Differential miRNA Expressions Linking Environmental Risk Factors to Triple-Negative Breast Cancer Stages at Diagnosis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Dataset and Measurements
2.2. miRNA Sequencing
2.3. miRNA Normalization
2.4. Differentially Expressed miRNA Among Environmental Risk Factors
2.5. Pathway Analysis
2.6. Statistical Analysis
3. Results
3.1. Data Preprocessing
3.2. Association Between Environmental Risk Factors and TNBC Stage
3.3. Differential Expression Analysis of miRNAs
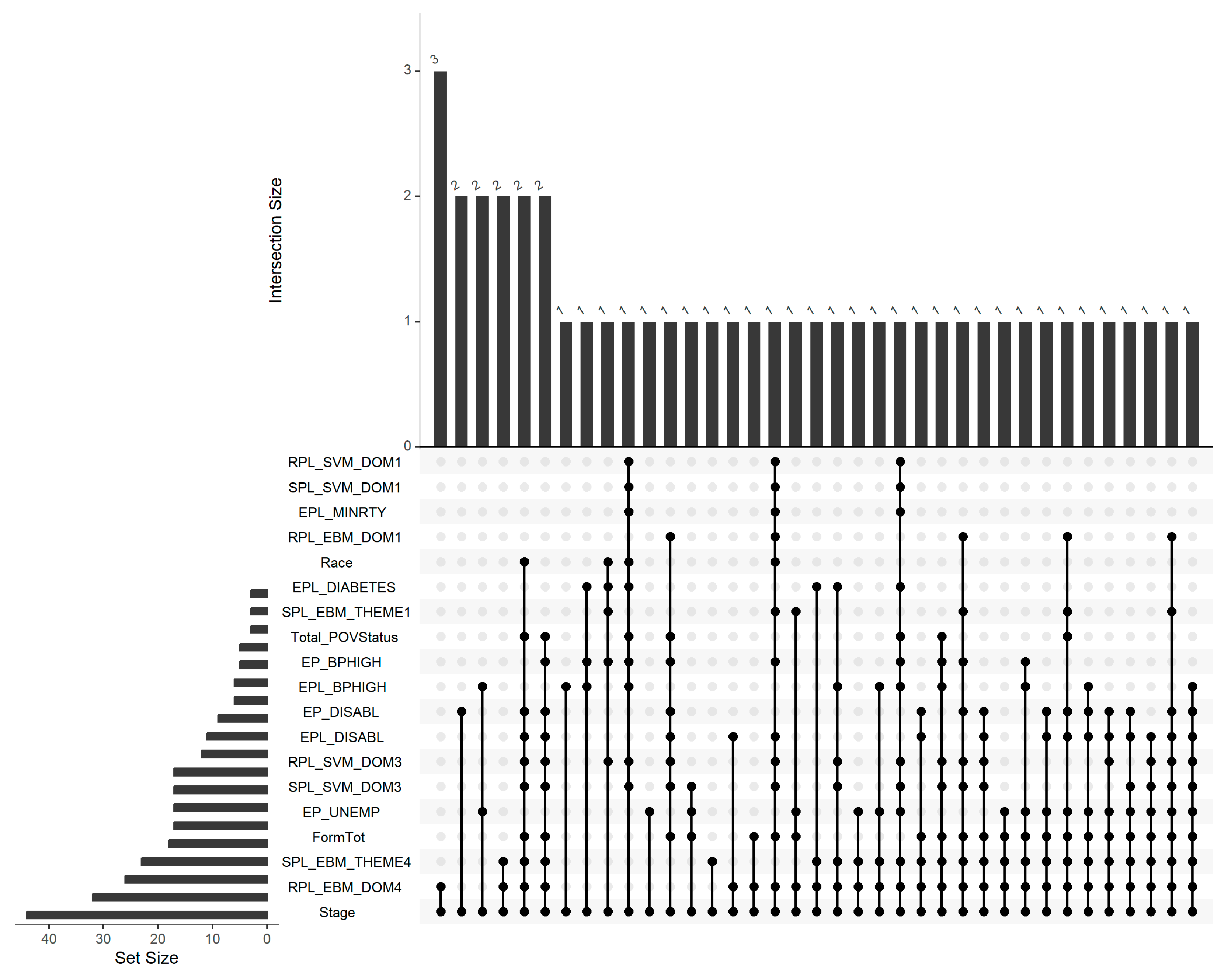
3.4. Shared miRNAs Across Risk Factors and Disease Stage
3.5. Functional and Pathway Enrichment Analysis
4. Discussions
- 8 miRNAs were associated with census tract-level racial groups (Supplementary Table S1).
- 102 and 196 miRNAs were linked to domains involving proximity to high-volume roads, railways, and airports, as well as their percentile ranks (Supplementary Tables S12 and S2, respectively).
- 40 and 56 miRNAs were associated with the percentage of individuals with high blood pressure values and their percentile ranks (Supplementary Tables S3 and S9, respectively).
- 22 and 19 miRNAs were associated with domains involving ozone, PM2.5, air toxics cancer risk, diesel particulate matter, and their respective percentile ranks (Supplementary Tables S8 and S4).
- 21 miRNAs were linked to the percentage of unemployed individuals (Supplementary Table S5).
- 82 miRNAs were associated with the community-level indicator of total poverty status (Supplementary Table S6).
- 102 and 126 miRNAs were associated with domains comprising English language proficiency, age groups (65+, ≤17), civilian disability status, and their percentile ranks (Supplementary Tables S7 and S10, respectively).
- 69 miRNAs were significantly associated with TNBC stage (Supplementary Table S11).
- 4 miRNAs each were associated with the percentile rank of minority population percentage and domains involving racial/ethnic minority representation and their percentile ranks (Supplementary Tables S13–S15).
- 27 and 24 miRNAs were associated with the percentage and percentile rank of the civilian noninstitutionalized population with disabilities (Supplementary Tables S17 and S16, respectively).
- 12 miRNAs were linked to the percentile rank of individuals with diabetes (Supplementary Table S18).
- 69 miRNAs were associated with the total concentration of hazardous air pollutant formaldehyde, considered as lifetime cancer risk from inhalation of air toxics by the National Air Toxics Assessment (Supplementary Table S19).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TNBC | Triple Negative Breast Cancer |
| miRNA | Micro Ribonucleic Acid |
| LTR | Louisiana Tumor Registry |
| FFPE | Formalin-Fixed, Paraffin-Embedded |
| DE | Differentially Expressed |
| NLM | National Library of Medicine |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| ERBB2 | Erb-B2 Receptor Tyrosine Kinase 2 |
| TMM | Trimmed Mean of M-values |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| PA | Pathway Analysis |
| FDR | False Discovery Rate |
| BH | Benjamini-Hochberg |
| RIN | RNA Integrity Number |
References
- Hiatt, R.A.; Brody, J.G. Environmental determinants of breast cancer. Annu. Rev. Public Health 2018, 39, 113–133. [Google Scholar] [CrossRef]
- Johnson-Thompson, M.C.; Guthrie, J. Ongoing research to identify environmental risk factors in breast carcinoma. Cancer 2000, 88, 1224–1229. [Google Scholar] [CrossRef]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015, 313, 165–173. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.; Luo, M.; Rizvi, N.; Bam, A.; Del Valle, L.; Yu, Q. Differentially expressed miRNAs mediate racial differences in diagnosis stage among triple negative breast cancer patients in Louisiana. Data Sci. Sci. 2025; submitted. [Google Scholar]
- Siddharth, S.; Sharma, D. Racial disparity and triple-negative breast cancer in African-American women: A multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers 2018, 10, 514. [Google Scholar] [CrossRef]
- Sugita, B.; Gill, M.; Mahajan, A.; Duttargi, A.; Kirolikar, S.; Almeida, R.; Regis, K.; Oluwasanmi, O.L.; Marchi, F.; Marian, C. Differentially expressed miRNAs in triple negative breast cancer between African-American and non-Hispanic white women. Oncotarget 2016, 7, 79274. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Gomez, S.L.; Keegan, T.H.; Kurian, A.W.; Clarke, C.A. Breast cancer mortality in African-American and non-Hispanic white women by molecular subtype and stage at diagnosis: A population-based study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Hanusek, K.; Karczmarski, J.; Litwiniuk, A.; Urbańska, K.; Ambrozkiewicz, F.; Kwiatkowski, A.; Martyńska, L.; Domańska, A.; Bik, W.; Paziewska, A. Obesity as a risk factor for breast cancer—The role of miRNA. Int. J. Mol. Sci. 2022, 23, 15683. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, Y.; Yu, F.; Ping, Z.; Liu, L. Body mass index increases the lymph node metastasis risk of breast cancer: A dose-response meta-analysis with 52904 subjects from 20 cohort studies. BMC Cancer 2020, 20, 601. [Google Scholar] [CrossRef]
- Dietze, E.C.; Chavez, T.A.; Seewaldt, V.L. Obesity and triple-negative breast cancer: Disparities, controversies, and biology. Am. J. Pathol. 2018, 188, 280–290. [Google Scholar] [CrossRef]
- Yang, Y.; Lynch, B.M.; Hodge, A.M.; Liew, D.; Mclean, C.A.; Seviiri, M.; Southey, M.C.; Hopper, J.L.; English, D.R.; Giles, G.G. Blood pressure and risk of breast cancer, overall and by subtypes: A prospective cohort study. J. Hypertens. 2017, 35, 1371–1380. [Google Scholar] [CrossRef]
- Bosco, J.L.; Palmer, J.R.; Boggs, D.A.; Hatch, E.E.; Rosenberg, L. Cardiometabolic factors and breast cancer risk in US black women. Breast Cancer Res. Treat. 2012, 134, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Largent, J.; McEligot, A.; Ziogas, A.; Reid, C.; Hess, J.; Leighton, N.; Peel, D.; Anton-Culver, H. Hypertension, diuretics and breast cancer risk. J. Hum. Hypertens. 2006, 20, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, N.; Goldgar, D.E.; Easton, D.F.; Rookus, M.; Brohet, R.; Antoniou, A.C.; Peock, S.; Evans, G.; Eccles, D.; Douglas, F. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS). J. Natl. Cancer Inst. 2006, 98, 535–544. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Gammon, M.D.; John, E.M. Reproductive factors and breast cancer. Epidemiol. Rev. 1993, 15, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Farhangi, A.; Rana, F. TNBC vs non-TNBC: A retrospective review of differences in mean age, family history, smoking history, and stage at diagnosis. Clin. Adv. Hematol. Oncol. 2014, 12, 377–381. [Google Scholar]
- Obeng-Gyasi, S.; Asad, S.; Fisher, J.L.; Rahurkar, S.; Stover, D.G. Socioeconomic and surgical disparities are associated with rapid relapse in patients with triple-negative breast cancer. Ann. Surg. Oncol. 2021, 28, 6500–6509. [Google Scholar] [CrossRef]
- Dreyer, M.S.; Nattinger, A.B.; McGinley, E.L.; Pezzin, L.E. Socioeconomic status and breast cancer treatment. Breast Cancer Res. Treat. 2018, 167, 1–8. [Google Scholar] [CrossRef]
- Halpern, M.T.; Bian, J.; Ward, E.M.; Schrag, N.M.; Chen, A.Y. Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer 2007, 110, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-l.; Wang, D.-w.; Yang, Z.-c.; Ma, R.; Li, Z.; Suo, W.; Zhao, Z.; Li, Z.-w. Marital status is an independent prognostic factor in inflammatory breast cancer patients: An analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res. Treat. 2019, 178, 379–388. [Google Scholar] [CrossRef]
- Wong, C.M.; Tsang, H.; Lai, H.K.; Thomas, G.N.; Lam, K.B.; Chan, K.P.; Zheng, Q.; Ayres, J.G.; Lee, S.Y.; Lam, T.H. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol. Biomark. Prev. 2016, 25, 839–845. [Google Scholar] [CrossRef]
- Prada, D.; Baccarelli, A.A.; Terry, M.B.; Valdéz, L.; Cabrera, P.; Just, A.; Kloog, I.; Caro, H.; García-Cuellar, C.; Sánchez-Pérez, Y. Long-term PM 2.5 exposure before diagnosis is associated with worse outcome in breast cancer. Breast Cancer Res. Treat. 2021, 188, 525–533. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef]
- Tüfekci, K.U.; Öner, M.G.; Meuwissen, R.L.J.; Genç, Ş. The role of microRNAs in human diseases. In miRNomics: MicroRNA Biology and Computational Analysis; Humana Press: Totowa, NJ, USA, 2014; pp. 33–50. [Google Scholar]
- Xu, J.; Wu, K.-j.; Jia, Q.-j.; Ding, X.-f. Roles of miRNA and IncRNA in triple-negative breast cancer. J. Zhejiang Univ.-Sci. B 2020, 21, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, Q.; Yang, H.; Zhang, X. New progress in the role of microRNAs in the diagnosis and prognosis of triple negative breast cancer. Front. Mol. Biosci. 2023, 10, 1162463. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention and Agency for Toxic Substances Disease Registry. Environmental Justice Index. Available online: https://atsdr.cdc.gov/place-health/php/eji/eji-technical-documentation.html (accessed on 27 March 2024).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Xia, Y. Statistical normalization methods in microbiome data with application to microbiome cancer research. Gut Microbes 2023, 15, 2244139. [Google Scholar] [CrossRef] [PubMed]
- Parise, C.A.; Bauer, K.R.; Caggiano, V. Variation in breast cancer subtypes with age and race/ethnicity. Crit. Rev. Oncol./Hematol. 2010, 76, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Lex, A.; Gehlenborg, N. Points of view: Sets and intersections. Nat. Methods 2014, 11, 779. [Google Scholar] [CrossRef]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of intersecting sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef]
- García-Campos, M.A.; Espinal-Enríquez, J.; Hernández-Lemus, E. Pathway analysis: State of the art. Front. Physiol. 2015, 6, 383. [Google Scholar] [CrossRef]
- Folger, O.; Jerby, L.; Frezza, C.; Gottlieb, E.; Ruppin, E.; Shlomi, T. Predicting selective drug targets in cancer through metabolic networks. Mol. Syst. Biol. 2011, 7, 501. [Google Scholar] [CrossRef]
- Wu, M.; Li, Q.; Wang, H. Identification of novel biomarkers associated with the prognosis and potential pathogenesis of breast cancer via integrated bioinformatics analysis. Technol. Cancer Res. Treat. 2021, 20, 1533033821992081. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, X.; Li, P.; Tan, J.; Wang, X.; Xiang, T.; Ren, G. miR-7-5p suppresses cell proliferation and induces apoptosis of breast cancer cells mainly by targeting REGγ. Cancer Lett. 2015, 358, 27–36. [Google Scholar] [CrossRef]
- Samara, M.; Thodou, E.; Patoulioti, M.; Poultsidi, A.; Thomopoulou, G.E.; Giakountis, A. Integrated miRNA Signatures: Advancing Breast Cancer Diagnosis and Prognosis. Biomolecules 2024, 14, 1352. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.; Blumenschein, G., Jr. Defining biomarkers to predict sensitivity to PI3K/Akt/mTOR pathway inhibitors in breast cancer. Cancer Treat. Rev. 2013, 39, 313–320. [Google Scholar] [CrossRef]
- Massihnia, D.; Galvano, A.; Fanale, D.; Perez, A.; Castiglia, M.; Incorvaia, L.; Listì, A.; Rizzo, S.; Cicero, G.; Bazan, V. Triple negative breast cancer: Shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget 2016, 7, 60712. [Google Scholar] [CrossRef] [PubMed]
- Giuli, M.; Giuliani, E.; Screpanti, I.; Bellavia, D.; Checquolo, S. Notch signaling activation as a hallmark for triple-negative breast cancer subtype. J. Oncol. 2019, 2019, 8707053. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, M.; Gonzalez-Perez, R.R. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2011, 1815, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Moretti, E.; Siclari, O.; Migliaccio, I.; Santarpia, L.; D’Incalci, M.; Piccolo, S.; Veronesi, A.; Zambelli, A.; Del Sal, G. Targeting triple negative breast cancer: Is p53 the answer? Cancer Treat. Rev. 2013, 39, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A basic review on estrogen receptor signaling pathways in breast cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
| Early Stage (n = 184) Counts (Row Percentage, %) | Advanced Stage (n = 250) Counts (Row Percentage, %) | p-Value * | |
|---|---|---|---|
| Race | 0.111 | ||
| African American | 104 (46.0) | 122 (54.0) | |
| Caucasian American | 80 (38.5) | 128 (61.5) | |
| EPL_MINRTY | 0.048 | ||
| EPL_MINRTY_1 (≤0.54) | 80 (48.5) | 85 (51.5) | |
| EPL_MINRTY_2 (>0.54) | 104 (38.8) | 164 (61.2) | |
| SPL_SVM_DOM1 | 0.048 | ||
| SPL_SVM_DOM1_1 (≤0.54) | 80 (48.5) | 85 (51.5) | |
| SPL_SVM_DOM1_2 (>0.54) | 104 (38.8) | 164 (61.2) | |
| RPL_SVM_DOM1 | 0.048 | ||
| RPL_SVM_DOM1_1 (≤0.54) | 80 (48.5) | 85 (51.5) | |
| RPL_SVM_DOM1_2 (>0.54) | 104 (38.8) | 164 (61.2) | |
| Total_PovStatus | 0.047 | ||
| Total_PovStatus_1 (<2350) | 33 (52.4) | 30 (47.6) | |
| Total_PovStatus_2 (≥2350, <6250) | 91 (36.8) | 156 (63.2) | |
| Total_PovStatus_3 (≥6250) | 33 (45.8) | 39 (54.2) | |
| Total_PovStatus_4 (missing) | 27 (51.9) | 25 (48.1) | |
| EPL_DIABETES | 0.033 | ||
| EPL_DIABETES_1 (≤0.44) | 43 (53.1) | 38 (46.9) | |
| EPL_DIABETES_2 (>0.44) | 141 (40.1) | 211 (58.9) | |
| RPL_EBM_DOM1 | 0.025 | ||
| RPL_EBM_DOM1_1 (<0.24) | 18 (62.1) | 11 (37.9) | |
| RPL_EBM_DOM1_2 (≥0.24, <0.52) | 62 (36.5) | 108 (63.5) | |
| RPL_EBM_DOM1_3 (≥0.52) | 104 (44.4) | 130 (55.6) | |
| EP_BPHIGH | 0.031 | ||
| EP_BPHIGH_1 (<35) | 48 (53.3) | 42 (46.7) | |
| EP_BPHIGH_2 (≥35, <39) | 34 (37.0) | 58 (63.0) | |
| EP_BPHIGH_3 (≥39, <44) | 56 (46.3) | 65 (53.7) | |
| EP_BPHIGH_4 (≥44) | 46 (35.4) | 84 (64.6) | |
| SPL_EBM_THEME1 | 0.022 | ||
| SPL_EBM_THEME1_1 (<1.26) | 19 (57.6) | 14 (42.4) | |
| SPL_EBM_THEME1_2 (≥1.26, <1.9) | 56 (35.0) | 104 (65.0) | |
| SPL_EBM_THEME1_3 (≥1.9) | 109 (45.4) | 131 (54.6) | |
| SPL_SVM_DOM3 | 0.019 | ||
| SPL_SVM_DOM3_1 (<1.75) | 53 (51.5) | 50 (48.5) | |
| SPL_SVM_DOM3_2 (≥1.75, <2) | 38 (38.0) | 62 (62.0) | |
| SPL_SVM_DOM3_3 (≥2, <2.25) | 49 (49.0) | 51 (51.0) | |
| SPL_SVM_DOM3_4 (≥2.25) | 44 (33.8) | 86 (66.2) | |
| EP_UNEMP | 0.018 | ||
| EP_UNEMP_1 (≤7) | 93 (37.5) | 155 (62.5) | |
| EP_UNEMP_2 (>7) | 90 (48.9) | 94 (51.1) | |
| FormTot | 0.009 | ||
| FormTot_1 (≤2500) | 123 (45.4) | 148 (54.6) | |
| FormTot_2 (>2500) | 34 (30.6) | 77 (69.4) | |
| FormTot_3 (missing) | 27 (51.9) | 25 (48.1) | |
| SPL_EBM_THEME4 | 0.006 | ||
| SPL_EBM_THEME4_1 (<1) | 83 (48.0) | 90 (52.0) | |
| SPL_EBM_THEME4_2 (≥1, <1.6) | 77 (44.3) | 97 (55.7) | |
| SPL_EBM_THEME4_3 (≥1.6) | 24 (27.9) | 62 (72.1) | |
| RPL_EBM_DOM4 | 0.006 | ||
| RPL_EBM_DOM4_1 (<0.48) | 82 (47.7) | 90 (52.3) | |
| RPL_EBM_DOM4_2 (≥0.48, <0.63) | 22 (38.6) | 35 (61.4) | |
| RPL_EBM_DOM4_3 (≥0.63, <0.81) | 56 (48.3) | 60 (51.7) | |
| RPL_EBM_DOM4_4 (≥0.81) | 24 (27.3) | 64 (72.7) | |
| EPL_DISABL | 0.005 | ||
| EPL_DISABL_1 (≤0.54) | 74 (52.1) | 68 (47.9) | |
| EPL_DISABL_2 (>0.54) | 110 (37.8) | 181 (62.2) | |
| EP_DISABL | 0.004 | ||
| EP_DISABL_1 (≤13) | 71 (53.0) | 64 (47.4) | |
| EP_DISABL_2 (>13) | 113 (38.0) | 185 (62.1) | |
| RPL_SVM_DOM3 | 0.004 | ||
| RPL_SVM_DOM3_1 (<0.35) | 65 (50.0) | 65 (50.0) | |
| RPL_SVM_DOM3_2 (≥0.35, <0.51) | 29 (36.3) | 51 (63.8) | |
| RPL_SVM_DOM3_3 (≥0.51, <0.74) | 53 (50.5) | 52 (49.5) | |
| RPL_SVM_DOM3_4 (≥0.74) | 37 (31.4) | 81 (68.6) | |
| EPL_BPHIGH | 0.003 | ||
| EPL_BPHIGH_1 (≤0.65) | 46 (57.5) | 34 (42.5) | |
| EPL_BPHIGH_2 (>0.65) | 138 (39.1) | 215 (60.9) |
| KEGG Pathway ID | Description Pathway | p-Adjust * | Gene Counts |
|---|---|---|---|
| has04010 | MAPK signaling | 1.195 × 10−18 | 278 |
| has04110 | Cell cycle | 4.524 × 10−17 | 154 |
| has04310 | Wnt signaling | 9.300 × 10−14 | 164 |
| has04151 | PI3K-Akt signaling | 6.328 × 10−12 | 314 |
| has05224 | Breast cancer | 2.839 × 10−10 | 137 |
| has04210 | Apoptosis | 9.172 × 10−10 | 127 |
| has04350 | TGF-beta signaling | 3.307 × 10−8 | 102 |
| has04115 | p53 signaling | 5.913 × 10−8 | 72 |
| has04340 | Hedgehog signaling | 1.605 × 10−6 | 54 |
| has04330 | Notch signaling pathway | 2.071 × 10−6 | 59 |
| has04215 | Apoptosis—multiple species | 1.536 × 10−5 | 32 |
| has04915 | Estrogen signaling pathway | 1.758 × 10−5 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bam, A.; Hu, Y.; Wu, X.; Luo, M.; Rizvi, N.; Del Valle, L.; Zea, A.H.; Hossain, F.; Danos, D.M.; Zabaleta, J.; et al. Differential miRNA Expressions Linking Environmental Risk Factors to Triple-Negative Breast Cancer Stages at Diagnosis. Cancers 2025, 17, 2618. https://doi.org/10.3390/cancers17162618
Bam A, Hu Y, Wu X, Luo M, Rizvi N, Del Valle L, Zea AH, Hossain F, Danos DM, Zabaleta J, et al. Differential miRNA Expressions Linking Environmental Risk Factors to Triple-Negative Breast Cancer Stages at Diagnosis. Cancers. 2025; 17(16):2618. https://doi.org/10.3390/cancers17162618
Chicago/Turabian StyleBam, Amjila, Yawen Hu, Xiaocheng Wu, Meng Luo, Nubaira Rizvi, Luis Del Valle, Arnold H. Zea, Fokhrul Hossain, Denise Moore Danos, Jovanny Zabaleta, and et al. 2025. "Differential miRNA Expressions Linking Environmental Risk Factors to Triple-Negative Breast Cancer Stages at Diagnosis" Cancers 17, no. 16: 2618. https://doi.org/10.3390/cancers17162618
APA StyleBam, A., Hu, Y., Wu, X., Luo, M., Rizvi, N., Del Valle, L., Zea, A. H., Hossain, F., Danos, D. M., Zabaleta, J., Ochoa, A., Miele, L., Trapido, E., & Yu, Q. (2025). Differential miRNA Expressions Linking Environmental Risk Factors to Triple-Negative Breast Cancer Stages at Diagnosis. Cancers, 17(16), 2618. https://doi.org/10.3390/cancers17162618







