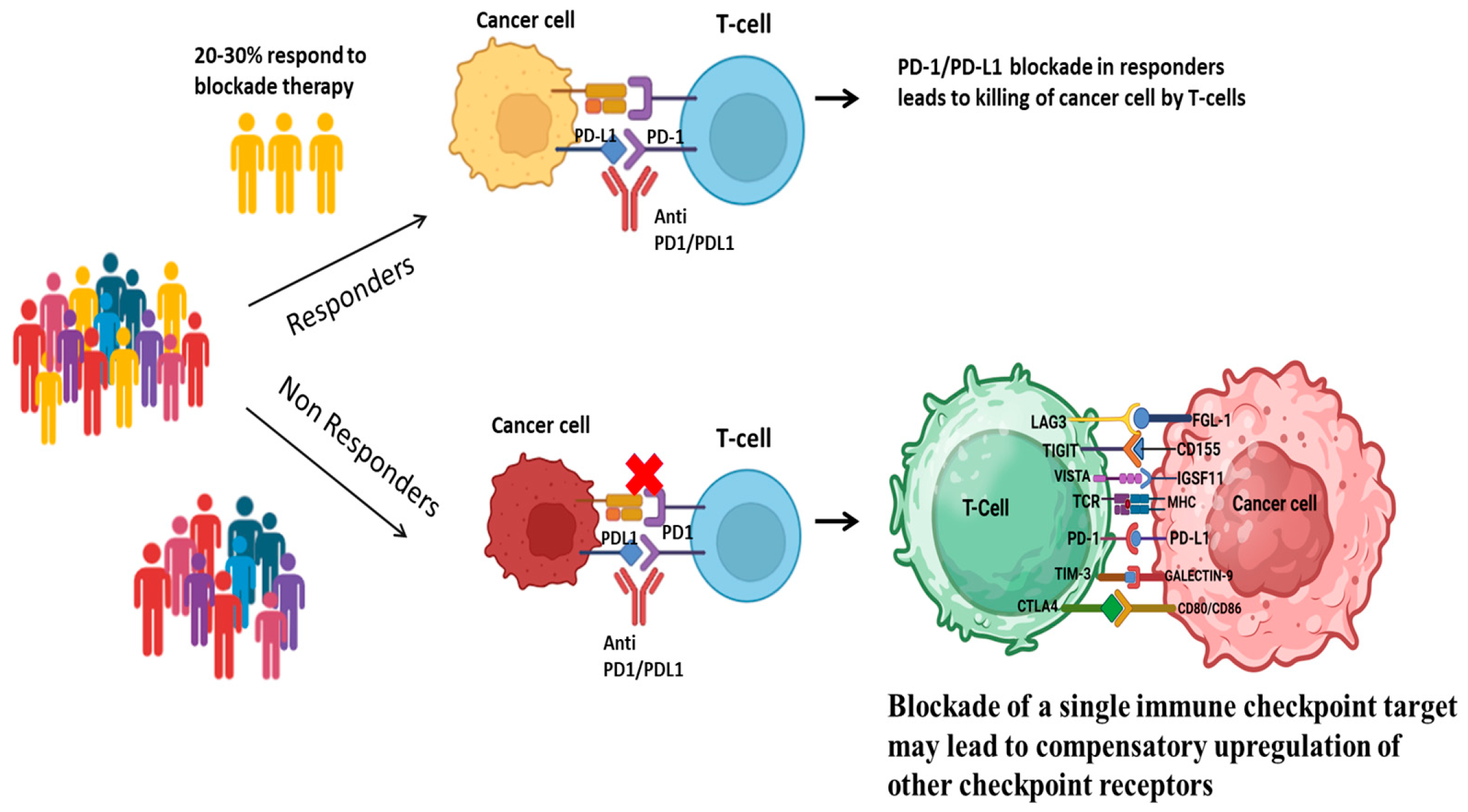
1.1. Overview of Immune Checkpoints in Cancer
1.2. The Emergence of Novel Immune Checkpoint Ligands
2. Structural and Functional Overview of IGSF11
2.1. Gene and Protein Structure
2.2. Tissue-Specific Expression
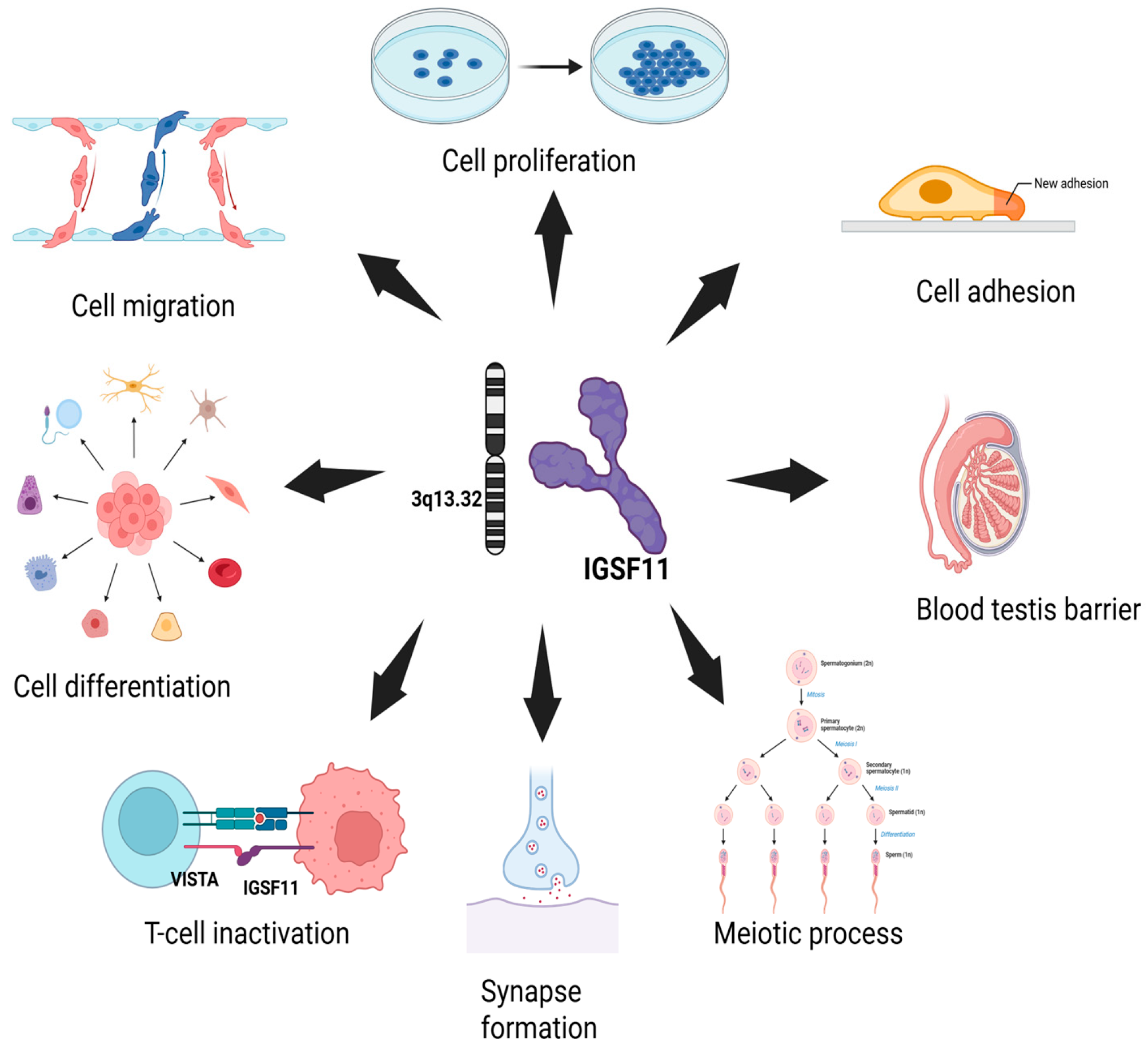
2.3. Known Physiological Roles
3. IGSF11 in Cancer: Expression Patterns and Clinical Correlations
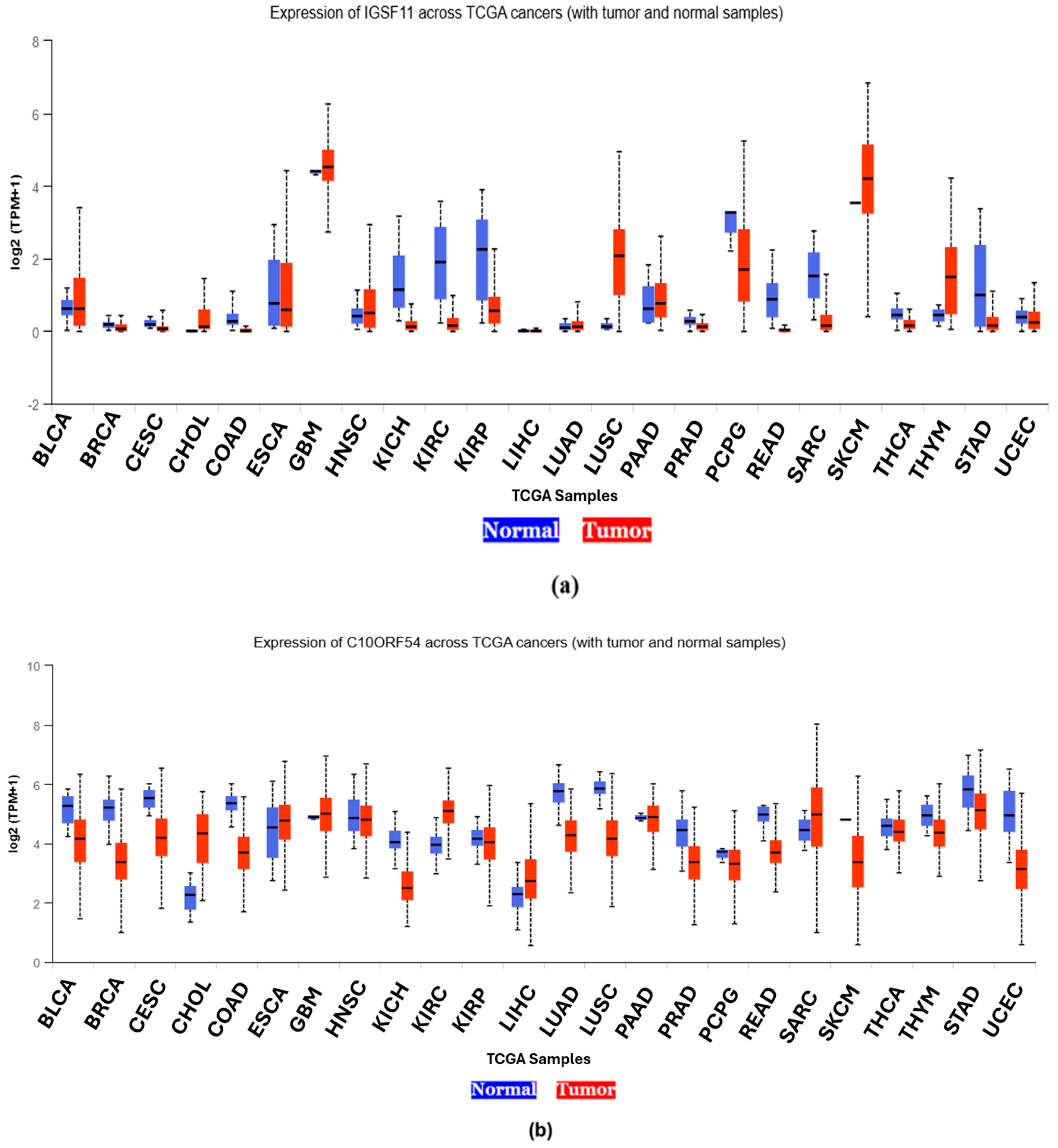
3.1. Differential Expression Across Cancer Types
3.1.1. Glioblastoma
3.1.2. Gastric Cancer
3.1.3. Melanoma
3.1.4. Breast Cancer
3.1.5. Pan-Cancer Expression Analysis of IGSF11 and VISTA Using UALCAN
3.2. Co-Expression with Other Immune Checkpoints
4. Mechanisms of IGSF11-Mediated Immune Modulation
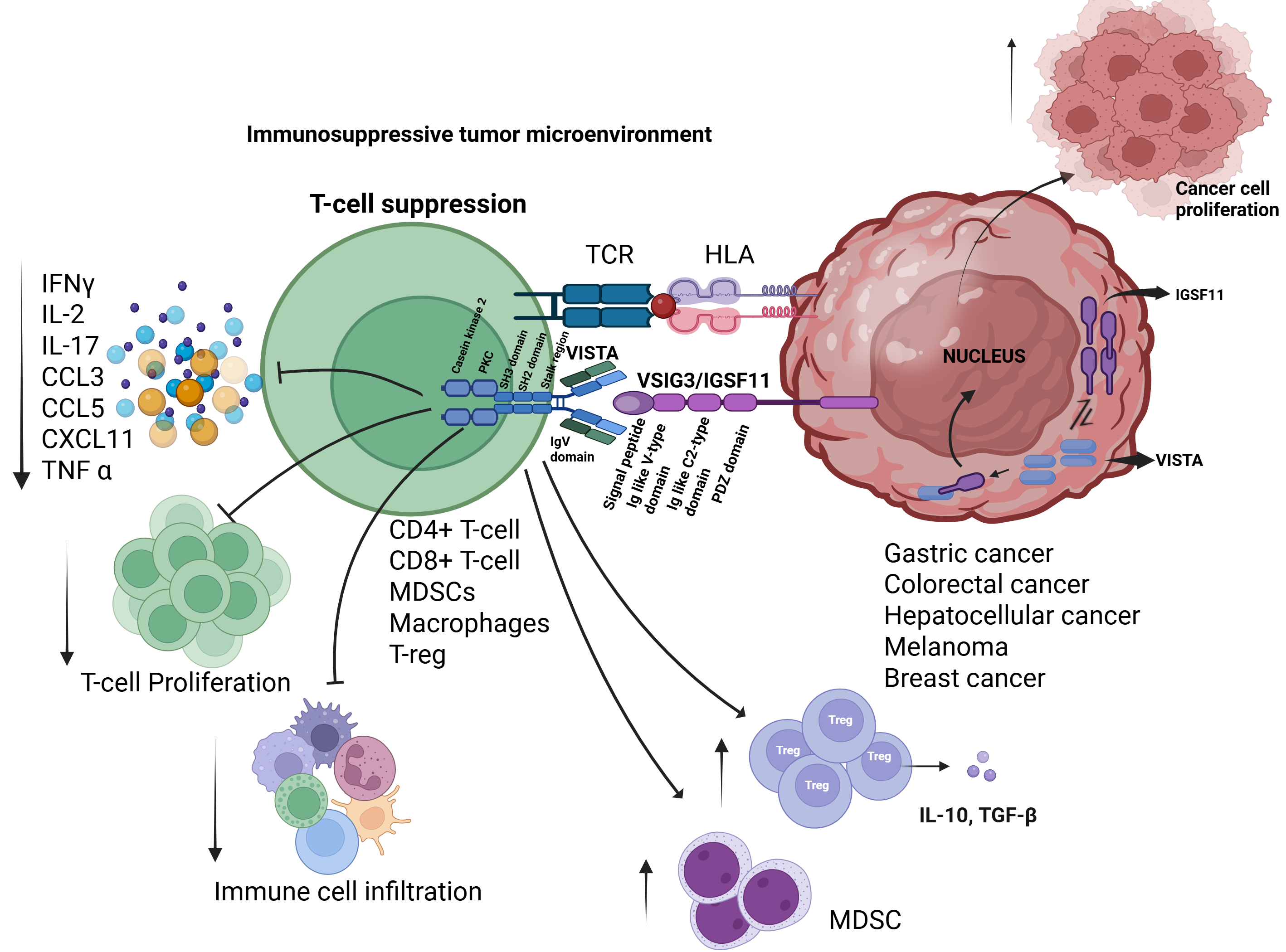
4.1. IGSF11-VISTA Signaling Axis
4.2. Role in T-Cell Suppression and Immune Evasion
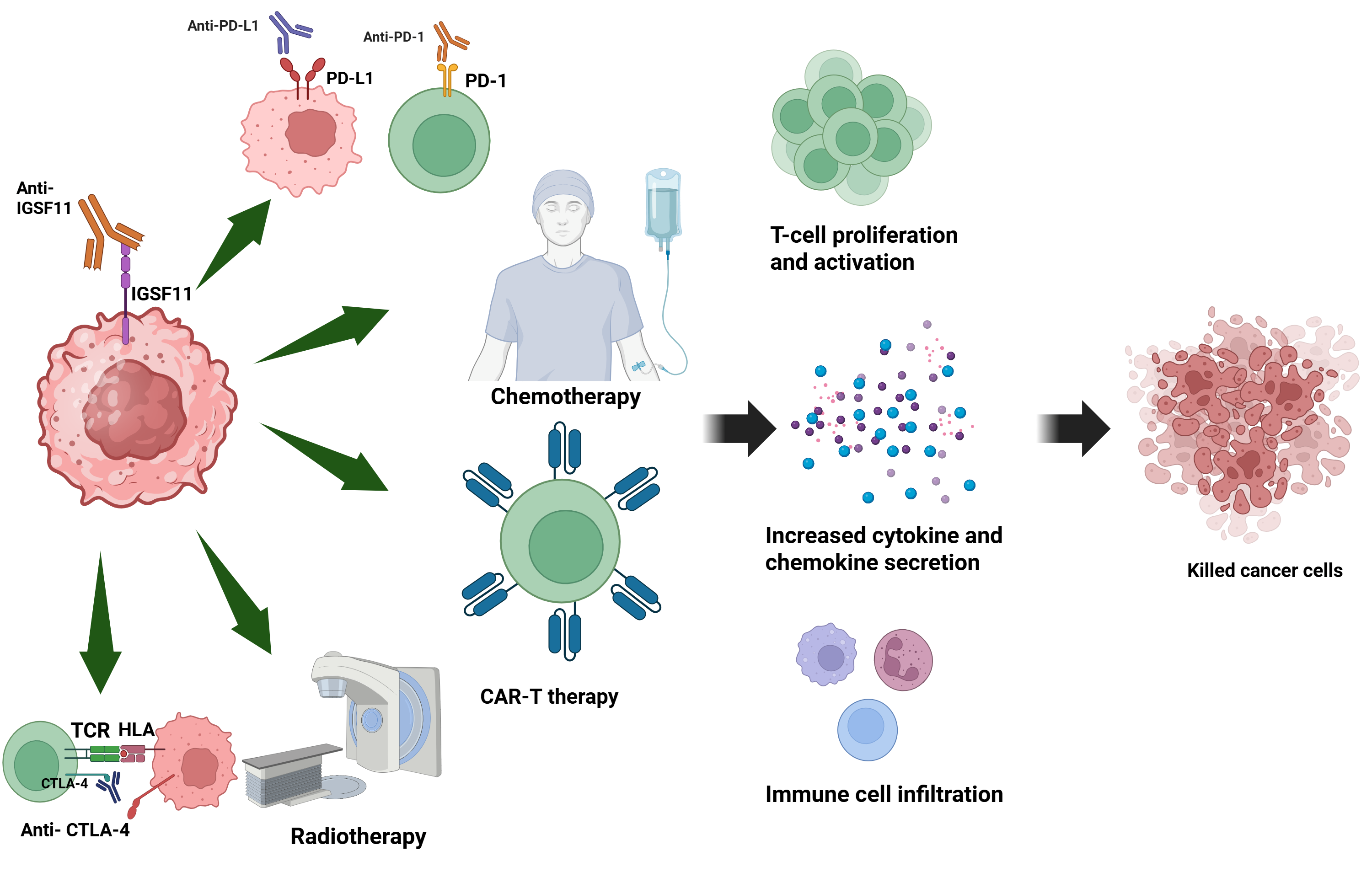
5. Preclinical and Clinical Insights into IGSF11 Targeting
5.1. Potential Therapeutic Strategies: Antibodies and Small Molecule Inhibitors
5.1.1. IMT-18
5.1.2. HMBD-002
5.1.3. SG7
5.1.4. K284-3046
5.1.5. Sinefungine (SFG)
5.2. Challenges and Opportunities in Drug Development
6. IGSF11 in the Tumor Microenvironment (TME)
6.1. IGSF11-VISTA Interaction Is a Driver of Cold Tumor Phenotype
6.2. Impact on Tumor Progression and Metastasis
6.3. Implications for Combination Immunotherapies
7. Future Perspectives and Research Directions
7.1. Gaps in Current Understanding
7.2. Clinical Translation: From Bench to Bedside
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IGSF11 | Immunoglobulin Superfamily Member 11 |
| ICIs | Immune checkpoint inhibitors |
| TCR | T-cell antigen receptor |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| FDA | Food and Drug Administration |
| PD-1 | Programmed Cell Death Protein 1 |
| PDL1 | Programmed Cell Death Ligand 1 |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| CD155 | Cluster of differentiation 155 |
| FGL1 | Fibrinogen Like 1 |
| TCGA | The Cancer Genome Atlas |
| NCDs | Noncommunicable diseases |
| irAEs | Immune-related adverse events |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| VISTA | V-domain immunoglobulin suppressor of T-cell activation |
| LAG-3 | Lymphocyte-activation gene 3 |
| TME | Tumor microenvironment |
| IHC | Immunohistochemical |
| SKCM | Skin cutaneous melanoma |
| GBM | Glioblastoma multiforme |
| CHOL | Cholangiocarcinoma |
| SARC | Sarcoma |
| LUSC | Lung squamous carcinoma |
| IL-17 | Interleukin-17 |
| CCL3 | Chemokine ligand 3 |
| CXCL11 | C-X-C motif chemokine 11 |
| CCL5 | Chemokine ligand 5 |
| TILs | Tumor-infiltrating lymphocytes |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumour Necrosis Factor alpha |
| IFN-γ | Interferon-gamma |
| MDSCs | Myeloid-derived suppressor cells |
| BLCA | Bladder urothelial carcinoma |
| BRCA | Breast invasive carcinoma |
| CESC | Cervical squamous cell carcinoma |
| COAD | Colon adenocarcinoma |
| ESCA | Esophageal carcinoma |
| HNSC | Head and Neck squamous cell carcinoma |
| KICH | Kidney Chromophobe |
| KIRC | Kidney renal clear cell carcinoma |
| KIRP | Kidney renal papillary cell carcinoma |
| LIHC | Liver hepatocellular carcinoma |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| PAAD | Pancreatic carcinoma |
| PRAD | Prostate adenocarcinoma |
| PCPG | Pheochromocytoma and paraganglioma |
| READ | Rectal adenocarcinoma |
| THCA | Thyroid carcinoma |
| THYM | Thymoma |
| STAD | Stomach adenocarcinoma |
| UCEC | Uterine Corpus Endometrial Carcinoma |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arafat Hossain, M. A comprehensive review of immune checkpoint inhibitors for cancer treatment. Int. Immunopharmacol. 2024, 143, 113365. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, H.; Chen, B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J. Cancer 2017, 8, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Chen, J.; Yan, H.; He, Q.; Luo, P.; Xu, Z.; Yang, X. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des. Dev. Ther. 2020, 14, 3625–3649. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhang, D.; Wu, S.; Xu, M.; Zhou, X.; Lu, X.J.; Ji, J. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: Mechanisms, predictive factors, and future perspectives. Biomark. Res. 2020, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Pourbagheri-Sigaroodi, A.; Kaveh, V.; Sheikholeslami, S.A.; Salari, S.; Bashash, D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: Recent advances and opportunities. Crit. Rev. Oncol. Hematol. 2021, 157, 103160. [Google Scholar] [CrossRef]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Resistance mechanisms to immune checkpoint inhibitors: Updated insights. Mol. Cancer 2025, 24, 20. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Benallaoua, M.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Kuchroo, V.; Zarour, H.M. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T-cell dysfunction in melanoma patients. J. Exp. Med. 2010, 207, 2175–2186. [Google Scholar] [CrossRef]
- Du, H.; Yi, Z.; Wang, L.; Li, Z.; Niu, B.; Ren, G. The co-expression characteristics of LAG3 and PD-1 on the T-cells of patients with breast cancer reveal a new therapeutic strategy. Int. Immunopharmacol. 2020, 78, 106113. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, B.; Gao, J.; Xin, N.; Wang, W.; Song, X.; Shao, Y.; Zhao, C. CD155 knockdown promotes apoptosis via AKT/Bcl-2/Bax in colon cancer cells. J. Cell. Mol. Med. 2018, 22, 131–140. [Google Scholar] [CrossRef]
- Kang, C.W.; Dutta, A.; Chang, L.Y.; Mahalingam, J.; Lin, Y.C.; Chiang, J.M.; Hsu, C.Y.; Huang, C.T.; Su, W.T.; Chu, Y.Y.; et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T-cells in colon cancer. Sci. Rep. 2015, 5, 15659. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, G.; Manick, B.; Hernandez, V.; Renelt, M.; Erickson, C.; Guan, J.; Singh, R.; Rollins, S.; Solorz, A.; et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019, 156, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Shekari, N.; Shanehbandi, D.; Baghbani, E.; Safaei, S.; Masoumi, J.; Baradaran, B.; Jalali, S.A. VSIG-3/IGSF11 silencing in A2058 melanoma cells simultaneously suppresses melanoma progression and induces anti-tumoral cytokine profile in human T-cells: In silico and in vitro study. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 3861–3880. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Suda, T.; Tsunoda, T.; Uchida, N.; Ura, K.; Kato, T.; Hasegawa, S.; Satoh, S.; Ohgi, S.; Tahara, H.; et al. Identification of immunoglobulin superfamily 11 (IGSF11) as a novel target for cancer immunotherapy of gastrointestinal and hepatocellular carcinomas. Cancer Sci. 2005, 96, 498–506. [Google Scholar] [CrossRef]
- Olbromski, M.; Mrozowska, M.; Piotrowska, A.; Smolarz, B.; Romanowicz, H. The VISTA/VSIG3/PSGL-1 axis: Crosstalk between immune effector cells and cancer cells in invasive ductal breast carcinoma. Cancer Immunol. Immunother. 2024, 73, 136. [Google Scholar] [CrossRef]
- Zhou, X.; Khan, S.; Huang, D.; Li, L. V-Set and immunoglobulin domain containing (VSIG) proteins as emerging immune checkpoint targets for cancer immunotherapy. Front. Immunol. 2022, 13, 938470. [Google Scholar] [CrossRef]
- Tang, X.Y.; Xiong, Y.L.; Shi, X.G.; Zhao, Y.B.; Shi, A.P.; Zheng, K.F.; Liu, Y.J.; Jiang, T.; Ma, N.; Zhao, J.B. IGSF11 and VISTA: A pair of promising immune checkpoints in tumor immunotherapy. Biomark. Res. 2022, 10, 49. [Google Scholar] [CrossRef]
- Eom, D.S.; Inoue, S.; Patterson, L.B.; Gordon, T.N.; Slingwine, R.; Kondo, S.; Watanabe, M.; Parichy, D.M. Melanophore migration and survival during zebrafish adult pigment stripe development require the immunoglobulin superfamily adhesion molecule Igsf11. PLoS Genet. 2012, 8, e1002899. [Google Scholar] [CrossRef]
- Kim, H.; Takegahara, N.; Walsh, M.C.; Choi, Y. CD44 Can Compensate for IgSF11 Deficiency by Associating with the Scaffold Protein PSD-95 during Osteoclast Differentiation. Int. J. Mol. Sci. 2020, 21, 2646. [Google Scholar] [CrossRef]
- Jang, S.; Oh, D.; Lee, Y.; Hosy, E.; Shin, H.; van Riesen, C.; Whitcomb, D.; Warburton, J.M.; Jo, J.; Kim, D.; et al. Synaptic adhesion molecule IgSF11 regulates synaptic transmission and plasticity. Nat. Neurosci. 2016, 19, 84–93. [Google Scholar] [CrossRef]
- Higashine, K.; Hashimoto, K.; Tsujimoto, E.; Oishi, Y.; Hayashi, Y.; Miyamoto, Y. Promotion of differentiation in developing mouse cerebellar granule cells by a cell adhesion molecule BT-IgSF. Neurosci. Lett. 2018, 686, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhu, G.; Yan, A.; He, J.; Liu, Y.; Li, L.; Yang, X.; Dong, C.; Kee, K. IGSF11 is required for pericentric heterochromatin dissociation during meiotic diplotene. PLoS Genet. 2021, 17, e1009778. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Padkjær, S.B.; Wang, J.; Sun, Z.; Shan, B.; Yang, L.; Chen, H.; Kang, L.; Madsen, D.; Li, X.; et al. Construction of a versatile expression library for all human single-pass transmembrane proteins for receptor pairings by high throughput screening. J. Biotechnol. 2017, 260, 18–30. [Google Scholar] [CrossRef]
- Deng, J.; Le Mercier, I.; Kuta, A.; Noelle, R.J. A New VISTA on combination therapy for negative checkpoint regulator blockade. J. Immunother. Cancer 2016, 4, 86. [Google Scholar] [CrossRef]
- Ghouzlani, A.; Rafii, S.; Karkouri, M.; Lakhdar, A.; Badou, A. The Promising IgSF11 Immune Checkpoint Is Highly Expressed in Advanced Human Gliomas and Associates to Poor Prognosis. Front. Oncol. 2021, 10, 608609. [Google Scholar] [CrossRef]
- Bi, J.; Chowdhry, S.; Wu, S.; Zhang, W.; Masui, K.; Mischel, P.S. Altered cellular metabolism in gliomas–an emerging landscape of actionable co-dependency targets. Nat. Rev. Cancer. 2020, 20, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Z.; Zhang, C.; Liu, X.; Yang, F.; Sun, L.; Liang, J.; Hu, H.; Liu, Y.; You, G.; et al. MEGF10, a Glioma Survival-Associated Molecular Signature, Predicts IDH Mutation Status. Dis. Markers 2018, 2018, 5975216. [Google Scholar] [CrossRef]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018, 20, 674–686. [Google Scholar] [CrossRef]
- Parkin, D.M. Global cancer statistics in the year 2000. Lancet Oncol. 2001, 2, 533–543. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Marzagalli, M.; Ebelt, N.D.; Manuel, E.R. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin. Cancer Biol. 2019, 59, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi Gorabi, A.; Sadat Ravari, M.; Sanaei, M.J.; Davaran, S.; Kesharwani, P.; Sahebkar, A. Immune checkpoint blockade in melanoma: Advantages, shortcomings and emerging roles of the nanoparticles. Int. Immunopharmacol. 2022, 113, 109300. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, D.; Yang, Q.; Wu, J.; Tian, H.; Ji, Z.; Chen, L.; Cai, J.; Li, Z.; Chen, Y. Global trends in BRCA-related breast cancer research from 2013 to 2022: A scientometric analysis. Front. Oncol. 2023, 13, 1197168. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Hacking, S.; Wu, D.; Lee, L.; Vitkovski, T.; Nasim, M. Nature and Significance of Stromal Differentiation, PD-L1, and VISTA in GIST. Pathol. Res. Pr. 2022, 229, 153703. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Chen, W.; Jiang, J.; Wang, L.; Li, T.; Sun, H.; Liu, J. Structural Basis of VSIG3: The Ligand for VISTA. Front. Immunol. 2021, 12, 625808. [Google Scholar] [CrossRef]
- Mehta, N.; Maddineni, S.; Kelly, R.L.; Lee, R.B.; Hunter, S.A.; Silberstein, J.L.; Parra Sperberg, R.A.; Miller, C.L.; Rabe, A.; Labanieh, L.; et al. An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA. Sci. Rep. 2020, 10, 15171. [Google Scholar] [CrossRef]
- Thakkar, D.; Paliwal, S.; Dharmadhikari, B.; Guan, S.; Liu, L.; Kar, S.; Tulsian, N.K.; Gruber, J.J.; DiMascio, L.; Paszkiewicz, K.H.; et al. Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J. Immunother. Cancer 2022, 10, e003382. [Google Scholar] [CrossRef] [PubMed]
- iOmx Therapeutics, A.G. iOmx Therapeutics Raises €65 Million in Series B Financing to Advance Pipeline of First-in-Class Cancer Immunotherapies. Available online: https://www.life-sciences-germany.com/product/imt-iomx-therapeutics-immodutome-antibody-cancer-drug-pharmaceutical-2001-33525.html (accessed on 26 June 2025).
- Hummingbird Bioscience. A Study of HMBD-002, a Monoclonal Antibody Targeting VISTA, as Monotherapy and Combined with Pembrolizumab, in Patients with Advanced Solid Tumors; ClinicalTrials.gov; Hummingbird Bioscience: Singapore, 2025.
- Bougras-Cartron, G.; Nadaradjane, A.; Joalland, M.P.; Lalier-Bretaudeau, L.; Raimbourg, J.; Cartron, P.F. Adenosine Methylation Level of miR-125a-5p Promotes Anti-PD-1 Therapy Escape through the Regulation of IGSF11/VSIG3 Expression. Cancers 2023, 15, 3188. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Mera, T.; Wang, L.; Faustman, D.L. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci. Rep. 2013, 3, 3153. [Google Scholar] [CrossRef]
- Zhao, X.; Rong, L.; Zhao, X.; Li, X.; Liu, X.; Deng, J.; Wu, H.; Xu, X.; Erben, U.; Wu, P.; et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Investig. 2012, 122, 4094–4104. [Google Scholar] [CrossRef]
- Zheng, L.; Fisher, G.; Miller, R.E.; Peschon, J.; Lynch, D.H.; Lenardo, M.J. Induction of apoptosis in mature T-cells by tumour necrosis factor. Nature 1995, 377, 348–351. [Google Scholar] [CrossRef]
- Donia, M.; Andersen, R.; Kjeldsen, J.W.; Fagone, P.; Munir, S.; Nicoletti, F.; Andersen, M.H.; Thor Straten, P.; Svane, I.M. Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T- Cells, Which Dampen CD8+ T-cell Antitumor Reactivity. Cancer Res. 2015, 75, 3747–3759. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Cell Lines or Patient Samples | Reference |
|---|---|---|
| Gastrointestinal cancer | Human gastric cell line MKN1, MKN28 and MKN45, MKN74, Kato III St-4 and human colon cancer cell line SNU-C4 | [19] |
| Hepatocellular cancer | Human hepatocellular carcinoma cell line SNU475 | [19] |
| Glioblastoma | Twenty glioma patients and thirty-two PBMC specimens | [30] |
| Melanoma | Human melanoma cell line A2058 | [18] |
| Breast cancer | Human breast cancer cell line Me16C, T47D, MCF7,BT474 SKBR3, MDAMB231, MDAMB231/BO2 | [20] |
| Abbreviation | Cancer Type | IGSF11 Expression | VISTA Expression |
|---|---|---|---|
| BLCA | Bladder urothelial carcinoma | High | High |
| BRCA | Breast invasive carcinoma | Low | Low |
| CESC | Cervical squamous cell carcinoma | Low | High |
| CHOL | Cholangiocarcinoma | High | High |
| COAD | Colon adenocarcinoma | Low | Low |
| ESCA | Esophageal carcinoma | High | High |
| GBM | Glioblastoma multiforme | High | High |
| HNSC | Head and Neck squamous cell carcinoma | High | High |
| KICH | Kidney Chromophobe | Low | Low |
| KIRC | Kidney renal clear cell carcinoma | Low | Low |
| KIRP | Kidney renal papillary cell carcinoma | Low | High |
| LIHC | Liver hepatocellular carcinoma | Low | Low |
| LUAD | Lung adenocarcinoma | High | Low |
| LUSC | Lung squamous cell carcinoma | High | Low |
| PAAD | Pancreatic carcinoma | High | High |
| PRAD | Prostate adenocarcinoma | Low | Low |
| PCPG | Pheochromocytoma and paraganglioma | High | High |
| READ | Rectal adenocarcinoma | Low | Low |
| SARC | Sarcoma | Low | High |
| SKCM | Skin cutaneous melanoma | High | High |
| THCA | Thyroid carcinoma | Low | High |
| THYM | Thymoma | High | High |
| STAD | Stomach adenocarcinoma | Low | High |
| UCEC | Uterine Corpus Endometrial Carcinoma | High | Low |
| Drug | Description | Phase Target | Target | Cancer Type | Status | Developed by |
|---|---|---|---|---|---|---|
| IMT-18 | mAb | Preclinical | IGSF11 | Targeting tumors that are resistant to PD-1/PD-L1 therapies | - | IOMx [45] |
| HMBD-002 | mAb | Phase I/II NCT05082610 | VISTA/IGSF11 | Advanced solid tumors | Active, Not Recruiting | Hummingbird Bioscience [46] |
| SG7 | mAb | Preclinical | VISTA/IGSF11 and PSGL1 | Colon cancer, melanoma | - | Mehta et al. 2021 [43] |
| K284-3046 | Small molecule | Preclinical | IGSF11 | In vitro assays | - | Xie et al. 2021 [42] |
| Sinefungin | Small molecule | Preclinical | IGSF11 | In vitro assays | - | Bougras-Cartron G et al. 2023 [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.; Kartikasari, A.E.R.; Telukutla, S.R.; Plebanski, M.; Banerjee, D. IGSF11-Mediated Immune Modulation: Unlocking a Novel Pathway in Emerging Cancer Immunotherapies. Cancers 2025, 17, 2636. https://doi.org/10.3390/cancers17162636
Srivastava S, Kartikasari AER, Telukutla SR, Plebanski M, Banerjee D. IGSF11-Mediated Immune Modulation: Unlocking a Novel Pathway in Emerging Cancer Immunotherapies. Cancers. 2025; 17(16):2636. https://doi.org/10.3390/cancers17162636
Chicago/Turabian StyleSrivastava, Sapna, Apriliana E. R. Kartikasari, Srinivasa Reddy Telukutla, Magdalena Plebanski, and Dibyendu Banerjee. 2025. "IGSF11-Mediated Immune Modulation: Unlocking a Novel Pathway in Emerging Cancer Immunotherapies" Cancers 17, no. 16: 2636. https://doi.org/10.3390/cancers17162636
APA StyleSrivastava, S., Kartikasari, A. E. R., Telukutla, S. R., Plebanski, M., & Banerjee, D. (2025). IGSF11-Mediated Immune Modulation: Unlocking a Novel Pathway in Emerging Cancer Immunotherapies. Cancers, 17(16), 2636. https://doi.org/10.3390/cancers17162636







