CT-Based Pericardial Composition Change as an Imaging Biomarker for Radiation-Induced Cardiotoxicity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
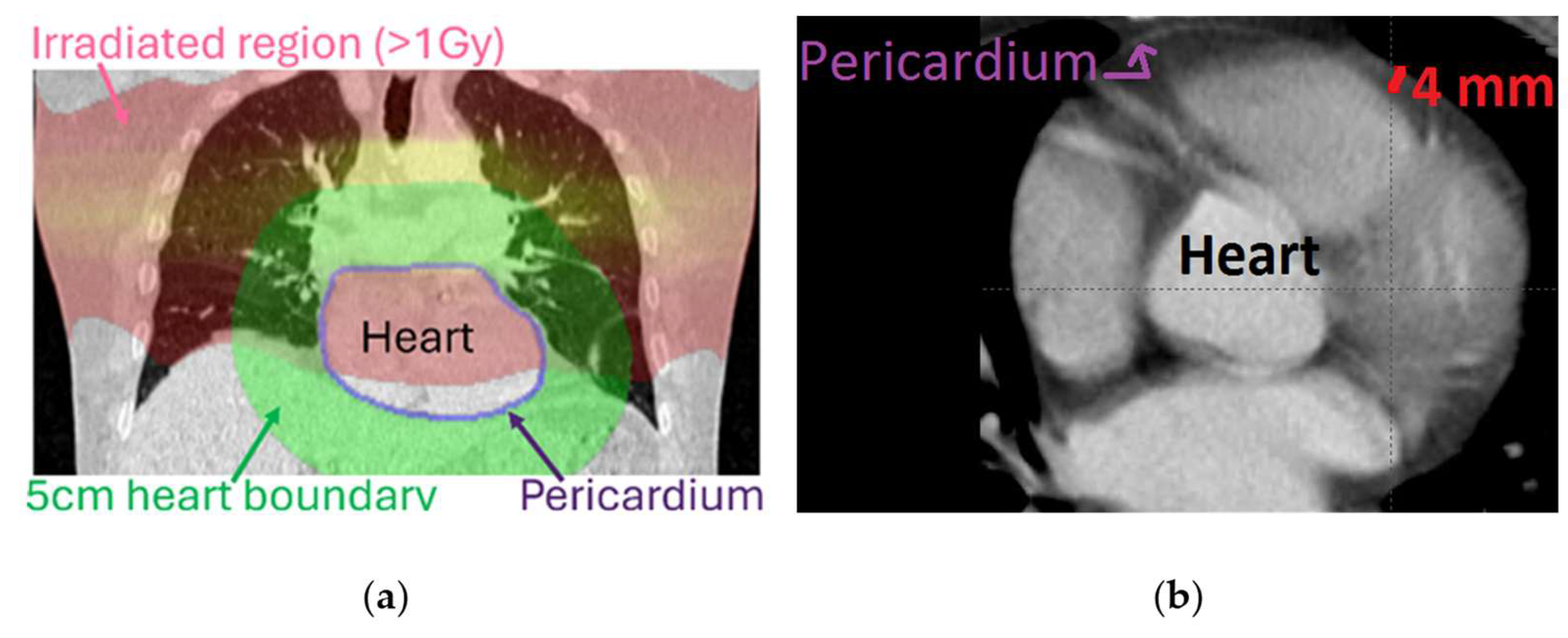
2.2. Image Analysis
- Batch1: Both baseline and follow-up CTs without contrast enhancement;
- Batch2: Both baseline and follow-up CTs with contrast enhancement;
- Batch3: Baseline CT with and follow-up CT without contrast enhancement; and
- Batch4: Follow-up CT with and baseline CT without contrast enhancement.
3. Results
3.1. Registration
3.2. Data Harmonization
3.3. Biomarker 1: Volume Associated to an HU Change
3.4. Biomarker 2: Tissue Mass Change
3.5. Biomarker 3: Tissue Volume Change
3.6. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| CVD | Cardiovascular diseases |
| EQD2 | Equivalent Dose in 2 Gy fractions |
| fx | fraction |
| HU | Hounsfield unit |
| ICD10 | International Classification of Diseases, 10th Revision |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RT | Radiotherapy |
Appendix A

Appendix B
| Manufacturer | Machine—Reconstruction Kernel/Method |
|---|---|
| GE Medical Systems | Revolution Apex—Standard |
| Revolution CT—Standard | |
| Discovery LS—Standard | |
| Revolution CT—STND# | |
| Discovery MI—Standard | |
| Discovery 710—Lung | |
| Discovery 710—Standard | |
| Philips | GEMINI TF TOF 64—C |
| GEMINI TF TOF 64—B | |
| GEMINI TF TOF 64—D | |
| GEMINI TF TOF 64T—B | |
| GEMINI TF TOF 16—B | |
| Brilliance 16P—C | |
| Brilliance 64—L | |
| Brilliance 64—C | |
| Brilliance 64—D | |
| Brilliance 64—B | |
| Brilliance 64—UB | |
| Brilliance 64—YA | |
| Precedence 16p—B | |
| iCT 256—B | |
| iCT 256—C | |
| Mx8000 IDT 16—C | |
| Iqon—Spectral CT—C | |
| Toshiba | Aquilion—FC11 |
| Aquilion—FC12 | |
| Aquilion—FC50 | |
| Aquilion—FC51 | |
| Aquilion PRIME—FC56 | |
| Aquilion PRIME—FC0B | |
| Aquilion ONE—FC0B | |
| Aquilion ONE—FC51 | |
| Aquilion ONE—FC56 | |
| Siemens | Biograph 40—B40f |
| Biograph 40—B30f | |
| Biograph 40—B19f | |
| Biograph 40—B20f | |
| Biograph 64—B40f | |
| Biograph 64—[‘130f’,’3’] | |
| Biograph 64—B30f | |
| Biograph 64—B19f | |
| Biograph 64—B70f | |
| Sensation 16—B40f | |
| Sensation 64—B30f | |
| SOMATOM Definition—B60f | |
| SOMATOM Definition Flash—B50f | |
| SOMATOM Definition Flash—[‘j70h’,’3’] | |
| SOMATOM Definition Flash—[‘170f’,’3’] | |
| SOMATOM Definition Flash—[‘130f’,’2’] | |
| SOMATOM Definition Edge—[‘170f’,’3’] | |
| SOMATOM Definition Edge—B40f | |
| SOMATOM Force—[‘Bv40d’,’3’] | |
| SOMATOM Force—[‘Qr40d’,’3’] | |
| SOMATOM Definition AS—B30f |
Appendix C
Appendix D




Appendix E
| Covariate | p Value | HR (95% Confidence Interval) | Median Survival Months |
|---|---|---|---|
| Mean pericardium dose | 0.0207 | 0.751 (0.583–0.969) | (≤mean) = 36.7 (>mean) = 26.6 |
| Mean heart dose | 0.0206 | 0.745 (0.571–0.971) | (≤mean) = 35.4 (>mean) = 26.7 |
| Baseline—follow-up mass change in Fat HU range (%) | 0.023 | 1.32 (1.04–1.67) | (≤mean) = 28.0 (>mean) = 36.7 |
| Baseline—follow-up volume change in Fluid HU range (%) | 0.0424 | 1.3 (0.994–1.7) | (≤0) = 28.3 (>0) = 36.0 |
| Baseline—follow-up volume change in Heme HU range (%) | 0.0101 | 1.38 (1.06–1.8) | (≤0) = 26.9 (>0) = 36.7 |
| Baseline—follow-up volume change in Fibrous HU range (%) | 0.0242 | 0.76 (0.598–0.965) | (≤mean) = 39.7 (>mean) = 28.6 |
| Pre-RT CVD events (yes = 1) | 0.0056 | 0.673 (0.488–0.926) | (≤0) = 36.7 (>0) = 24.1 |
| Number of CVD events before the end of RT | 0.0056 | 0.673 (0.488–0.926) | (≤0) = 36.7 (>0) = 24.1 |
| Patient sex (female = 1) | 0.00472 | 1.41 (1.1–1.8) | (≤0) = 26.7333 (>0) = 41.4667 |
| Post-RT CVD events (yes = 1) | 0.168 | 1.68 (0.932–3.03) | (≤0) = 31.4 (>0) = 69.1 |
| Number of CVD events after the end of RT | 0.168 | 1.68 (0.932–3.03) | (≤0) = 31.4 (>0) = 69.1 |
| Covariate | p Value | HR (95% Confidence Interval) | Median Months to Post-RT CVD |
|---|---|---|---|
| Baseline—follow-up mass change in Fluid HU range (%) | 0.0027 | 11 (0.0217–5.58 × 103) | (≤mean) = 8.7 (>mean) = 38.0 |
| Baseline—follow-up mass change in Heme HU range (%) | 0.0027 | 11 (0.0217–5.58 × 103) | (≤mean) = 8.7 (>mean) = 38.0 |
| Baseline—follow-up mass change in Fibrous HU range (%) | 0.139 | 2.82 (0.292–27.1) | (≤mean) = 16.7 (>mean) = 38.0 |
| Skewness in HU-change histogram for the heart | 0.0197 | 0.296 (0.0675–1.29) | (≤mean) = 48.4 (>mean) = 22.3 |
| Mean in HU-change histogram for pericardium | 0.0544 | 2.89 (0.591–14.2) | (≤median) = 16.7 (>median) = 41.5 |
References
- Aboumsallem, J.P.; Moslehi, J.; De Boer, R.A. Reverse Cardio-Oncology: Cancer Development in Patients With Cardiovascular Disease. J. Am. Heart Assoc. 2020, 9, e013754. [Google Scholar] [CrossRef] [PubMed]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Aboumsallem, J.P.; Moore, K.J.; De Boer, R.A. Reverse cardio-oncology: Exploring the effects of cardiovascular disease on cancer pathogenesis. J. Mol. Cell. Cardiol. 2022, 163, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pouvreau, P.; Taleb, I.; Fontaine, A.; Edouard, L.; Gibson, N.; Yaouanq, M.; Boudoussier, A.; Petit, A.; Vinh-Hung, V.; Sargos, P.; et al. Heart is a heavy burden: Cardiac toxicity in radiation oncology. Support. Care Cancer 2024, 32, 769. [Google Scholar] [CrossRef]
- Stoltzfus, K.C.; Zhang, Y.; Sturgeon, K.; Sinoway, L.I.; Trifiletti, D.M.; Chinchilli, V.M.; Zaorsky, N.G. Fatal heart disease among cancer patients. Nat. Commun. 2020, 11, 2011. [Google Scholar] [CrossRef]
- Logotheti, S.; Pavlopoulou, A.; Rudsari, H.K.; Galow, A.-M.; Kafalı, Y.; Kyrodimos, E.; Giotakis, A.I.; Marquardt, S.; Velalopoulou, A.; Verginadis, I.I.; et al. Intercellular pathways of cancer treatment-related cardiotoxicity and their therapeutic implications: The paradigm of radiotherapy. Pharmacol. Ther. 2024, 260, 108670. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Lancellotti, P.; Nkomo, V.T.; Badano, L.P.; Bergler-Klein, J.; Bogaert, J.; Davin, L.; Cosyns, B.; Coucke, P.; Dulgheru, R.; Edvardsen, T.; et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 721–740. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; De Marco, G.; Natale, G.; Patanè, V.; De Chiara, M.; Buono, M.; Russo, G.M.; Monti, R.; Balestrucci, G.; et al. Role of Cardiac Biomarkers in Non-Small Cell Lung Cancer Patients. Diagnostics 2023, 13, 400. [Google Scholar] [CrossRef]
- Velusamy, R.; Nolan, M.; Murphy, A.; Thavendiranathan, P.; Marwick, T.H. Screening for Coronary Artery Disease in Cancer Survivors. JACC CardioOncol. 2023, 5, 22–38. [Google Scholar] [CrossRef]
- Abravan, A.; Faivre-Finn, C.; Gomes, F.; Van Herk, M.; Price, G. Comorbidity in patients with cancer treated at The Christie. Br. J. Cancer 2024, 131, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, E.; Zhang, S.; Zakariaee, R.; Guthier, C.V.; Hakimian, B.; Mirhadi, A.; Kamrava, M.; Padda, S.K.; Lewis, J.H.; Nikolova, A.; et al. Left Anterior Descending Coronary Artery Radiation Dose Association With All-Cause Mortality in NRG Oncology Trial RTOG 0617. Int. J. Radiat. Oncol. 2023, 115, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.; Yegya-Raman, N.; Kegelman, T.P.; Kallan, M.J.; Roshkovan, L.; Katz, S.; Ky, B.; Fradley, M.; Xiao, Y.; Lee, S.H.; et al. Cardiovascular Substructure Dose and Cardiac Events following Proton- and Photon-Based Chemoradiotherapy for Non-Small Cell Lung Cancer. Adv. Radiat. Oncol. 2023, 8, 101235. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Reddy, Y.N.V. The Role of the Pericardium in Heart Failure. JACC Heart Fail. 2019, 7, 574–585. [Google Scholar] [CrossRef]
- Klein, A.L.; Ming Wang, T.K.; Reyaldeen, R. Mortality and the Pericardial Sac: Are We Only Scratching the Surface? J. Am. Coll. Cardiol. 2020, 76, 2632–2634. [Google Scholar] [CrossRef]
- Lorenzo-Esteller, L.; Ramos-Polo, R.; Pons Riverola, A.; Morillas, H.; Berdejo, J.; Pernas, S.; Pomares, H.; Asiain, L.; Garay, A.; Martínez Pérez, E.; et al. Pericardial Disease in Patients with Cancer: Clinical Insights on Diagnosis and Treatment. Cancers 2024, 16, 3466. [Google Scholar] [CrossRef]
- Mori, S.; Bertamino, M.; Guerisoli, L.; Stratoti, S.; Canale, C.; Spallarossa, P.; Porto, I.; Ameri, P. Pericardial effusion in oncological patients: Current knowledge and principles of management. Cardio-Oncol. 2024, 10, 8. [Google Scholar] [CrossRef]
- Sigvardt, F.L.; Hansen, M.L.; Kristensen, S.L.; Gustafsson, F.; Ghanizada, M.; Schou, M.; Køber, L.; Torp-Pedersen, C.; Gislason, G.H.; Madelaire, C. Risk Factors for Morbidity and Mortality Following Hospitalization for Pericarditis. J. Am. Coll. Cardiol. 2020, 76, 2623–2631. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Farkas, D.K.; Ehrenstein, V.; Bhaskaran, K.; Bøtker, H.E.; Sørensen, H.T. Pericarditis as a Marker of Occult Cancer and a Prognostic Factor for Cancer Mortality. Circulation 2017, 136, 996–1006. [Google Scholar] [CrossRef]
- Thor, M.; Deasy, J.O.; Hu, C.; Gore, E.; Bar-Ad, V.; Robinson, C.; Wheatley, M.; Oh, J.H.; Bogart, J.; Garces, Y.I.; et al. Modeling the Impact of Cardiopulmonary Irradiation on Overall Survival in NRG Oncology Trial RTOG 0617. Clin. Cancer Res. 2020, 26, 4643–4650. [Google Scholar] [CrossRef]
- Xue, J.; Han, C.; Jackson, A.; Hu, C.; Yao, H.; Wang, W.; Hayman, J.; Chen, W.; Jin, J.; Kalemkerian, G.P.; et al. Doses of radiation to the pericardium, instead of heart, are significant for survival in patients with non-small cell lung cancer. Radiother. Oncol. 2019, 133, 213–219. [Google Scholar] [CrossRef]
- Chang, L.-K.; Kuo, Y.-W.; Wu, S.-G.; Chung, K.-P.; Shih, J.-Y. Recurrence of pericardial effusion after different procedure modalities in patients with non-small-cell lung cancer. ESMO Open 2022, 7, 100354. [Google Scholar] [CrossRef]
- Wang, K.; Eblan, M.J.; Deal, A.M.; Lipner, M.; Zagar, T.M.; Wang, Y.; Mavroidis, P.; Lee, C.B.; Jensen, B.C.; Rosenman, J.G.; et al. Cardiac Toxicity After Radiotherapy for Stage III Non–Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J. Clin. Oncol. 2017, 35, 1387–1394. [Google Scholar] [CrossRef]
- Heine, M.; Agrawal, A.; Wensink, E.; Wang, T.K.M.; Klein, A. The Role of the Advanced Practice Provider in a Pericardial Center of Excellence. Curr. Cardiol. Rep. 2025, 27, 69. [Google Scholar] [CrossRef]
- Kivity, S.; Baran, T.Z.; Reuveni, M.M.; Irony, A.; Adler, L.; Alder, Y.; Parikh, R.; Kivity, S. The Longitudinal Incidence of Pericarditis in 1.6 Million Patients: A 20-Year Study. Am. J. Cardiol. 2024, 223, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Wang, T.K.M.; Cremer, P.C.; Abbate, A.; Adler, Y.; Asher, C.; Brucato, A.; Chetrit, M.; Hoit, B.; Jellis, C.L.; et al. Pericardial Diseases. JACC Cardiovasc. Imaging 2024, 17, 937–988. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.M.; Williams, P.L.; Williams, M.P.; Edwards, A.J.; Roobottom, C.A.; Morgan-Hughes, G.J.; Manghat, N.E. Imaging the pericardium: Appearances on ECG-gated 64-detector row cardiac computed tomography. Br. J. Radiol. 2010, 83, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Szpakowski, N.; Desai, M.Y. Radiation-Associated Pericardial Disease. Curr. Cardiol. Rep. 2019, 21, 97. [Google Scholar] [CrossRef]
- Tarsitano, M.G.; Pandozzi, C.; Muscogiuri, G.; Sironi, S.; Pujia, A.; Lenzi, A.; Giannetta, E. Epicardial Adipose Tissue: A Novel Potential Imaging Marker of Comorbidities Caused by Chronic Inflammation. Nutrients 2022, 14, 2926. [Google Scholar] [CrossRef]
- Marandi, R. PERSIMUNE Health Informatics. Available online: https://github.com/PERSIMUNE (accessed on 1 July 2025).
- Keszei, A.P.; Berkels, B.; Deserno, T.M. Survey of Non-Rigid Registration Tools in Medicine. J. Digit. Imaging 2017, 30, 102–116. [Google Scholar] [CrossRef]
- Smith, A.G.; Han, E.; Petersen, J.; Olsen, N.A.F.; Giese, C.; Athmann, M.; Dresbøll, D.B.; Thorup-Kristensen, K. RootPainter: Deep learning segmentation of biological images with corrective annotation. New Phytol. 2022, 236, 774–791. [Google Scholar] [CrossRef] [PubMed]
- Wasserthal, J.; Breit, H.-C.; Meyer, M.T.; Pradella, M.; Hinck, D.; Sauter, A.W.; Heye, T.; Boll, D.T.; Cyriac, J.; Yang, S.; et al. TotalSegmentator: Robust Segmentation of 104 Anatomic Structures in CT Images. Radiol. Artif. Intell. 2023, 5, e230024. [Google Scholar] [CrossRef] [PubMed]
- Orlhac, F.; Eertink, J.J.; Cottereau, A.-S.; Zijlstra, J.M.; Thieblemont, C.; Meignan, M.; Boellaard, R.; Buvat, I. A Guide to ComBat Harmonization of Imaging Biomarkers in Multicenter Studies. J. Nucl. Med. 2022, 63, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: Comparison of attenuation values with unenhanced CT. Abdom. Radiol. 2017, 42, 702–709. [Google Scholar] [CrossRef]
- Cetin, M.S. Effectiveness of computed tomography attenuation values in characterization of pericardial effusion. Anatol. J. Cardiol. 2017, 17, 322–327. [Google Scholar] [CrossRef]
- Haseltine, J.M.; Apte, A.; Jackson, A.; Yorke, E.; Yu, A.F.; Plodkowski, A.; Wu, A.; Peleg, A.; Al-Sadawi, M.; Iocolano, M.; et al. Association of cardiac calcium burden with overall survival after radiotherapy for non-small cell lung cancer. Phys. Imaging Radiat. Oncol. 2023, 25, 100410. [Google Scholar] [CrossRef]
- Hoey, E.T.D.; Shahid, M.; Watkin, R.W. Computed tomography and magnetic resonance imaging evaluation of pericardial disease. Quant. Imaging Med. Surg. 2016, 6, 274–284. [Google Scholar] [CrossRef]
- Wang, K.; Malkin, H.E.; Patchett, N.D.; Pearlstein, K.A.; Heiling, H.M.; McCabe, S.D.; Deal, A.M.; Mavroidis, P.; Oakey, M.; Fenoli, J.; et al. Coronary Artery Calcifications and Cardiac Risk After Radiation Therapy for Stage III Lung Cancer. Int. J. Radiat. Oncol. 2022, 112, 188–196. [Google Scholar] [CrossRef]
- Carlsson, M.; Cain, P.; Holmqvist, C.; Stahlberg, F.; Lundback, S.; Arheden, H. Total heart volume variation throughout the cardiac cycle in humans. Am. J. Physiol.-Heart Circ. Physiol. 2004, 287, H243–H250. [Google Scholar] [CrossRef]
- Carlsson, M.; Rosengren, A.; Ugander, M.; Ekelund, U.; Cain, P.A.; Arheden, H. Center of volume and total heart volume variation in healthy subjects and patients before and after coronary bypass surgery. Clin. Physiol. Funct. Imaging 2005, 25, 226–233. [Google Scholar] [CrossRef]
- Smooth Noisy Data in MATLAB. Available online: https://www.mathworks.com/help/matlab/ref/smoothdata.html (accessed on 10 June 2025).
- Creed, J.; Gerke, T.; Berglund, A. MatSurv: Survival analysis and visualization in MATLAB. J. Open Source Softw. 2020, 5, 1830. [Google Scholar] [CrossRef]
- Dhore-Patil, A.; Urina-Jassir, D.; Samson, R.; Le Jemtel, T.H.; Oparil, S. Epicardial Adipose Tissue Thickness and Preserved Ejection Fraction Heart Failure. Curr. Hypertens. Rep. 2024, 26, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Hussain, K.; Shlofmitz, E. Pericardial Calcification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538342/ (accessed on 6 May 2025).
- Ling, L.H.; Oh, J.K.; Breen, J.F.; Schaff, H.V.; Danielson, G.K.; Mahoney, D.W.; Seward, J.B.; Tajik, A.J. Calcific Constrictive Pericarditis: Is It Still with Us? Ann. Intern. Med. 2000, 132, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Shimony, A.; Fox, B.D.; Langleben, D.; Rudski, L.G. Incidence and Significance of Pericardial Effusion in Patients With Pulmonary Arterial Hypertension. Can. J. Cardiol. 2013, 29, 678–682. [Google Scholar] [CrossRef]
- Welch, T.D. Constrictive pericarditis: Diagnosis, management and clinical outcomes. Heart 2018, 104, 725–731. [Google Scholar] [CrossRef]
- Yeneneh, B.T.; Allen, S.; Panse, P.; Mookadam, F.; Rule, W. Constrictive Pericarditis 5 Months after Radiation Therapy in a 62-Year-Old Woman with Esophageal Cancer. Tex. Heart Inst. J. 2017, 44, 411–415. [Google Scholar] [CrossRef]
- Vemireddy, L.P.; Jain, N.; Aqeel, A.; Jeelani, H.M.; Shayuk, M. Lung Adenocarcinoma Presenting as Malignant Pericardial Effusion/Tamponade. Cureus 2021, 13, e13762. [Google Scholar] [CrossRef]
- Milevoj Kopcinovic, L.; Culej, J. Pleural, peritoneal and pericardial effusions—A biochemical approach. Biochem. Medica 2014, 24, 123–137. [Google Scholar] [CrossRef]
- Nardi-Agmon, I.; Zer, A.; Peysakhovich, Y.; Margalit, I.; Kornowski, R.; Peled, N.; Iakobishvili, Z. Development of Pericardial Effusion in Non-small Cell Lung Cancer Is Associated with the Presence of EGFR/ALK Mutations. Isr. Med. Assoc. J. IMAJ 2022, 24, 135–139. [Google Scholar]
- de Jesus, M.; Chanda, A.; Grabauskas, T.; Kumar, M.; Kim, A.S. Cardiovascular disease and lung cancer. Front. Oncol. 2024, 14, 1258991. [Google Scholar] [CrossRef]
- Forbes, N.; Terrones-Campos, C.; Smith, A.; Reekie, J.; Darkner, S.; Maraldo, M.; Pøhl, M.; Risumlund, S.; Specht, L.; Bentzen, S.M.; et al. Cardiac dose-volume analysis of 9,411 patients with registry data for cardiovascular disease and overall survival. medRxiv 2024. [Google Scholar] [CrossRef]
- Walls, G.M.; Bergom, C.; Mitchell, J.D.; Rentschler, S.L.; Hugo, G.D.; Samson, P.P.; Robinson, C.G. Cardiotoxicity following thoracic radiotherapy for lung cancer. Br. J. Cancer 2025, 132, 311–325. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Yang, J.; Zhang, S.; Zhang, P.; Yang, Y. Lung cancer and risk of cardiovascular mortality. Front. Cardiovasc. Med. 2025, 11, 1491912. [Google Scholar] [CrossRef]
- Roberts, W.C. Pericardial heart disease: Its morphologic features and its causes. Proc. Bayl. Univ. Med. Cent. 2005, 18, 38–55. [Google Scholar] [CrossRef]






| Characteristic | n | Characteristic | n | Characteristic | n |
|---|---|---|---|---|---|
| Sex | Mean heart dose | Patients with | |||
| Female | 266 | >10 Gy | 68 | History of RT for other primary cancers 3 | 0 |
| Male | 210 | <1 Gy | 188 | History of previous primary cancers | 178 |
| Age at treatment 1 | Max heart dose 2 | Pre-RT cardiovascular diseases | 91 | ||
| ≥65 | 381 | >15 Gy | 292 | Post-RT cardiovascular diseases | 11 |
| Diagnosis | Mean pericardium dose | Survival ≤2 years post-RT | 171 | ||
| SCLC | 63 | >10 Gy | 87 | Survival >5 years post-RT | 28 |
| NSCLC | 413 | <1 Gy | 159 | Image contrast enhancement | |
| RT fractions | Contrast enhanced baseline CTs | 260 | |||
| ≤8 | 183 | Contrast enhanced follow-up CTs | 426 |
| Tissue Composition/s | HU Range | Label |
|---|---|---|
| Calcification, calcified constrictive tissue, malignancy | HU ≥ 130 | Calcification |
| Fibrosis, constrictive tissue, adjacent myocardial tissue | 129 ≥ HU ≥ 65 | Fibrous |
| Normal pericardium, thickened pericardium, hemopericardium | 64 ≥ HU ≥ 13 | Heme |
| Effusion, normal fluid | 12 ≥ HU ≥ −5 | Fluid |
| Fat (including normal fatty tissue) | HU ≤ −6 | Fat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modiri, A.; Vogelius, I.R.; Campos, C.T.; Kutnar, D.; Jeudy, J.; Pohl, M.; Dickfeld, T.-M.L.; Bentzen, S.M.; Sawant, A.; Petersen, J. CT-Based Pericardial Composition Change as an Imaging Biomarker for Radiation-Induced Cardiotoxicity. Cancers 2025, 17, 2635. https://doi.org/10.3390/cancers17162635
Modiri A, Vogelius IR, Campos CT, Kutnar D, Jeudy J, Pohl M, Dickfeld T-ML, Bentzen SM, Sawant A, Petersen J. CT-Based Pericardial Composition Change as an Imaging Biomarker for Radiation-Induced Cardiotoxicity. Cancers. 2025; 17(16):2635. https://doi.org/10.3390/cancers17162635
Chicago/Turabian StyleModiri, Arezoo, Ivan R. Vogelius, Cynthia Terrones Campos, Denis Kutnar, Jean Jeudy, Mette Pohl, Timm-Michael L. Dickfeld, Soren M. Bentzen, Amit Sawant, and Jens Petersen. 2025. "CT-Based Pericardial Composition Change as an Imaging Biomarker for Radiation-Induced Cardiotoxicity" Cancers 17, no. 16: 2635. https://doi.org/10.3390/cancers17162635
APA StyleModiri, A., Vogelius, I. R., Campos, C. T., Kutnar, D., Jeudy, J., Pohl, M., Dickfeld, T.-M. L., Bentzen, S. M., Sawant, A., & Petersen, J. (2025). CT-Based Pericardial Composition Change as an Imaging Biomarker for Radiation-Induced Cardiotoxicity. Cancers, 17(16), 2635. https://doi.org/10.3390/cancers17162635







