The Role of Pyk2 Kinase in Glioblastoma Progression and Therapeutic Targeting
Simple Summary
Abstract
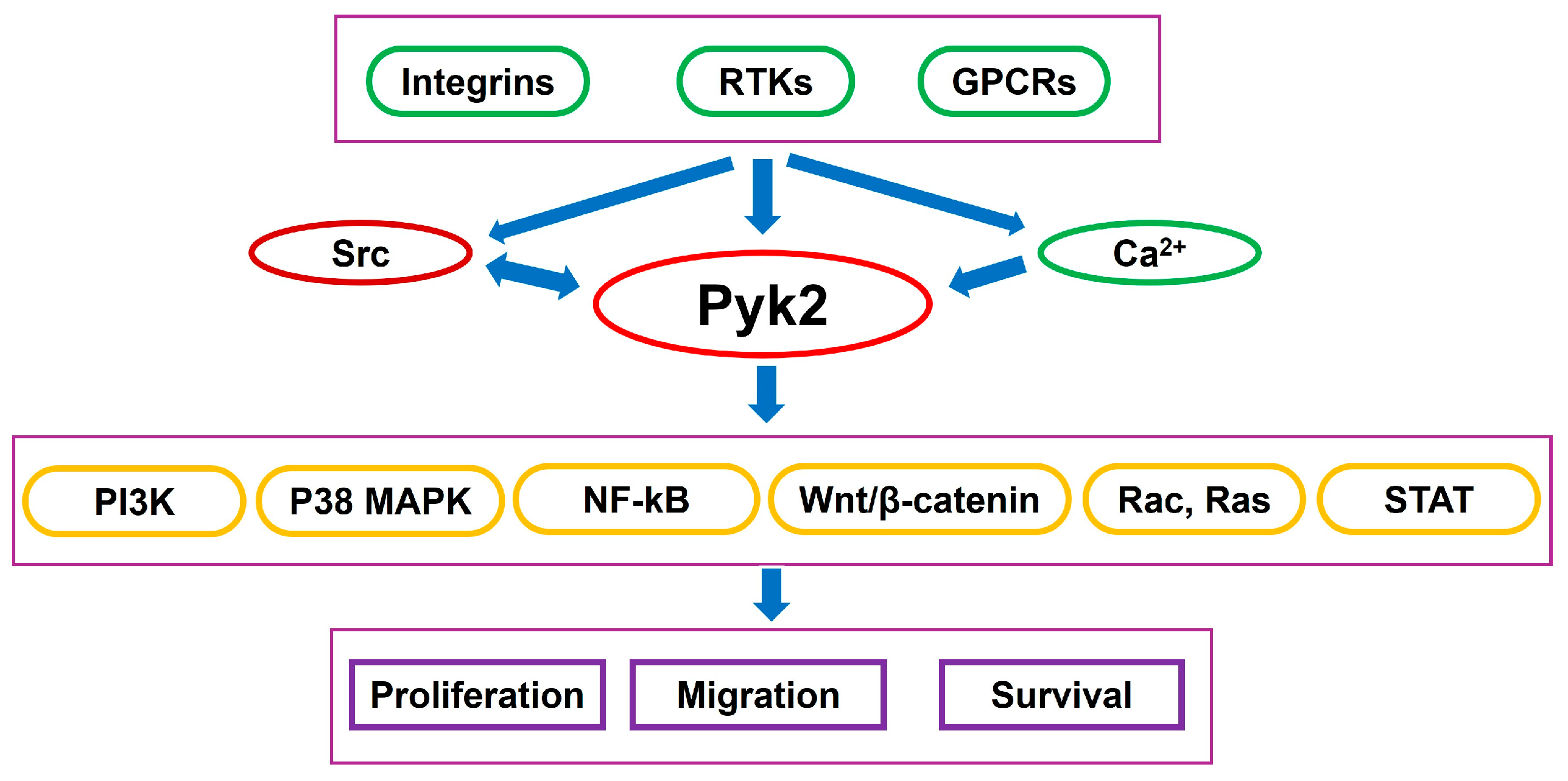
1. Introduction
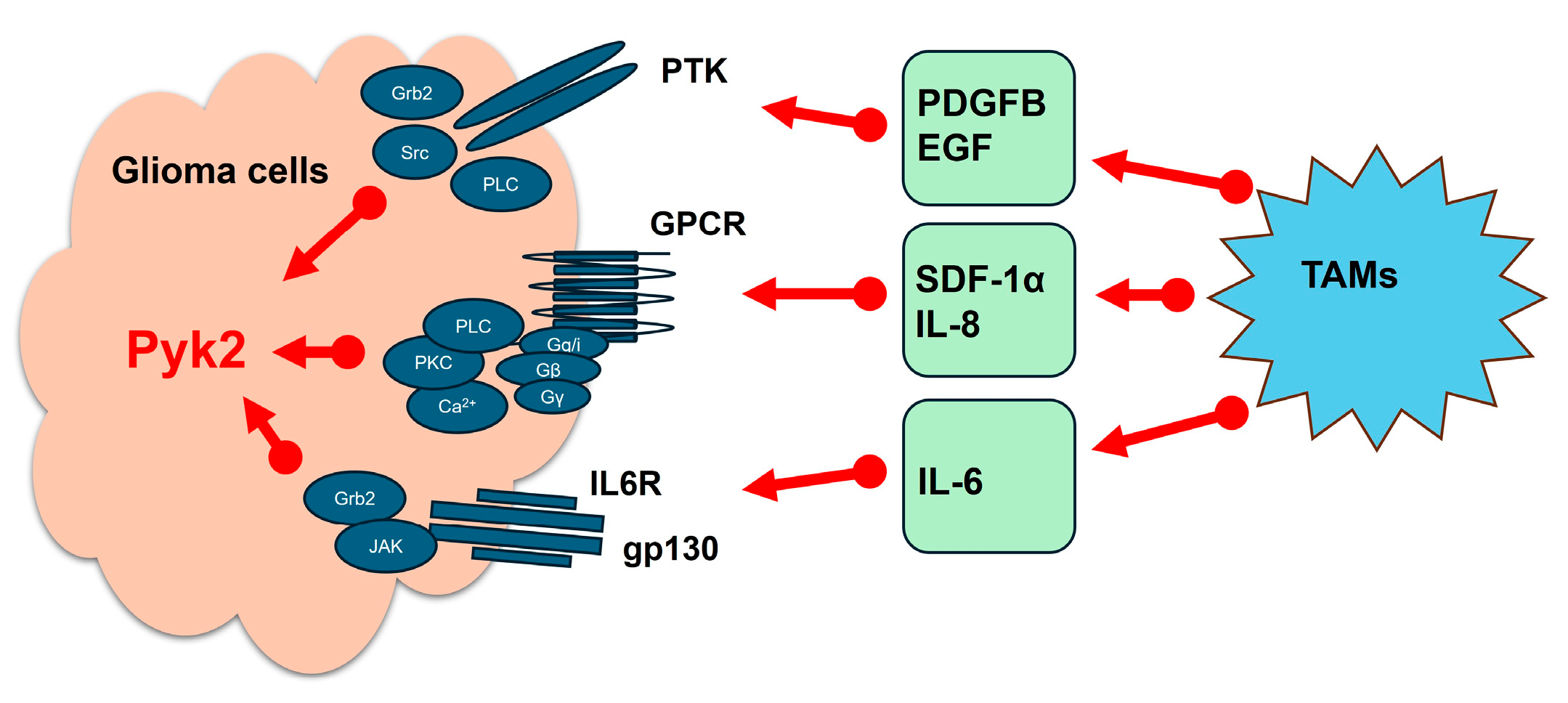
2. The Role of Pyk2 in Microglia-Induced Glioma Invasion
3. Regulation of Glioma Cell Invasion and Proliferation by Microglia-Derived Cytokines and Chemokines Through Pyk2 Signaling
3.1. Pyk2 and Invadopodia Formation
3.2. Pyk2-Dependent Cell Migration
3.3. Pyk2 and Glioma Proliferation
4. Pyk2 and GBM Recurrence
5. Pyk2 and Resistance to TMZ
5.1. Enhanced Cytotoxicity Through Pyk2 Inhibition
5.2. Influence of NF1 Status on Treatment Response
5.3. Apoptosis and Alternative Death Pathways
5.4. Effects on Invasion and Invadopodia Activity
5.5. In Vivo Efficacy of the Combinatorial Approach
5.6. Clinical Implications and Future Directions
6. Ethnicity and Sex-Specific Variability in Pyk2 Signaling
6.1. Sex Hormones and Differential Pyk2 Signaling
6.2. The Genetic Landscape of the Puerto Rican Population
6.3. Toward Precision Medicine: Future Research Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| Pyk2 | Proline-rich tyrosine kinase 2 |
| TAMs | Tumor-associated myeloid cells |
| EGF | Epidermal growth factor |
| PDGFβ | Platelet-derived growth factor |
| SDF-1α | Stromal cell-derived factor 1α |
| IL | Interleukin |
| NF1 | Neurofibromatosis 1 |
| FAK | Focal adhesion kinase |
| TMZ | Temozolomide |
| DNA | Deoxyribonucleic acid |
| BER | Base excision repair |
| MMR | DNA mismatch repair |
| MGMT | O6-methylguanine-DNA methyltransferase |
| Akt | Protein kinase B |
| Wnt | Wingless and Int-1, glycoproteins |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription protein, transcription factor |
| Ras | Ras family of GTPases, G-protein |
| Rac | Rho family of GTPases, G-protein |
| MAP | Mitogen-activated protein |
| NF-kB | Nuclear factor kappa B |
| RTKs | Receptor tyrosine kinases |
| GPCRs | G-protein-coupled receptors |
| Src | Non-receptor tyrosine kinase |
| PI3K | Phosphoinositide 3-kinase |
| MCM | Microglia-conditioned medium |
| MMPs | Matrix metalloproteinases |
| ECM | Extracellular matrix |
| RAF | Serine/threonine-specific protein kinases |
| GDNF | Glial cell-derived neurotrophic factor |
References
- Hatoum, A.; Mohamed, R.; Zakieh, O. The unique invasiveness of glioblastoma and possible drug targets on extracellular matrix. Cancer Manag. Res. 2019, 11, 1843–1855. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [PubMed]
- Cantidio, F.S.; Gil, G.O.B.; Queiroz, I.N.; Regalin, M. Glioblastoma—Treatment and Obstacles. Rep. Pract. Oncol. Radiother. 2022, 27, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Sacko, O.; Benouaich-Amiel, A.; Brandicourt, P.; Niaré, M.; Charni, S.; Cavandoli, C.; Brauge, D.; Catalaa, I.; Brenner, A.; Moyal, E.C.-J.; et al. The Impact of Surgery on the Survival of Patients with Recurrent Glioblastoma. Asian J. Neurosurg. 2021, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Li, J.; Huang, T.; Wang, Y.; Chang, L.; Zheng, W.; Ma, Y.; Chen, F.; Gong, X.; et al. Genomic analysis of primary and recurrent gliomas reveals clinical outcome related molecular features. Sci. Rep. 2019, 9, 16058. [Google Scholar] [CrossRef]
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.C.; Pentheroudakis, G. ESMO Guidelines Working Group. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 93–101. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma-a comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Guo, G.; Sun, Y.; Hong, R.; Xiong, J.; Lu, Y.; Liu, Y.; Lu, J.; Zhang, Z.; Guo, C.; Nan, Y.; et al. IKBKE enhances TMZ-chemoresistance through upregulation of MGMT expression in glioblastoma. Clin. Transl. Oncol. 2020, 22, 1252–1262. [Google Scholar] [CrossRef]
- Harder, B.G.; Peng, S.; Sereduk, C.P.; Sodoma, A.M.; Kitange, G.J.; Loftus, J.C.; Sarkaria, J.N.; Tran, N.L. Inhibition of phosphatidylinositol 3-kinase by PX-866 suppresses temozolomide-induced autophagy and promotes apoptosis in glioblastoma cells. Mol. Med. 2019, 25, 49. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Momin, A.A.; Mendes, T.; Barthe, P.; Faure, C.; Hong, S.; Yu, P.; Kadaré, G.; Jaremko, M.; Girault, J.A.; Jaremko, Ł.; et al. PYK2 senses calcium through a disordered dimerization and calmodulin-binding element. Commun. Biol. 2022, 5, 800. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.; Yang, Z.; Kloss, J. The Pyk2 FERM regulates Pyk2 complex formation and phosphorylation. Cell Signal. 2011, 23, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.C.; Yang, Z.; Tran, N.L.; Kloss, J.; Viso, C.; Berens, M.E.; Lipinski, C.A. The Pyk2 FERM domain as a target to inhibit glioma migration. Mol. Cancer Ther. 2009, 8, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Paulino, V.M.; Yang, Z.; Kloss, J.; Ennis, M.J.; Armstrong, B.A.; Loftus, J.C.; Tran, N.L. TROY (TNFRSF19) is overexpressed in advanced glial tumors and promotes glioblastoma cell invasion via Pyk2-Rac1 signaling. Mol. Cancer Res. 2010, 8, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Tran, N.L.; Menashi, E.; Rohl, C.; Kloss, J.; Bay, R.C.; Berens, M.E.; Loftus, J.C. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia 2005, 7, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Avraham, H.; Park, S.-Y.; Schinkmann, K.; Avraham, S. RAFTK/Pyk2-mediated cellular signalling. Cell. Signal. 2000, 12, 123–133. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Hanks, S.K.; Hunter, T.; van der Geer, P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 1994, 372, 786–791. [Google Scholar] [CrossRef]
- Anand, A.R.; Bradley, R.; Ganju, R.K. LPS-induced MCP-1 expression in human microvascular endothelial cells is mediated by the tyrosine kinase, Pyk2 via the p38 MAPK/NF-kappaB-dependent pathway. Mol. Immunol. 2009, 46, 962–968. [Google Scholar] [CrossRef]
- Rolón-Reyes, K.; Kucheryavykh, Y.V.; Cubano, L.A.; Inyushin, M.; Skatchkov, S.N.; Eaton, M.J.; Harrison, J.K.; Kucheryavykh, L.Y. Microglia Activate Migration of Glioma Cells through a Pyk2 Intracellular Pathway. PLoS ONE 2015, 10, e0131059. [Google Scholar] [CrossRef]
- Hecker, T.P.; Ding, Q.; Rege, T.A.; Hanks, S.K.; Gladson, C.L. Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene 2004, 23, 3962–3971. [Google Scholar] [CrossRef]
- Nuñez, R.E.; Del Valle, M.M.; Ortiz, K.; Almodovar, L.; Kucheryavykh, L. Microglial Cytokines Induce Invasiveness and Proliferation of Human Glioblastoma through Pyk2 and FAK Activation. Cancers 2021, 13, 6160. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Tran, N.L.; Viso, C.; Kloss, J.; Yang, Z.; Berens, M.E.; Loftus, J.C. Extended survival of Pyk2 or FAK deficient orthotopic glioma xenografts. J. Neurooncol. 2008, 90, 181–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortiz-Rivera, J.; Velez Crespo, G.; Inyushin, M.; Kucheryavykh, Y.; Kucheryavykh, L. Pyk2/FAK Signaling Is Upregulated in Recurrent Glioblastoma Tumors in a C57BL/6/GL261 Glioma Implantation Model. Int. J. Mol. Sci. 2023, 24, 13467. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rivera, J.; Nuñez, R.; Kucheryavykh, Y.; Kucheryavykh, L. The PYK2 inhibitor PF-562271 enhances the effect of temozolomide on tumor growth in a C57Bl/6-Gl261 mouse glioma model. J. Neurooncol. 2023, 161, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Babu, D.; Madigubba, S.; Panigrahi, M.; Phanithi, P.B. Tyrphostin A9 attenuates glioblastoma growth by suppressing PYK2/EGFR-ERK signaling pathway. J. Neurooncol. 2023, 163, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Okitsu-Sakurayama, S.; Higa-Nakamine, S.; Torihara, H.; Higashiyama, S.; Yamamoto, H. Roles of Pyk2 in signal transduction after gonadotropin-releasing hormone receptor stimulation. J. Cell Physiol. 2021, 236, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.B.; Lee, I.T.; Lin, Y.J.; Wang, S.Y.; Hsiao, L.D.; Yang, C.M. Silica Nanoparticles Shed Light on Intriguing Cellular Pathways in Human Tracheal Smooth Muscle Cells: Revealing COX-2/PGE2 Production through the EGFR/Pyk2 Signaling Axis. Biomedicines 2024, 12, 107. [Google Scholar] [CrossRef]
- Lyu, A.; Humphrey, R.S.; Nam, S.H.; Durham, T.A.; Hu, Z.; Arasappan, D.; Horton, T.M.; Ehrlich, L.I.R. Integrin signaling is critical for myeloid-mediated support of T-cell acute lymphoblastic leukemia. Nat. Commun. 2023, 14, 6270. [Google Scholar] [CrossRef]
- Man, K.N.M.; Bartels, P.; Henderson, P.B.; Kim, K.; Shi, M.; Zhang, M.; Ho, S.Y.; Nieves-Cintron, M.; Navedo, M.F.; Horne, M.C.; et al. α1-Adrenergic receptor-PKC-Pyk2-Src signaling boosts L-type Ca2+ channel CaV1.2 activity and long-term potentiation in rodents. eLife 2023, 12, 79648. [Google Scholar] [CrossRef]
- Fischer, P.; Hilfiker-Kleiner, D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S414–S427. [Google Scholar] [CrossRef]
- Graeber, M.B.; Scheithauer, B.W.; Kreutzberg, G.W. Microglia in brain tumors. Glia 2002, 40, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Graeber, M.B. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012, 14, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Morris, R.J.; Steinson, E. The multifactorial roles of microglia and macrophages in the maintenance and progression of glioblastoma. J. Neuroimmunol. 2021, 357, 577633. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Streit, W.J. Microglia: Biology and pathology. Acta Neuropathol. 2010, 119, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Gabrusiewicz, K.; Ellert-Miklaszewska, A.; Lipko, M.; Sielska, M.; Frankowska, M.; Kaminska, B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE 2011, 6, e23902. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.S.; Glass, R.; Synowitz, M.; Rooijen, N.V.; Kettenmann, H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of matalloprotease-2. J. Neuropathol. Exp. Neurol. 2005, 64, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.S.; Vinnakota, K.; Chirasani, S.; Synowitz, M.; Raguet, H.; Stock, K.; Sliwa, M.; Lehmann, S.; Kälin, R.; van Rooijen, N.; et al. Gliomas induce and exploit microglial MT1-MMPexpression for tumor expansion. Proc. Natl. Acad. Sci. USA 2009, 106, 12530–12535. [Google Scholar] [CrossRef] [PubMed]
- Watters, J.J.; Schartner, J.M.; Badie, B. Microglia function in brain tumors. J. Neurosci. Res. 2005, 81, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S. Molecular mechanisms of glioma invasiveness: The role of proteases. Nat. Rev. Cancer. 2003, 3, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Wick, W.; Weller, M. Malignant glioma biology: Role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc. Res. Tech. 2001, 52, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.; Betsholtz, C. The importance of microglia in the development of the vasculature in the central nervous system. Vasc. Cell 2013, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Rempel, S.A.; Rosenblum, M.L.; Mikkelsen, T.; Yan, P.S.; Ellis, K.D.; Golembieski, W.A.; Sameni, M.; Rozhin, J.; Ziegler, G.; Sloane, B.F. Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 1994, 54, 6027–6031. [Google Scholar] [PubMed]
- Mai, J.; Sameni, M.; Mikkelsen, T.; Sloane, B.F. Degradation of extracellular matrix protein tenascin-C by cathepsin B: An interaction involved in the progression of gliomas. Biol. Chem. 2002, 383, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Flannery, T.; Gibson, D.; Mirakhur, M.; McQuaid, S.; Greenan, C.; Trimble, A.; Walker, B.; McCormick, D.; Johnston, P.G. The Clinical Significance of Cathepsin S Expression in Human Astrocytomas. Am. J. Path. 2003, 163, 175–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thota, B.; Arimappamagan, A.; Kandavel, T.; Shastry, A.H.; Pandey, P.; Chandramouli, B.A.; Hegde, A.S.; Kondaiah, P.; Santosh, V. STAT-1 expression is regulated by IGFBP-3 in malignant glioma cells and is a strong predictor of poor survival in patients with glioblastoma. J. Neurosurg. 2014, 121, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Zhu, Y.S.; Mei, P.J.; Sun, S.G.; Zhang, H.; Chen, H.F.; Chen, C.; Miao, F.-A. Cullin1 regulates proliferation, migration and invasion of glioma cells. Med. Oncol. 2014, 31, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, Z.; Mo, J.; Sang, B.; Shi, Q.; Hu, J.; Xie, S.; Zhan, W.; Lu, D.; Yang, M.; et al. GOLPH3 promotes glioblastoma cell migration and invasion via the mTOR-YB1 pathway in vitro. Mol. Carcinog. 2015, 54, 1252–1263. [Google Scholar] [CrossRef]
- La Porta, C. AQP1 is not only a water channel: It contributes to cell migration through Lin7/beta-catenin. Cell Adh. Migr. 2010, 4, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Chilukuri, K.; Brady, O.A.; Boulos, M.I.; Kappes, J.C.; Galileo, D.S. L1 stimulation of human glioma cell motility correlates with FAK activation. J. Neurooncol. 2011, 105, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, C.; Hackmann, O.; Delic, S.; Schmidt, N.; Reifenberger, G.; Riemenschneider, M.J. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011, 122, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, M.; Yu, S.; Hai, L.; Zhang, L.; Zhang, C.; Zhao, P.; Zhou, H.; Wang, S.; Yang, X. Arg kinase mediates CXCL12/CXCR4-induced invadopodia formation and invasion of glioma cells. Exp. Cell Res. 2020, 389, 111893. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Tran, N.L.; Bay, C.; Kloss, J.; McDonough, W.S.; Beaudry, C.; Berens, M.E.; Loftus, J.C. Differential role of proline-rich tyrosine kinase 2 and focal adhesion kinase in determining glioblastoma migration and proliferation. Mol. Cancer Res. 2003, 1, 323–332. [Google Scholar]
- Hu, C.; Chen, X.; Wen, J.; Gong, L.; Liu, Z.; Wang, J.; Liang, J.; Hu, F.; Zhou, Q.; Wei, L.; et al. Antitumor effect of focal adhesion kinase inhibitor PF562271 against human osteosarcoma in vitro and in vivo. Cancer Sci. 2017, 108, 1347–1356. [Google Scholar] [CrossRef]
- Darmanis, S.; Sloan, S.A.; Croote, D.; Mignardi, M.; Chernikova, S.; Samghababi, P.; Zhang, Y.; Neff, N.; Kowarsky, M.; Caneda, C.; et al. Single-Cell RNA-Seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017, 21, 1399–1410. [Google Scholar] [CrossRef]
- Brooks, L.J.; Parrinello, S. Vascular regulation of glioma stem-like cells: A balancing act. Curr. Opin. Neurobiol. 2017, 47, 8–15. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Arismendi-Morillo, G.; Zuccoli, G.; Lee, D.C.; Duraj, T.; Elsakka, A.M.; Maroon, J.C.; Mukherjee, P.; Ta, L.; Shelton, L.; et al. Metabolic management of microenvironment acidity in glioblastoma. Front. Oncol. 2022, 12, 968351. [Google Scholar] [CrossRef]
- Gil-Henn, H.; Girault, J.A.; Lev, S. PYK2, a hub of signaling networks in breast cancer progression. Trends Cell Biol. 2024, 34, 312–326. [Google Scholar] [CrossRef]
- Lee, D.; Hong, J.-H. Activated PyK2 and Its Associated Molecules Transduce Cellular Signaling from the Cancerous Milieu for Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 15475. [Google Scholar] [CrossRef]
- Alanio, C.; Binder, Z.A.; Chang, R.B.; Nasrallah, M.P.; Delman, D.; Li, J.H.; Tang, O.Y.; Zhang, L.Y.; Zhang, J.V.; Wherry, E.J.; et al. Immunologic Features in De Novo and Recurrent Glioblastoma Are Associated with Survival Outcomes. Cancer Immunol. Res. 2022, 10, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Chanoch-Myers, R.; Mathewson, N.D.; Myskiw, C.; Atta, L.; Bussema, L.; Eichhorn, S.W.; Greenwald, A.C.; Kinker, G.S.; Rodman, C.; et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell 2021, 39, 779–792.e11. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rivera, J.; Albors, A.; Kucheryavykh, Y.; Harrison, J.K.; Kucheryavykh, L. The Dynamics of Tumor-Infiltrating Myeloid Cell Activation and the Cytokine Expression Profile in a Glioma Resection Site during the Post-Surgical Period in Mice. Brain Sci. 2022, 12, 893. [Google Scholar] [CrossRef]
- Pombo Antunes, A.R.; Scheyltjens, I.; Lodi, F.; Messiaen, J.; Antoranz, A.; Duerinck, J.; Kancheva, D.; Martens, L.; De Vlaminck, K.; Van Hove, H.; et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat. Neurosci. 2021, 24, 595–610. [Google Scholar] [CrossRef]
- Friebel, E.; Kapolou, K.; Unger, S.; Núñez, N.G.; Utz, S.; Rushing, E.J.; Regli, L.; Weller, M.; Greter, M.; Tugues, S.; et al. Single-Cell Mapping of Human Brain Cancer Reveals Tumor-Specific Instruction of Tissue-Invading Leukocytes. Cell 2020, 181, 1626–1642. [Google Scholar] [CrossRef]
- Gu, F.; Li, Q.; Gao, Q.; Jiang, J.; Zhu, K.; Huang, X.; Pan, J.; Yan, J.; Hu, J.; Wang, Z.; et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol. Cancer 2011, 10, 150. [Google Scholar] [CrossRef]
- Chien, C.H.; Hsueh, W.T.; Chuang, J.Y.; Chang, K.Y. Dissecting the mechanism of temozolomide resistance and its association with the regulatory roles of intracellular reactive oxygen species in glioblastoma. J. Biomed. Sci. 2021, 28, 18. [Google Scholar] [CrossRef]
- Kang, H.; Lee, H.; Kim, D.; Kim, B.; Kang, J.; Kim, H.Y.; Youn, H.; Youn, B. Targeting glioblastoma stem cells to overcome chemoresistance: An overview of current therapeutic strategies. Biomedicines 2022, 10, 1308. [Google Scholar] [CrossRef]
- Perazzoli, G.; Prados, J.; Ortiz, R.; Caba, O.; Cabeza, L.; Berdasco, M.; Gónzalez, B.; Melguizo, C. Temozolomide resistance in glioblastoma cell lines: Implication of MGMT, MMR, P-glycoprotein and CD133 expression. PLoS ONE 2015, 10, e0140131. [Google Scholar] [CrossRef]
- Mir, S.E.; De Witt Hamer, P.C.; Krawczyk, P.M.; Balaj, L.; Claes, A.; Niers, J.M.; Van Tilborg, A.A.G.; Zwinderman, A.H.; Geerts, D.; Kaspers, G.J.L. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 2010, 18, 244–257. [Google Scholar] [CrossRef]
- Hirose, Y.; Berger, M.S.; Pieper, R.O. p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001, 61, 1957–1963. [Google Scholar] [PubMed]
- Gousias, K.; Theocharous, T.; Simon, M. Mechanisms of cell cycle arrest and apoptosis in glioblastoma. Biomedicines 2022, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Errico, A.; Stocco, A.; Riccardi, V.M.; Gambalunga, A.; Bassetto, F.; Grigatti, M.; Ferlosio, A.; Tadini, G.; Garozzo, D.; Ferraresi, S.; et al. Neurofibromin deficiency and extracellular matrix cooperate to increase transforming potential through FAK-dependent signaling. Cancers 2021, 13, 2329. [Google Scholar] [CrossRef] [PubMed]
- Bergoug, M.; Doudeau, M.; Godin, F.; Mosrin, C.; Vallée, B.; Bénédetti, H. Neurofibromin structure, functions regulation. Cells 2020, 9, 2365. [Google Scholar] [CrossRef]
- Whittaker, S.R.; Theurillat, J.P.; Van Allen, E.; Wagle, N.; Hsiao, J.; Cowley, G.S.; Schadendorf, D.; Root, D.E.; Garraway, L.A. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov. 2013, 3, 350–362. [Google Scholar] [CrossRef]
- Kweh, F.; Zheng, M.; Kurenova, E.; Wallace, M.; Golubovskaya, V.; Cance, W.G. Neurofibromin physically interacts with the N-terminal domain of focal adhesion kinase. Mol. Carcinog. 2009, 48, 1005–1017. [Google Scholar] [CrossRef]
- Dai, C.; Santagata, S.; Tang, Z.; Shi, J.; Cao, J.; Kwon, H.; Bronson, R.T.; Whitesell, L.; Lindquist, S. Loss of tumor suppressor NF1 activates HSF1 to promote carcinogenesis. J. Clin. Investig. 2012, 122, 3742–3754. [Google Scholar] [CrossRef]
- de Bruin, E.C.; Cowell, C.; Warne, P.H.; Jiang, M.; Saunders, R.E.; Melnick, M.A.; Gettinger, S.; Walther, Z.; Wurtz, A.; Heynen, G.J.; et al. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer Discov. 2014, 4, 606–619. [Google Scholar] [CrossRef]
- Harder, A. MEK inhibitors—Novel targeted therapies of neurofibromatosis associated benign and malignant lesions. Biomark. Res. 2021, 9, 26. [Google Scholar] [CrossRef]
- Lefranc, F.; Facchini, V.; Kiss, R. Proautophagic drugs: A novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist 2007, 12, 1395–1403. [Google Scholar] [CrossRef]
- Shen, W.; Hu, J.A.; Zheng, J.S. Mechanism of temozolomide-induced antitumour effects on glioma cells. J. Int. Med. Res. 2014, 42, 164–172. [Google Scholar] [CrossRef]
- Bustany, S.; Cahu, J.; Guardiola, P.; Sola, B. Cyclin D1 sensitizes myeloma cells to endoplasmic reticulum stress-mediated apoptosis by activating the unfolded protein response pathway. BMC Cancer 2015, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Balkenende, A.; Verschoor, T.; Lallemand, F.; Michalides, R. Cyclin D1 overexpression enhances radiation-induced apoptosis and radiosensitivity in a breast tumor cell line. Cancer Res. 1999, 59, 1134–1140. [Google Scholar]
- Linder, S.; Cervero, P.; Eddy, R.; Condeelis, J. Mechanisms and roles of podosomes and invadopodia. Nat. Rev. Mol. Cell Biol. 2023, 24, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Dinevska, M.; Gazibegovic, N.; Morokoff, A.P.; Kaye, A.H.; Drummond, K.J.; Mantamadiotis, T.; Stylli, S.S. Inhibition of radiation and temozolomide-induced glioblastoma invadopodia activity using ion channel drugs. Cancers 2020, 12, 2888. [Google Scholar] [CrossRef]
- Trog, D.; Yeghiazaryan, K.; Fountoulakis, M.; Friedlein, A.; Moenkemann, H.; Haertel, N.; Schueller, H.; Breipohl, W.; Schild, H.; Leppert, D.; et al. Pro-invasive gene regulating effect of irradiation and combined temozolomide-radiation treatment on surviving human malignant glioma cells. Eur. J. Pharmacol. 2006, 542, 8–15. [Google Scholar] [CrossRef]
- Roberts, W.G.; Ung, E.; Whalen, P.; Cooper, B.; Hulford, C.; Autry, C.; Richter, D.; Emerson, E.; Lin, J.; Kath, J.; et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008, 68, 1935–1944. [Google Scholar] [CrossRef]
- Liu, J.; Xue, L.; Xu, X.; Luo, J.; Zhang, S. FAK-targeting PROTAC demonstrates enhanced antitumor activity against KRAS mutant non-small cell lung cancer. Exp. Cell Res. 2021, 408, 112868. [Google Scholar] [CrossRef]
- Jones, S.; Siu, L.; Bendell, J.; Cleary, J.; Razak, A.; Infante, J.; Pandya, S.; Bedard, P.; Pierce, K.; Houk, B.; et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2015, 33, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Camidge, D.R.; Mileshkin, L.R.; Chen, E.X.; Hicks, R.J.; Rischin, D.; Fingert, H.; Pierce, K.J.; Xu, H.; Robert, W.G.; et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J. Clin. Oncol. 2012, 30, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.; Mayol del Valle, M.; Kucheryavykh, L. Ethnicity-Based Variations in Focal Adhesion Kinase Signaling in Glioblastoma Gene Expression: A Study of the Puerto Rican Hispanic Population. Int. J. Mol. Sci. 2024, 25, 4947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrano, A.; Juarez, J.J.; Incontri, D.; Ibarra, A.; Cazares, H.G. Sex-Specific Differences in Glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef]
- Walsh, K.M.; Neff, C.; Bondy, M.L.; Kruchko, C.; Huse, J.T.; Amos, C.I.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. Influence of County-Level Geographic/Ancestral Origin on Glioma Incidence and Outcomes in US Hispanics. Neuro-Oncology 2023, 25, 398–406. [Google Scholar] [CrossRef]
- Qi, Z.-Y.; Shao, C.; Zhang, X.; Hui, G.-Z.; Wang, Z. Exogenous and Endogenous Hormones in Relation to Glioma inWomen: A Meta-Analysis of 11 Case-Control Studies. PLoS ONE 2013, 8, e68695. [Google Scholar] [CrossRef]
- Lan, Y.-L.; Wang, X.; Lou, J.-C.; Ma, B.-B.; Xing, J.-S.; Zou, S.; Zhang, B. Update on the Effect of Exogenous Hormone Use on Glioma Risk in Women: A Meta-Analysis of Case-Control and Cohort Studies. J. Neurooncol. 2018, 137, 357–365. [Google Scholar] [CrossRef]
- Karlsson, S.A.; Studer, E.; Kettunen, P.; Westberg, L. Neural Androgen Receptors Modulate Gene Expression and Social Recognition But Not Social Investigation. Front. Behav. Neurosci. 2016, 10, 41. [Google Scholar] [CrossRef]
- Lu, S.; Simon, N.G.; Wang, Y.; Hu, S. Neural Androgen Receptor Regulation: Effects of Androgen and Antiandrogen. J. Neurobiol. 1999, 41, 505–512. [Google Scholar] [CrossRef]
- Via, M.; Gignoux, C.R.; Roth, L.A.; Fejerman, L.; Galanter, J.; Choudhry, S.; Toro-Labrador, G.; Viera-Vera, J.; Oleksyk, T.K.; Beckman, K.; et al. History Shaped the Geographic Distribution of Genomic Admixture on the Island of Puerto Rico. PLoS ONE 2011, 6, e16513. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, S.; Burchard, E.G.; Borrell, L.N.; Tang, H.; Gomez, I.; Naqvi, M.; Nazario, S.; Torres, A.; Casal, J.; Martinez-Cruzado, J.C.; et al. Ancestry–Environment Interactions and Asthma Risk among Puerto Ricans. Am. J. Respir. Crit. Care Med. 2006, 174, 1088–1093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tortolero-Luna, G.; Torres-Cintrón, C.R.; Alvarado-Ortiz, M.; Ortiz-Ortiz, K.J.; Zavala-Zegarra, D.E.; Mora-Piñero, E. Incidence of Thyroid Cancer in Puerto Rico and the US by Racial/Ethnic Group, 2011–2015. BMC Cancer 2019, 19, 637. [Google Scholar] [CrossRef]
- Witkop, C.J.; Nuñez Babcock, M.; Rao, G.H.; Gaudier, F.; Summers, C.G.; Shanahan, F.; Harmon, K.R.; Townsend, D.; Sedano, H.O.; King, R.A. Albinism and Hermansky-Pudlak Syndrome in Puerto Rico. Bol. Asoc. Medica Puerto Rico 1990, 82, 333–339. [Google Scholar]
- Gahr, S.; Stoehr, R.; Geissinger, E.; Ficker, J.H.; Brueckl, W.M.; Gschwendtner, A.; Gattenloehner, S.; Fuchs, F.S.; Schulz, C.; Rieker, R.J.; et al. EGFR Mutational Status in a Large Series of Caucasian European NSCLC Patients: Data from Daily Practice. Br. J. Cancer 2013, 109, 1821–1828. [Google Scholar] [CrossRef]
- Zheng, R.; Yin, Z.; Alhatem, A.; Lyle, D.; You, B.; Jiang, A.S.; Liu, D.; Jobbagy, Z.; Wang, Q.; Aisner, S.; et al. Epidemiologic Features of NSCLC Gene Alterations in Hispanic Patients from Puerto Rico. Cancers 2020, 12, 3492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucheryavykh, L.; Kucheryavykh, Y. The Role of Pyk2 Kinase in Glioblastoma Progression and Therapeutic Targeting. Cancers 2025, 17, 2611. https://doi.org/10.3390/cancers17162611
Kucheryavykh L, Kucheryavykh Y. The Role of Pyk2 Kinase in Glioblastoma Progression and Therapeutic Targeting. Cancers. 2025; 17(16):2611. https://doi.org/10.3390/cancers17162611
Chicago/Turabian StyleKucheryavykh, Lilia, and Yuriy Kucheryavykh. 2025. "The Role of Pyk2 Kinase in Glioblastoma Progression and Therapeutic Targeting" Cancers 17, no. 16: 2611. https://doi.org/10.3390/cancers17162611
APA StyleKucheryavykh, L., & Kucheryavykh, Y. (2025). The Role of Pyk2 Kinase in Glioblastoma Progression and Therapeutic Targeting. Cancers, 17(16), 2611. https://doi.org/10.3390/cancers17162611




