The Role of mHealth Applications in Uro-Oncology: A Systematic Review and Future Directions
Simple Summary
Abstract
1. Introduction
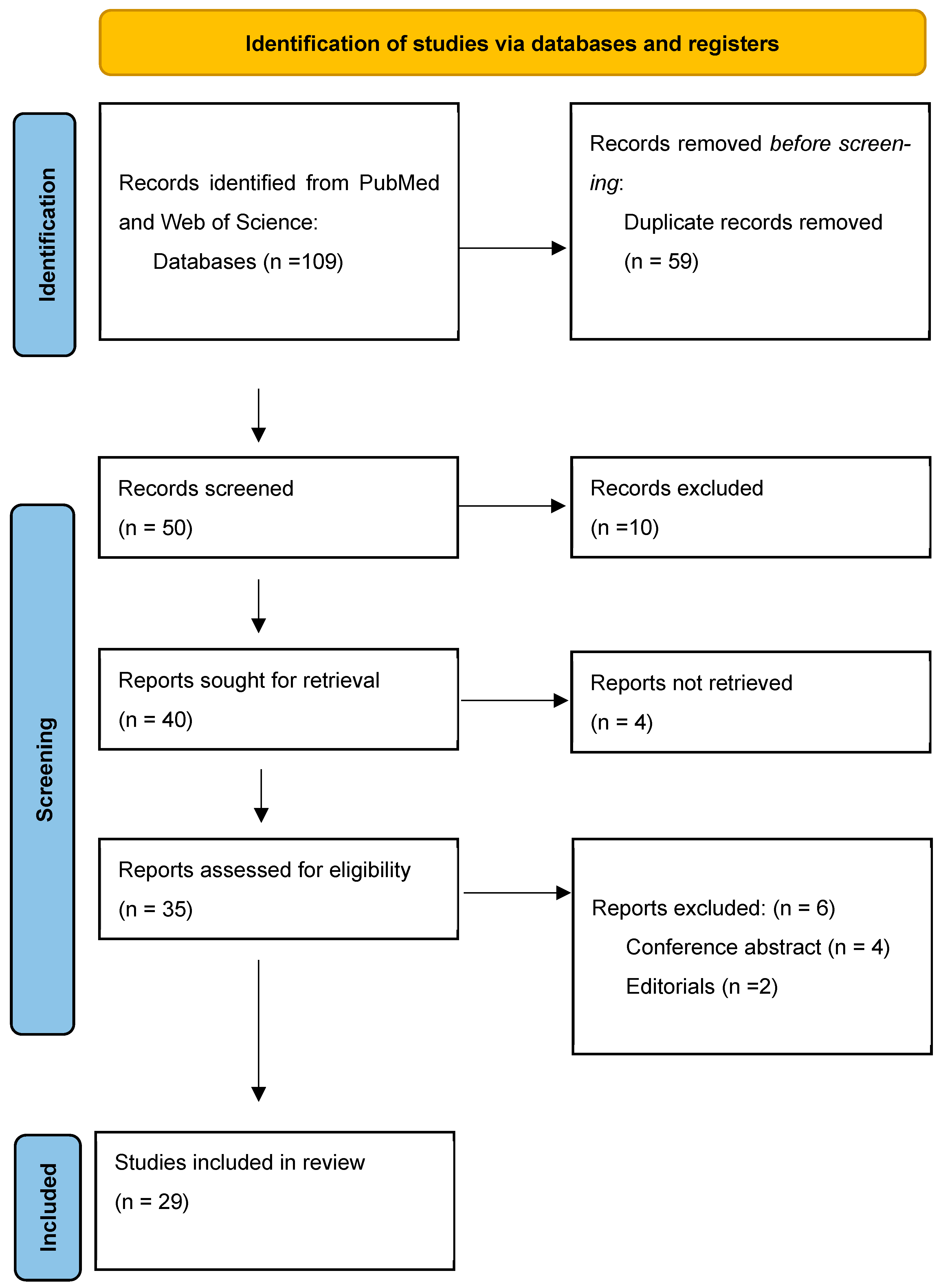
2. Material and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Selection Process
2.5. Data Extraction
- -
- Study characteristics: year of publication, study design, and sample size.
- -
- Participant characteristics: type of cancer and stage (localized, locally advanced, metastatic) and age range.
- -
- Intervention details: type of mHealth application, features, and functionality.
- -
- Comparators: description of control or comparison interventions (if applicable).
- -
- Key findings related to treatment outcomes, symptom management, adherence, quality of life, and patient satisfaction.
2.6. Data Synthesis
- -
- Types of mHealth applications used in uro-oncology.
- -
- Reported benefits in terms of patient outcomes and care quality.
- -
- Barriers to the effective implementation of mHealth technologies.
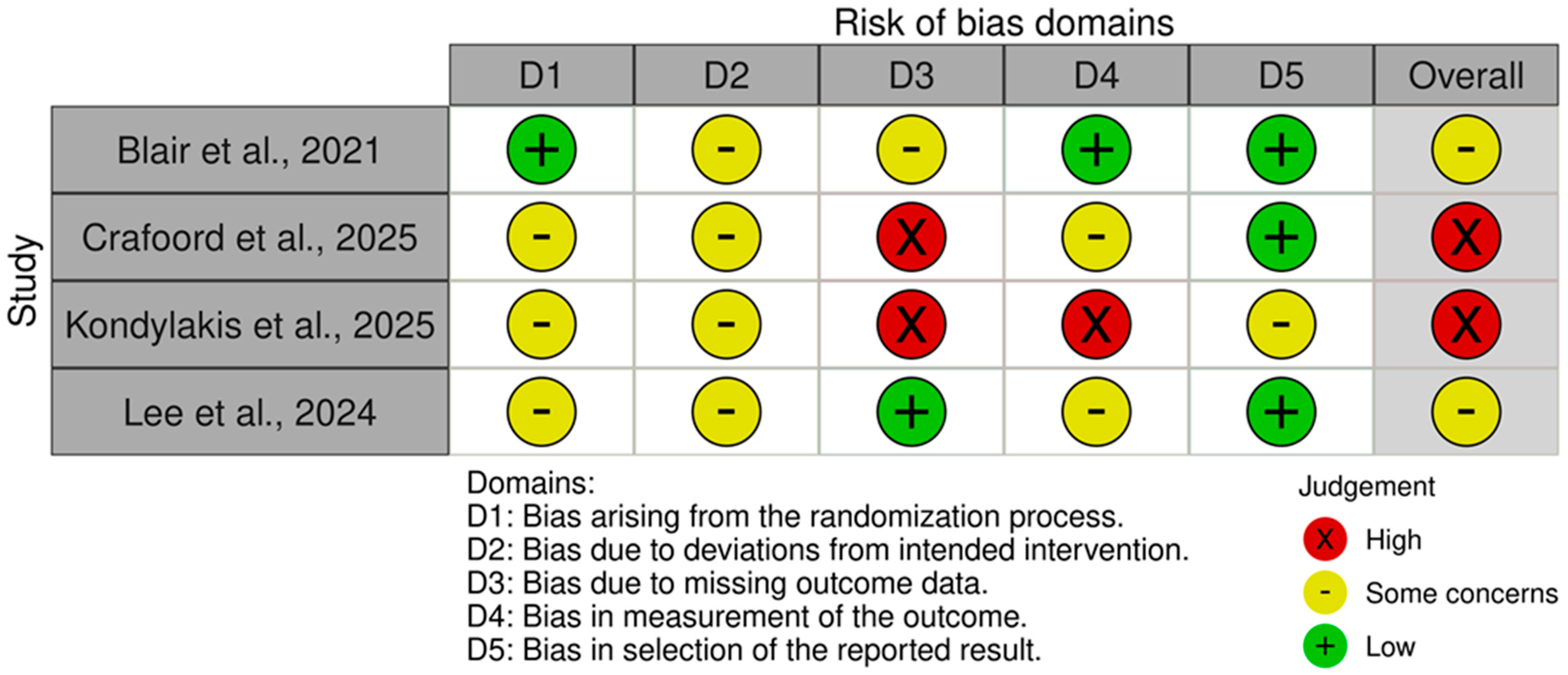
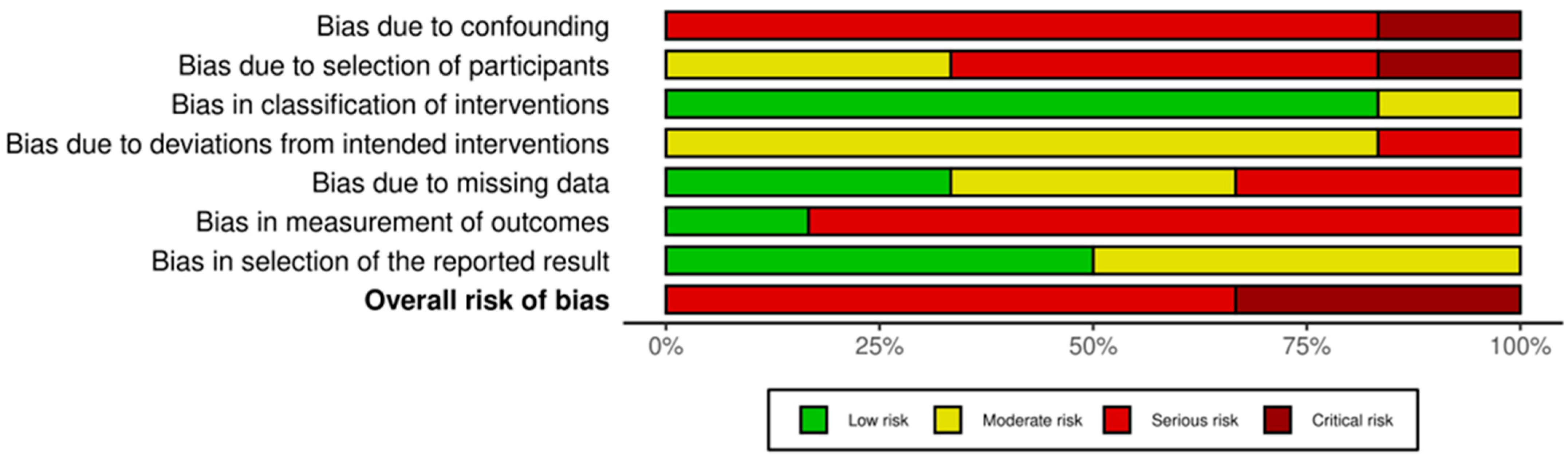
2.7. Risk of Bias Assessment
3. Results
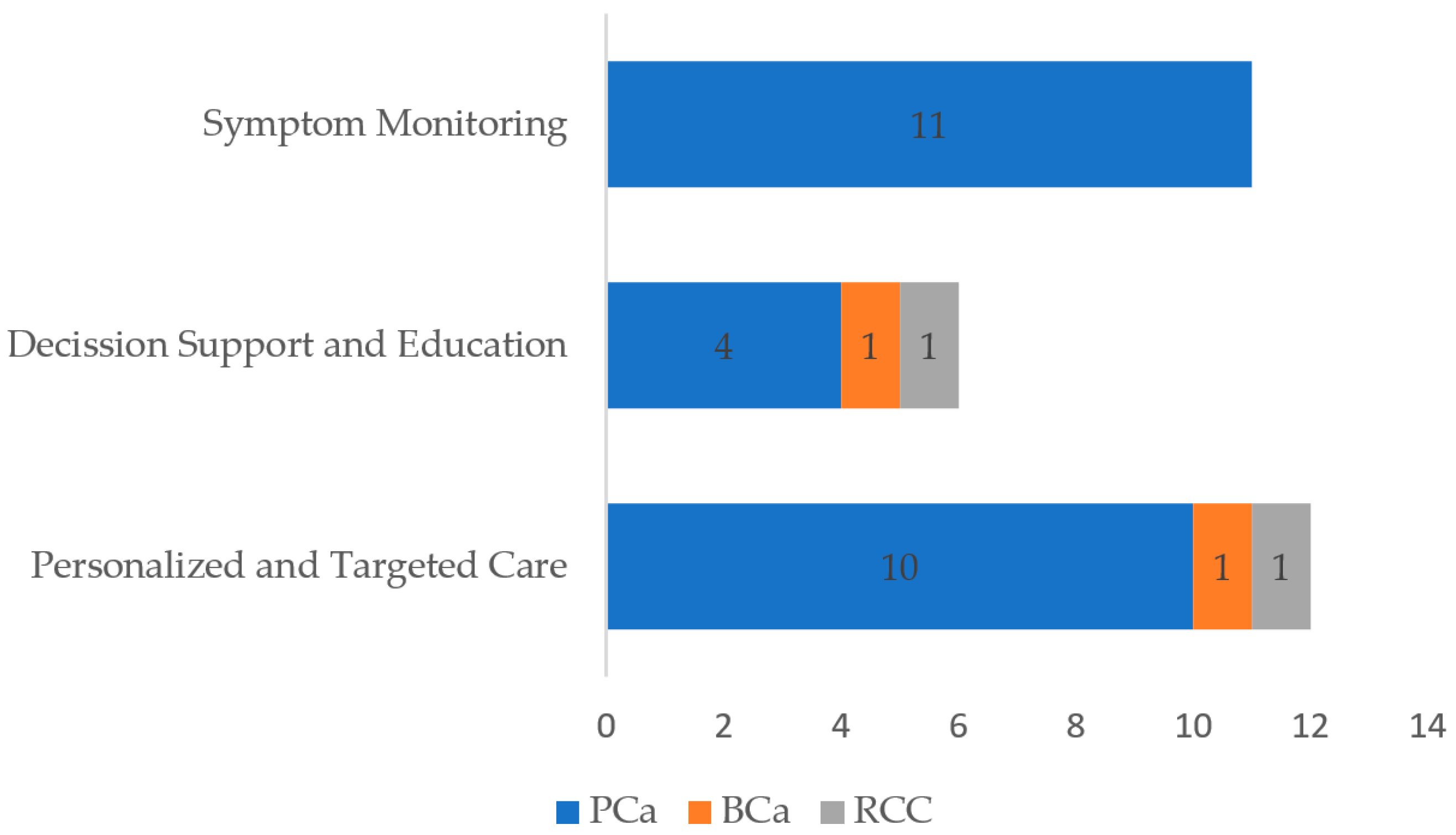
3.1. Role of mHealth Interventions
3.1.1. Symptom Monitoring and Management
| Study and Year | Study Design | Sample Size | Age Range | Urological Malignancy | Study Intervention | Comparator | Key Findings |
|---|---|---|---|---|---|---|---|
| Amor-García et al., 2020 [10] | Cross-sectional descriptive observational study | 46 smartphone applications | N/A (mHealth app study) | Genitourinary malignancies (prostate, testicular, bladder, kidney, cervical, ovarian, uterine, endometrial, vulva) | Evaluation of mobile health (mHealth) application quality for patients | N/A (descriptive study) | Avg. MARS score 2.98; most apps informative; “Engagement” domain scored lowest. |
| Belarmino et al., 2018 [22] | Qualitative usability study | Not specified | N/S | Prostate cancer (post-robot-assisted radical prostatectomy) | Mobile health application to monitor recovery and patient-reported outcomes | N/A (usability study) | App was feasible/usable; high questionnaire response/compliance rates for post-op activities. |
| Bergerot et al., 2025 [23] | Longitudinal pilot clinical trial | 50 patients | Median 59 years (range: 32–88) | Metastatic renal cell carcinoma (mRCC) | Mindfulness application (CARINAE) | No control group specified for causality | App feasible/acceptable; improvements in emotional symptoms, fatigue, mindfulness, and HRQoL. |
| Blair et al., 2021 [24] | Pilot randomized controlled trial (RCT), mixed-methods | 44 participants in intervention arm | Mean 63 years (SD 11; range: 40–85) | Prostate cancer (41%), also breast and colorectal cancer | App-based brisk walking intervention | Control group with usual care | Intervention feasible/acceptable; health coaching group showed increase in daily steps/moderate-intensity physical activity. |
| Camacho-Rivera et al., 2019 [25] | Cross-sectional observational study | 473 residents | Over 18 years old | Prostate and colorectal cancer screening | Evaluation of smartphone and health app use | N/A (descriptive study) | No noteworthy differences in smartphone/health app access across age groups; education predicted health app access. |
| Carhuapoma et al., 2021 [26] | Pre-test/post-test design, with qualitative and quantitative methods | Target of 158 patient-decision partner dyads | N/S | Prostate cancer | Multicomponent mHealth decision aid intervention to facilitate partner involvement | Enhanced usual care (EUC) group | Protocol for RCT evaluating mHealth decision aid on HRQL-PSY, decision conflict, regret. |
| Crafoord et al., 2020 [21] | Mixed-methods study | 75 prostate cancer patients, 74 breast cancer patients | Prostate cancer: median 72 years (range 44–81) | Prostate cancer (also breast cancer) | Interaktor interactive app for symptom self-management | N/A (descriptive study of use and perception) | High adherence to daily symptom reporting; app perceived as easy to use and supportive for self-care. |
| Crafoord et al., 2025 [27] | Two parallel, open-label randomized controlled trials (RCTs) | 75 intervention group and 75 control group for prostate cancer (P-RCT) | N/S | Prostate cancer (P-RCT), also breast cancer (B-RCT) | Interaktor interactive app for patient-reported outcomes (ePRO) and interactive support | Control group with standard care | Interaktor reduced QALYs loss at low cost; ePROs associated with lower symptom burden; nurses reported no increased workload. |
| Hälleberg Nyman et al., 2017 [28] | Qualitative descriptive study, part of an experimental study | 28 patients (17 used the app) | 57 to 77 years | Prostate cancer | Interaktor app for daily symptom reporting and self-care | Historical control group with standard care | App users experienced more mutual care participation, felt more active, and had continuous health service contact. |
| Jin et al., 2024 [29] | Non-randomized pilot feasibility and acceptability study | 18 patients | N/S | Prostate cancer (undergoing radiotherapy) | Smart water bottle and app (HidrateSpark 3) to improve bladder filling adherence | Retrospectively matched controls (compliance data in intervention arm only) | Bladder filling compliance met; high patient engagement (83% used >50% treatments). |
| Kelmendi et al., 2024 [30] | Single-arm, descriptive feasibility study (qualitative and quantitative) | 11 patients | Range 57 to 75 years (mean 66, median 68) | Prostate cancer | Complex intervention with ePROs, self-management advice in an app, and nurse support in primary care | N/A (single-arm study) | Nurse support + app intervention feasible in prostate cancer patients; valued personalized support; high app symptom reporting adherence. |
| Kennedy et al., 2025 [31] | Mixed-methods study with embedded design, part of a pilot RCT | 44 participants in intervention arm | Mean 63 years (SD 11; range 40–85) | Prostate cancer (41%), also breast and colorectal cancer | App-based brisk walking intervention (APROACH) | Control arm with usual care | Behavioral support intervention showed high fidelity in BCT delivery; app useful for habit formation, but use decreased over time. |
| Kondylakis et al., 2025 [32] | Feasibility randomized controlled trial (RCT) | 50 patients (23 intervention, 27 control), 39 completed trial | Intervention group: mean 45.38 (SD 26.2, range 12–91 years); control group: mean 56.66 (SD 27, range 12–91 years) | Prostate cancer (three in intervention, two in control), bladder cancer (two in intervention, four in control); also includes cardiac and orthopedic surgeries | CARINAE digital solution for perioperative stress and anxiety reduction | Control group with standard care | CARINAE feasible for stress/anxiety management; trend for lower stress; difference in HADS depression in one hospital; provider involvement crucial. |
| La Rocca et al., 2025 [33] | Cross-sectional descriptive observational study | 10 mobile health applications (MHAs) | N/A (app study) | Bladder cancer | Review and evaluation of MHAs for bladder cancer | N/A (descriptive study) | MHAs for BCa showed suboptimal quality (low MARS scores); less than one-third adhered to EAU guidelines; 100% covered BCa definition/treatment. |
| Lai et al., 2024 [12] | Retrospective genetic analysis of CAFs-RGs and predictive nomogram construction | 554 samples (386 PCa, 52 normal adjacent) plus data from 199 and 248 PCa patients from public databases | N/S | Prostate cancer | Development of a nomogram to predict clinical outcome and radiotherapy prognosis | N/A (predictive model development study) | Identified CAFsRGs predicting PCa prognosis/radiotherapy response; developed high-accuracy nomogram/online app for BRFS. |
| Langius-Eklöf et al., 2017 [16] | Prospective, randomized, controlled trial | For prostate cancer, related study had 66 intervention and 64 control | Over 18 years old | Prostate cancer | Interaktor interactive application for daily symptom reporting and self-care | Control group with standard care | Protocol for RCT evaluating Interaktor’s effect on symptom burden, QoL, health literacy, disease progression, costs. |
| Langius-Eklöf et al., 2017 [34] | Description of logged data and interviews, compared with a historical control group | 66 patients; 53 interviewed | Mean age 69 years | Prostate cancer | Interaktor interactive application for symptom management during radiotherapy | Historical control group | High adherence (87%); app easy to use, provided security; facilitated self-management/person-centered care. |
| Lee et al., 2024 [19] | Randomized, single-blind, waiting-list controlled trial | 48 patients (24 experimental, 24 control); 46 included in final analysis | Mean age 68.83 (SD 7.09) years | Prostate cancer | 4-week nurse-led mobile program on exercise and diet | Waiting-list control group with usual care | Program improved MetS components (glucose, abdominal circumference), body composition; significant effect on irritative/obstructive urinary HRQoL. |
| Martini et al., 2024 [17] | Prospective non-randomized study | 122 patients (62 in optimized pathway, 60 in standard care) | 64–65 years | Prostate cancer (post-radical prostatectomy) | Optimized perioperative program with a personalized mobile application for preparation and recovery | Standard of care (SOC) group | App-based program improved 6-week continence rate (92% vs. 75%, p = 0.01); fewer grade ≥ 2 complications; increased same-day discharge; high usability/satisfaction. |
| Mohseni et al., 2023 [8] | Two-phase app development and usability evaluation | Phase 1: 15 specialists; Phase 2: 21 patients, 10 specialists | Specialists: mean 44.90 ± 3.51 years; patients: not specified. | Prostate cancer | Development of a mobile application for electronic patient-reported outcomes | N/A (development and usability evaluation study) | App for ePROs/side effect reporting developed; high satisfaction among patients/specialists; app functions deemed necessary. |
| Nabi et al., 2020 [35] | Qualitative usability study with focus groups and in-depth interviews | Five patients, five physicians | Patients: mean 62 years (range 45–75) | Prostate cancer | Evaluation of an mHealth mobile application (name not specified) | N/A (usability study) | Patients appreciated holistic care; registration difficulties (60%); physicians underestimated patient tech ability; patients comfortable documenting exercise/diet. |
| Obro et al., 2022 [36] | Qualitative usability study, with individual and group interviews | Four urological nurses and one physician; patient number not specified | Nurses: between 30 and 52 years; physician: not specified | Low risk localized prostate cancer | 19-week mHealth coaching program | N/A (qualitative usability study) | Nurses found coaching increased autonomy/attentiveness; lack of mHealth competencies reduced motivation. |
| Peng et al., 2024 [20] | Retrospective study, with assignment by clinical eligibility | 112 patients (56 per group) | Older men, exact range not specified | Prostate cancer (post-radical prostatectomy) | Mobile internet management for continuous care | Control group with standard care | Mobile internet management improved patient knowledge, emotional well-being, and self-care abilities. |
| Pereira-Azevedo et al., 2018 [11] | Review article | N/A (review) | N/A (review) | Prostate cancer | Discussion on eHealth and mHealth in detection and active surveillance (e.g., risk calculators, monitoring apps) | N/A (review) | eHealth market growing but underutilized; RPCRC, PRIAS, Follow MyPSA are value-added tools. |
| Roman Souza et al., 2024 [37] | Pilot clinical trial | 20 patients | Range 49 to 82 years, mean 66 years (SD 11) | Stage IV renal cell carcinoma (RCC) | Mobile health (mHealth) application for education and symptom management (educational modules and algorithm) | N/A (feasibility and acceptability study) | App met acceptability/feasibility criteria; knowledge test score changes after educational modules. |
| Sundberg et al., 2015 [38] | Feasibility study | Nine patients | Mean age 69 years | Prostate cancer | Interactive ICT platform (mobile application) for symptom assessment and management | N/A (feasibility study) | App feasible/acceptable; relevant questionnaire/self-care advice; alerts led to nurse contact/support; facilitated patient participation/communication. |
| Sundberg et al., 2017 [18] | Non-randomized controlled study (historically controlled) | 130 patients (66 intervention, 64 control) | Mean 69 years (range 52–82) | Prostate cancer | Interaktor interactive application for early detection, reporting, and symptom management | Control group with standard care | Intervention group had lower fatigue/nausea; reduced emotional functioning, insomnia, urinary symptoms; app facilitated real-time communication. |
| Sundberg et al., 2021 [39] | Quasi-experimental design, with historical control group | 130 patients (66 intervention group, 64 control group) | Targeted population “middle-aged or older men” | Prostate cancer | Interaktor application for symptom reporting and self-care support | Control group with standard care | Intervention group showed improvements in advanced health literacy skills. |
| Tran et al., 2020 [40] | Single-arm pilot feasibility trial | 29 patients analyzed (out of 30 consented) | Median 55 years | Prostate cancer | Digital health application (Strength Through Insight) for collecting ePROs | N/A (single-arm study) | App feasible (86% satisfactory completion); patients reported ease of use, preference for text messages, increased symptom awareness. |
3.1.2. Decision-Support and Educational Tools
3.1.3. Personalized and Targeted Care
3.2. Effectiveness of mHealth Interventions
3.2.1. Symptom Control and Management
3.2.2. Patient Engagement and Adherence to Treatment
3.2.3. Impact on Quality of Life
3.3. Feasibility and Challenges of mHealth Tools
3.4. Risk of Bias of Included Studies
4. Discussion
5. Recommendations and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICT | Information and Communication Technologies |
| WHO | World Health Organization |
| eHealth | Electronic Health |
| mHealth | Mobile Health |
| Apps | Applications |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| WoS | Web of Science |
| MeSH | Medical Subject Heading |
| RCT | Randomized Controlled Trial |
| RoB-2 | Risk of Bias Tool 2 |
| NRSIs | Non-Randomized Studies of Intervention |
| ROBINS-I | Risk of Bias in Non-Randomized Studies of Intervention |
| MARS | Mobile Application Rating Scale |
| mRCC | Metastatic Renal Cell Carcinoma |
| HRQoL | Health-related Quality of Life |
| EUC | Enhanced Usual Care |
| HRQL-PSY | Psychosocial Health-related Quality of Life |
| ePRO | Electronic Patient Reported Outcomes |
| QALYs | Quality-adjusted Life Years |
| SD | Standard Deviation |
| BCT | Behavior Change Technique |
| HADS | Hospital Anxiety and Depression Scale |
| MHA | Mobile Health Application |
| BCa | Bladder Cancer |
| EAU | European Association of Urology |
| CAFs-RGs | Cancer-associated Fibroblast-Related Genes |
| PCa | Prostate Cancer |
| BRFS | Biochemical Recurrence-free Survival |
| MetS | Metabolic Syndrome |
| SOC | Standard of Care |
| RPCRC | Rotterdam Prostate Cancer Risk Calculator |
| PRIAS | Prostate Cancer Research International Active Surveillance |
| RCC | Renal Cell Carcinoma |
| ADT | Androgen Deprivation Therapy |
| FBS | Fasting Blood Glucose |
| AC | Abdominal Circumference |
| RP | Radical Prostatectomy |
References
- World Health Organization. Global Observatory for eHealth. Available online: https://www.who.int/observatories/global-observatory-for-ehealth (accessed on 26 May 2025).
- Oh, H.; Rizo, C.; Enkin, M.; Jadad, A.; Powell, J.; Pagliari, C. What Is eHealth (3): A Systematic Review of Published Definitions. J. Med. Internet Res. 2005, 7, e110. [Google Scholar] [CrossRef]
- Nasi, G.; Cucciniello, M.; Guerrazzi, C. The Role of Mobile Technologies in Health Care Processes: The Case of Cancer Supportive Care. J. Med. Internet Res. 2015, 17, e26. [Google Scholar] [CrossRef]
- Kamel Boulos, M.N.; Brewer, A.C.; Karimkhani, C.; Buller, D.B.; Dellavalle, R.P. Mobile Medical and Health Apps: State of the Art, Concerns, Regulatory Control and Certification. Online J. Public Health Inform. 2014, 5, 229. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Katz, A. Quality of Life for Men With Prostate Cancer. Cancer Nurs. 2007, 30, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pachman, D.R.; Barton, D.L.; Swetz, K.M.; Loprinzi, C.L. Troublesome Symptoms in Cancer Survivors: Fatigue, Insomnia, Neuropathy, and Pain. J. Clin. Oncol. 2012, 30, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Ayatollahi, H.; Arefpour, A.M. Electronic Patient-Reported Outcome (ePRO) Application for Patients with Prostate Cancer. PLoS ONE 2023, 18, e0289974. [Google Scholar] [CrossRef]
- Ernsting, C.; Dombrowski, S.U.; Oedekoven, M.; O´Sullivan, J.L.; Kanzler, M.; Kuhlmey, A.; Gellert, P. Using Smartphones and Health Apps to Change and Manage Health Behaviors: A Population-Based Survey. J. Med. Internet Res. 2017, 19, e101. [Google Scholar] [CrossRef] [PubMed]
- Amor-García, M.Á.; Collado-Borrell, R.; Escudero-Vilaplana, V.; Melgarejo-Ortuño, A.; Herranz-Alonso, A.; Arranz Arija, J.Á.; Sanjurjo-Sáez, M. Assessing Apps for Patients with Genitourinary Tumors Using the Mobile Application Rating Scale (MARS): Systematic Search in App Stores and Content Analysis. JMIR mHealth uHealth 2020, 8, e17609. [Google Scholar] [CrossRef]
- Pereira-Azevedo, N.M.; Venderbos, L.D.F. eHealth and mHealth in Prostate Cancer Detection and Active Surveillance. Transl. Androl. Urol. 2018, 7, 170–181. [Google Scholar] [CrossRef]
- Lai, C.; Wu, Z.; Li, Z.; Huang, X.; Hu, Z.; Yu, H.; Yuan, Z.; Shi, J.; Hu, J.; Mulati, Y.; et al. Single-Cell Analysis Extracted CAFs-Related Genes to Established Online App to Predict Clinical Outcome and Radiotherapy Prognosis of Prostate Cancer. Clin. Transl. Oncol. 2024, 26, 1240–1255. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Langius-Eklöf, A.; Christiansen, M.; Lindström, V.; Blomberg, K.; Hälleberg Nyman, M.; Wengström, Y.; Sundberg, K. Adherence to Report and Patient Perception of an Interactive App for Managing Symptoms During Radiotherapy for Prostate Cancer: Descriptive Study of Logged and Interview Data. JMIR Cancer 2017, 3, e18. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Kesch, C.; Touzani, A.; Calleris, G.; Buhas, B.; Abou-Zahr, R.; Rahota, R.-G.; Pradère, B.; Tollon, C.; Beauval, J.-B.; et al. Personalized Mobile App–Based Program for Preparation and Recovery After Radical Prostatectomy: Initial Evidence for Improved Outcomes From a Prospective Nonrandomized Study. J. Med. Internet Res. 2024, 26, e55429. [Google Scholar] [CrossRef]
- Sundberg, K.; Wengström, Y.; Blomberg, K.; Hälleberg-Nyman, M.; Frank, C.; Langius-Eklöf, A. Early Detection and Management of Symptoms Using an Interactive Smartphone Application (Interaktor) during Radiotherapy for Prostate Cancer. Support. Care Cancer 2017, 25, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, J.; Oh, E.G.; Lee, J.; Park, C.; Choi, Y.D. Effectiveness of a Nurse-Led Mobile-Based Health Coaching Program for Patients With Prostate Cancer at High Risk of Metabolic Syndrome: Randomized Waitlist Controlled Trial. JMIR mHealth uHealth 2024, 12, e47102. [Google Scholar] [CrossRef]
- Peng, S.; Wei, Y.; Ye, L.; Jin, X.; Huang, L. Application of Mobile Internet Management in the Continuing Care of Patients after Radical Prostatectomy. Sci. Rep. 2024, 14, 31520. [Google Scholar] [CrossRef]
- Crafoord, M.-T.; Fjell, M.; Sundberg, K.; Nilsson, M.; Langius-Eklöf, A. Engagement in an Interactive App for Symptom Self-Management during Treatment in Patients With Breast or Prostate Cancer: Mixed Methods Study. J. Med. Internet Res. 2020, 22, e17058. [Google Scholar] [CrossRef]
- Belarmino, A.; Walsh, R.; Alshak, M.; Patel, N.; Wu, R.; Hu, J.C. Feasibility of a Mobile Health Application To Monitor Recovery and Patient-Reported Outcomes after Robot-Assisted Radical Prostatectomy. Eur. Urol. Oncol. 2019, 2, 425–428. [Google Scholar] [CrossRef]
- Bergerot, C.D.; Bergerot, P.G.; Philip, E.J.; Malhotra, J.; Castro, D.V.; Govindarajan, A.; Fuzita, W.H.; França, M.V.D.S.; Azeredo, A.C.D.; Anjos, G.M.D.; et al. Feasibility and Acceptability of a Mindfulness App-Based Intervention among Patients with Metastatic Renal Cell Carcinoma: A Multinational Study. Oncol. 2025, 30, oyae309. [Google Scholar] [CrossRef]
- Blair, C.K.; Harding, E.; Wiggins, C.; Kang, H.; Schwartz, M.; Tarnower, A.; Du, R.; Kinney, A.Y. A Home-Based Mobile Health Intervention to Replace Sedentary Time With Light Physical Activity in Older Cancer Survivors: Randomized Controlled Pilot Trial. JMIR Cancer 2021, 7, e18819. [Google Scholar] [CrossRef]
- Camacho-Rivera, M.; Rice, S.L.; Oh, S.; Paris, M.; Akpara, E.; Molina, J.; Obadina, M.; Mcmillan, S.; Aracena, J.L.M.; Morency, J.; et al. Cancer Health Impact Program (CHIP): Identifying Social and Demographic Associations of mHealth Access and Cancer Screening Behaviors Among Brooklyn, New York, Residents. CANCER Epidemiol. Biomark. Prev. 2019, 28, 478–485. [Google Scholar] [CrossRef]
- Carhuapoma, L.R.; Thayer, W.M.; Elmore, C.E.; Gildersleeve, J.; Singh, T.; Shaukat, F.; Uveges, M.K.; Gray, T.; Chu, C.; Song, D.; et al. Employing a Mobile Health Decision Aid to Improve Decision-Making for Patients with Advanced Prostate Cancer and Their Decision Partners/Proxies: The CHAMPION Randomized Controlled Trial Study Design. Trials 2021, 22, 631. [Google Scholar] [CrossRef]
- Crafoord, M.-T.; Ekstrand, J.; Sundberg, K.; Nilsson, M.I.; Fjell, M.; Langius-Eklöf, A. Mobile Electronic Patient-Reported Outcomes and Interactive Support During Breast and Prostate Cancer Treatment: Health Economic Evaluation From Two Randomized Controlled Trials. JMIR Cancer 2025, 11, e53539. [Google Scholar] [CrossRef] [PubMed]
- Hälleberg Nyman, M.; Frank, C.; Langius-Eklöf, A.; Blomberg, K.; Sundberg, K.; Wengström, Y. Patients’ Perspective on Participation in Care With or Without the Support of a Smartphone App During Radiotherapy for Prostate Cancer: Qualitative Study. JMIR mHealth uHealth 2017, 5, e107. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Montoya, C.; Rich, B.J.; Taswell, C.S.; Noy, M.; Kwon, D.; Spieler, B.; Mahal, B.; Abramowitz, M.; Yechieli, R.; et al. A Smart Water Bottle and Companion App (HidrateSpark 3) to Improve Bladder-Filling Compliance in Patients With Prostate Cancer Receiving Radiotherapy: Nonrandomized Trial of Feasibility and Acceptability. JMIR Cancer 2024, 10, e51061. [Google Scholar] [CrossRef] [PubMed]
- Kelmendi, N.; Langius-Eklöf, A.; Taloyan, M.; Sundberg, K.; Craftman, Å.; Nilsson, M. A Digital and Nurse-Led Support Intervention, First Year after Prostate Cancer Treatment: A Single-Arm Feasibility Study in a Swedish Primary Care Setting. BMC Prim. Care 2024, 25, 409. [Google Scholar] [CrossRef]
- Kennedy, F.; Smith, S.; Beeken, R.J.; Buck, C.; Williams, S.; Martin, C.; Lally, P.; Fisher, A. An App-Based Intervention With Behavioral Support to Promote Brisk Walking in People Diagnosed With Breast, Prostate, or Colorectal Cancer (APPROACH): Process Evaluation Study. JMIR Cancer 2025, 11, e64747. [Google Scholar] [CrossRef]
- Kondylakis, H.; Giglioli, I.A.C.; Katehakis, D.; Aldemir, H.; Zikas, P.; Papagiannakis, G.; Hors-Fraile, S.; González-Sanz, P.L.; Apostolakis, K.; Stephanidis, C.; et al. Stress Reduction in Perioperative Care: Feasibility Randomized Controlled Trial. J. Med. Internet Res. 2025, 27, e54049. [Google Scholar] [CrossRef]
- La Rocca, R.; Di Mauro, E.; Falcone, A.; Sicignano, E.; Cirillo, L.; Reccia, P.; Romano, L.; Olivetta, M.; Mattiello, G.; Crocetto, F.; et al. Analysis and Adherence to Guidelines of Mobile Health Application for Bladder Cancer, Where Are We? Arch. Ital. Urol. Androl. 2025, 97, 13455. [Google Scholar] [CrossRef]
- Langius-Eklöf, A.; Crafoord, M.-T.; Christiansen, M.; Fjell, M.; Sundberg, K. Effects of an Interactive mHealth Innovation for Early Detection of Patient-Reported Symptom Distress with Focus on Participatory Care: Protocol for a Study Based on Prospective, Randomised, Controlled Trials in Patients with Prostate and Breast Cancer. BMC Cancer 2017, 17, 466. [Google Scholar] [CrossRef]
- Nabi, J.; Cone, E.B.; Vasavada, A.; Sun, M.; Kilbridge, K.L.; Kibel, A.S.; Berry, D.L.; Trinh, Q.-D. Mobile Health App for Prostate Cancer Patients on Androgen Deprivation Therapy: Qualitative Usability Study. JMIR mHealth uHealth 2020, 8, e20224. [Google Scholar] [CrossRef]
- Obro, L.F.; Osther, P.J.S.; Ammentorp, J.; Pihl, G.T.; Krogh, P.G.; Handberg, C. Healthcare Professionals’ Experiences and Perspectives of Facilitating Self-Management Support for Patients with Low-Risk Localized Prostate Cancer via mHealth and Health Coaching. Int. J. Environ. Res. Public Health 2022, 20, 346. [Google Scholar] [CrossRef]
- Roman Souza, G.; Turner, K.; Gullapalli, K.; Paravathaneni, M.; Ionescu, F.; Semaan, A.; DeJesus, A.B.; Trujillo, G.; Le, C.; Kim, Y.; et al. Feasibility of a Smartphone Application for Education and Symptom Management of Patients With Renal Cell Carcinoma on Combined Tyrosine Kinase and Immune Checkpoint Inhibitors. JCO Clin. Cancer Inform. 2024, 8, e2400044. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, K.; Eklöf, A.L.; Blomberg, K.; Isaksson, A.-K.; Wengström, Y. Feasibility of an Interactive ICT-Platform for Early Assessment and Management of Patient-Reported Symptoms during Radiotherapy for Prostate Cancer. Eur. J. Oncol. Nurs. 2015, 19, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, K.; Lindström, V.; Petersson, L.-M.; Langius-Eklöf, A. Supporting Health Literacy Using an Interactive App for Symptom Management during Radiotherapy for Prostate Cancer. Patient Educ. Couns. 2021, 104, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Dicker, A.; Leiby, B.; Gressen, E.; Williams, N.; Jim, H. Utilizing Digital Health to Collect Electronic Patient-Reported Outcomes in Prostate Cancer: Single-Arm Pilot Trial. J. Med. Internet Res. 2020, 22, e12689. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Luque, M.Á.; Rivero-Belenchón, I.; Congregado-Ruiz, C.B.; Escobar-Rodríguez, G.A.; Delgado-Granados, F.J.; Rivas-González, J.A.; Medina-López, R.A. The Role of mHealth Applications in Uro-Oncology: A Systematic Review and Future Directions. Cancers 2025, 17, 2613. https://doi.org/10.3390/cancers17162613
Gómez-Luque MÁ, Rivero-Belenchón I, Congregado-Ruiz CB, Escobar-Rodríguez GA, Delgado-Granados FJ, Rivas-González JA, Medina-López RA. The Role of mHealth Applications in Uro-Oncology: A Systematic Review and Future Directions. Cancers. 2025; 17(16):2613. https://doi.org/10.3390/cancers17162613
Chicago/Turabian StyleGómez-Luque, Miguel Ángel, Inés Rivero-Belenchón, Carmen Belén Congregado-Ruiz, German Antonio Escobar-Rodríguez, Francisco Javier Delgado-Granados, Jose Antonio Rivas-González, and Rafael Antonio Medina-López. 2025. "The Role of mHealth Applications in Uro-Oncology: A Systematic Review and Future Directions" Cancers 17, no. 16: 2613. https://doi.org/10.3390/cancers17162613
APA StyleGómez-Luque, M. Á., Rivero-Belenchón, I., Congregado-Ruiz, C. B., Escobar-Rodríguez, G. A., Delgado-Granados, F. J., Rivas-González, J. A., & Medina-López, R. A. (2025). The Role of mHealth Applications in Uro-Oncology: A Systematic Review and Future Directions. Cancers, 17(16), 2613. https://doi.org/10.3390/cancers17162613







