Timing Matters: A Systematic Review of Early Versus Delayed Palliative Care in Advanced Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction and Management
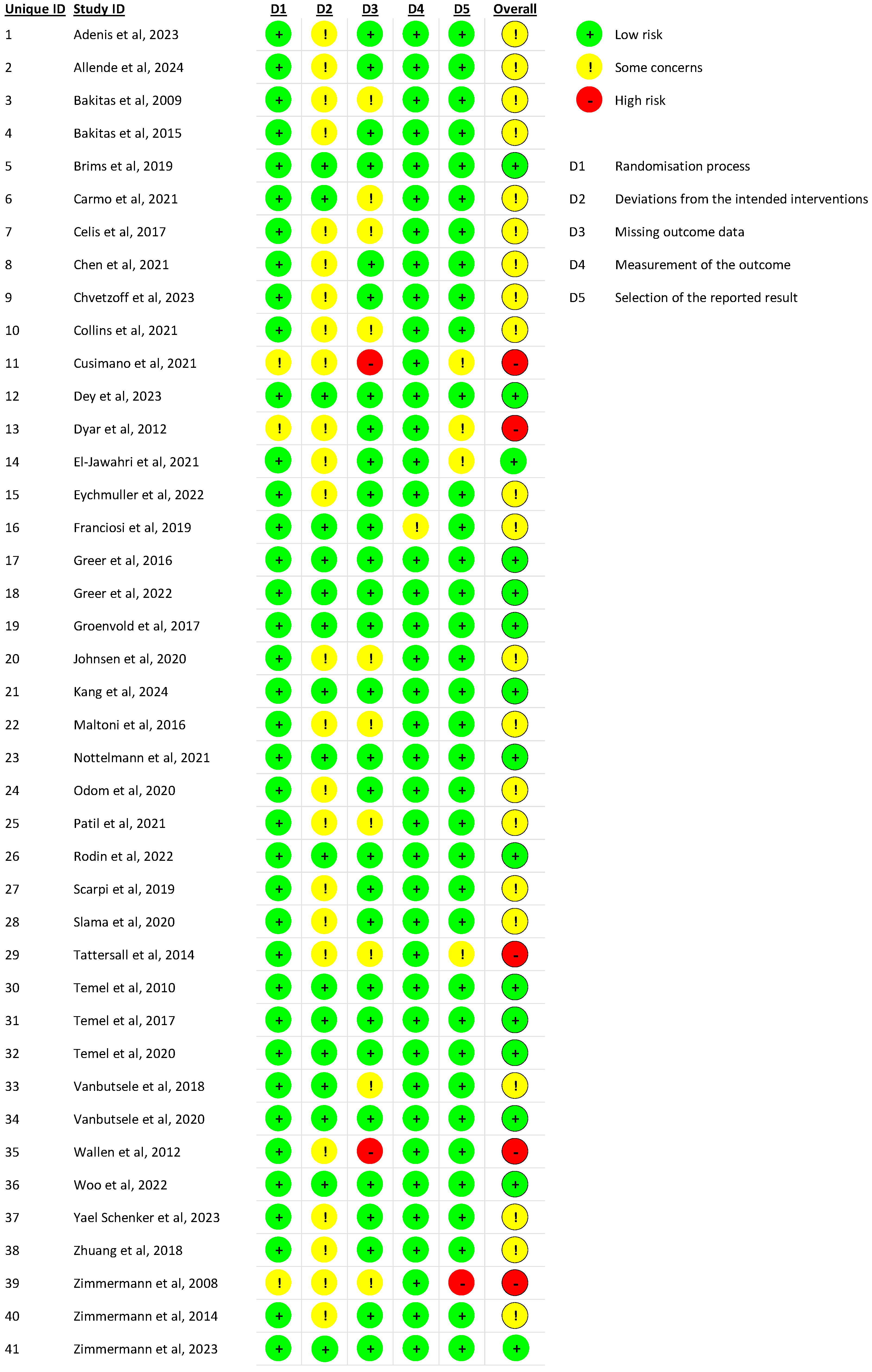
2.5. Risk of Bias Assessment
3. Results
3.1. Characteristics of Included Studies
3.2. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bizuayehu, H.M.; Ahmed, K.Y.; Kibret, G.D.; Dadi, A.F.; Belachew, S.A.; Bagade, T.; Tegegne, T.K.; Venchiarutti, R.L.; Kibret, K.T.; Hailegebireal, A.H.; et al. Global disparities of cancer and its projected burden in 2050. JAMA Netw. Open 2024, 7, e2443198. [Google Scholar] [CrossRef]
- Bergerot, C.D.; Pal, S.K. Shining a light on the psychological burden of cancer. Nat. Med. 2022, 28, 637–638. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Marlin, A.; Yoshida, T.; Ullrich, A. Palliative Care: The World Health Organization’s global perspective. J. Pain Symptom Manag. 2002, 24, 91–96. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. Integrating palliative care into the trajectory of cancer care. Nat. Rev. Clin. Oncol. 2016, 13, 159–171. [Google Scholar] [CrossRef]
- Kircher, C.E.; Hanna, T.P.; Tranmer, J.; Goldie, C.E.; Ross-White, A.; Moulton, E.; Flegal, J.; Goldie, C.L. Defining “early palliative care” for adults diagnosed with a life-limiting illness: A scoping review. BMC Palliat. Care 2025, 24, 93. [Google Scholar] [CrossRef]
- Huffman, J.L.; Harmer, B. End-of-Life Care. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shi, Z.; Du, M.; Zhu, S.; Lei, Y.; Xu, Q.; Li, W.; Gu, W.; Zhao, N.; Chen, Y.; Liu, W.; et al. Factors influencing accessibility of palliative care: A systematic review and meta-analysis. BMC Palliat. Care 2025, 24, 80. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Adenis, A.; Da Silva, A.; Ben Abdelghani, M.; Bourgeois, V.; Bogart, E.; Turpin, A.; Evin, A.; Proux, A.; Galais, M.-P.; Jaraudias, C.; et al. Early palliative care and overall survival in patients with metastatic upper gastrointestinal cancers (EPIC): A multicentre, open-label, randomised controlled phase 3 trial. eClinicalMedicine 2024, 74, 102470. [Google Scholar] [CrossRef] [PubMed]
- Allende, S.; Turcott, J.G.; Verástegui, E.; Rodríguez-Mayoral, O.; Flores-Estrada, D.; Pérez Camargo, D.A.; Ramos-Ramírez, M.; Martínez-Hernández, J.-N.; Oñate-Ocaña, L.F.; Pina, P.S.; et al. Early Incorporation to Palliative Care (EPC) in Patients With Advanced Non-Small Cell Lung Cancer: The PACO Randomized Clinical Trial. Oncologist 2024, 29, e1373–e1385. [Google Scholar] [CrossRef]
- Bakitas, M.; Lyons, K.D.; Hegel, M.T.; Balan, S.; Brokaw, F.C.; Seville, J.; Hull, J.G.; Li, Z.; Tosteson, T.D.; Byock, I.R.; et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009, 302, 741–749. [Google Scholar] [CrossRef]
- Bakitas, M.A.; Tosteson, T.D.; Li, Z.; Lyons, K.D.; Hull, J.G.; Li, Z.; Dionne-Odom, J.N.; Frost, J.; Dragnev, K.H.; Hegel, M.T.; et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015, 33, 1438–1445. [Google Scholar] [CrossRef]
- Brims, F.; Gunatilake, S.; Lawrie, I.; Marshall, L.; Fogg, C.; Qi, C.; Creech, L.; Holtom, N.; Killick, S.; Yung, B.; et al. RESPECT-Meso investigators Early specialist palliative care on quality of life for malignant pleural mesothelioma: A randomised controlled trial. Thorax 2019, 74, 354–361. [Google Scholar] [CrossRef]
- do Carmo, T.M.; Paiva, B.S.R.; de Oliveira, C.Z.; Nascimento, M.S.D.A.; Paiva, C.E. The feasibility and benefit of a brief psychosocial intervention in addition to early palliative care in patients with advanced cancer to reduce depressive symptoms: A pilot randomized controlled clinical trial. BMC Cancer 2017, 17, 564. [Google Scholar] [CrossRef]
- Soto-Perez-de-Celis, E.; Chavarri-Guerra, Y.; Ramos-Lopez, W.A.; Alcalde-Castro, J.; Covarrubias-Gomez, A.; Navarro-Lara, Á.; Quiroz-Friedman, P.; Sánchez-Román, S.; Alcocer-Castillejos, N.; Aguilar-Velazco, J.C.; et al. Patient Navigation to Improve Early Access to Supportive Care for Patients with Advanced Cancer in Resource-Limited Settings: A Randomized Controlled Trial. Oncologist 2021, 26, 157–164. [Google Scholar] [CrossRef]
- Chen, M.; Yang, L.; Yu, H.; Yu, H.; Wang, S.; Tian, L.; Liu, S. Early Palliative Care in Patients With Non-Small-Cell Lung Cancer: A Randomized Controlled Trial in Southwest China. Am. J. Hosp. Palliat. Care 2022, 39, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Chvetzoff, G.; Bouleuc, C.; Lardy-Cléaud, A.; Saltel, P.; Dieras, V.; Morelle, M.; Guastalla, J.-P.; Tredan, O.; Rebattu, P.; Pop, S.; et al. Impact of early palliative care on additional line of chemotherapy in metastatic breast cancer patients: Results from the randomized study OSS. Support. Care Cancer 2022, 31, 82. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Sundararajan, V.; Le, B.; Mileshkin, L.; Hanson, S.; Emery, J.; Philip, J. The feasibility of triggers for the integration of Standardised, Early Palliative (STEP) Care in advanced cancer: A phase II trial. Front. Oncol. 2022, 12, 991843. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Sajewycz, K.; Harle, I.; Giroux, J.; Hanna, T.; Willing, S.; Martin, V.; Francis, J.-A. Acceptability and Feasibility of Early Palliative Care Among Women with Advanced Epithelial Ovarian Cancer: A Randomized Controlled Pilot Study. J. Obstet. Gynaecol. Can. 2021, 43, 707–715. [Google Scholar] [CrossRef]
- Dey, T.; Mukerjee, A.; Rai, B.; Arora, M.; Kumar, D.; Srinivasa, G.Y.; Ghoshal, S. Early integration of palliative care in cervical cancer: Experiences from a pilot study. J. Family Med. Prim. Care 2023, 12, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Dyar, S.; Lesperance, M.; Shannon, R.; Sloan, J.; Colon-Otero, G. A nurse practitioner directed intervention improves the quality of life of patients with metastatic cancer: Results of a randomized pilot study. J. Palliat. Med. 2012, 15, 890–895. [Google Scholar] [CrossRef]
- El-Jawahri, A.; LeBlanc, T.; VanDusen, H.; Traeger, L.; Greer, J.A.; Pirl, W.F.; Jackson, V.A.; Telles, J.; Rhodes, A.; Spitzer, T.R.; et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: A randomized clinical trial. JAMA 2016, 316, 2094–2103. [Google Scholar] [CrossRef]
- Eychmüller, S.; Zwahlen, S.; Fliedner, M.C.; Jüni, P.; Aebersold, D.M.; Aujesky, D.; Fey, M.F.; Maessen, M.; Trelle, S. Single early palliative care intervention added to usual oncology care for patients with advanced cancer: A randomized controlled trial (SENS Trial). Palliat. Med. 2021, 35, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, V.; Maglietta, G.; Degli Esposti, C.; Caruso, G.; Cavanna, L.; Bertè, R.; Bacchini, G.; Bocchi, L.; Piva, E.; Monfredo, M.; et al. Early palliative care and quality of life of advanced cancer patients-a multicenter randomized clinical trial. Ann. Palliat. Med. 2019, 8, 381–389. [Google Scholar] [CrossRef]
- Greer, J.A.; Tramontano, A.C.; McMahon, P.M.; Pirl, W.F.; Jackson, V.A.; El-Jawahri, A.; Parikh, R.B.; Muzikansky, A.; Gallagher, E.R.; Temel, J.S. Cost Analysis of a Randomized Trial of Early Palliative Care in Patients with Metastatic Nonsmall-Cell Lung Cancer. J. Palliat. Med. 2016, 19, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Moy, B.; El-Jawahri, A.; Jackson, V.A.; Kamdar, M.; Jacobsen, J.; Lindvall, C.; Shin, J.A.; Rinaldi, S.; Carlson, H.A.; et al. Randomized Trial of a Palliative Care Intervention to Improve End-of-Life Care Discussions in Patients With Metastatic Breast Cancer. J. Natl. Compr. Cancer Netw. 2022, 20, 136–143. [Google Scholar] [CrossRef]
- Groenvold, M.; Petersen, M.A.; Damkier, A.; Neergaard, M.A.; Nielsen, J.B.; Pedersen, L.; Sjøgren, P.; Strömgren, A.S.; Vejlgaard, T.B.; Gluud, C.; et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: The Danish Palliative Care Trial. Palliat. Med. 2017, 31, 814–824. [Google Scholar] [CrossRef]
- Johnsen, A.T.; Petersen, M.A.; Sjøgren, P.; Pedersen, L.; Neergaard, M.A.; Damkier, A.; Gluud, C.; Fayers, P.; Lindschou, J.; Strömgren, A.S.; et al. Exploratory analyses of the Danish Palliative Care Trial (DanPaCT): A randomized trial of early specialized palliative care plus standard care versus standard care in advanced cancer patients. Support. Care Cancer 2020, 28, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Kang, J.H.; Koh, S.-J.; Kim, Y.J.; Seo, S.; Kim, J.H.; Cheon, J.; Kang, E.J.; Song, E.-K.; Nam, E.M.; et al. Early integrated palliative care in patients with advanced cancer: A randomized clinical trial. JAMA Netw. Open 2024, 7, e2426304. [Google Scholar] [CrossRef]
- Maltoni, M.; Scarpi, E.; Dall’Agata, M.; Zagonel, V.; Bertè, R.; Ferrari, D.; Broglia, C.M.; Bortolussi, R.; Trentin, L.; Valgiusti, M.; et al. Early Palliative Care Italian Study Group (EPCISG) Systematic versus on-demand early palliative care: Results from a multicentre, randomised clinical trial. Eur. J. Cancer 2016, 65, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nottelmann, L.; Groenvold, M.; Vejlgaard, T.B.; Petersen, M.A.; Jensen, L.H. Early, integrated palliative rehabilitation improves quality of life of patients with newly diagnosed advanced cancer: The Pal-Rehab randomized controlled trial. Palliat. Med. 2021, 35, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Dionne-Odom, J.N.; Ejem, D.B.; Wells, R.; Azuero, A.; Stockdill, M.L.; Keebler, K.; Sockwell, E.; Tims, S.; Engler, S.; Kvale, E.; et al. Effects of a Telehealth Early Palliative Care Intervention for Family Caregivers of Persons With Advanced Heart Failure: The ENABLE CHF-PC Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e202583. [Google Scholar] [CrossRef]
- Slama, O.; Pochop, L.; Sedo, J.; Svancara, J.; Sedova, P.; Svetlakova, L.; Demlova, R.; Vyzula, R. Effects of Early and Systematic Integration of Specialist Palliative Care in Patients with Advanced Cancer: Randomized Controlled Trial PALINT. J. Palliat. Med. 2020, 23, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Singhai, P.; Noronha, V.; Bhattacharjee, A.; Deodhar, J.; Salins, N.; Joshi, A.; Menon, N.S.; Abhyankar, A.; Khake, A.; et al. Effect of early palliative care on quality of life of advanced head and neck cancer patients: A phase III trial. J. Natl. Cancer Inst. 2021, 113, 1228–1237. [Google Scholar] [CrossRef]
- Rodin, R.; Swami, N.; Pope, A.; Hui, D.; Hannon, B.; Le, L.W.; Zimmermann, C. Impact of early palliative care according to baseline symptom severity: Secondary analysis of a cluster-randomized controlled trial in patients with advanced cancer. Cancer Med. 2022, 11, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Scarpi, E.; Dall’Agata, M.; Zagonel, V.; Gamucci, T.; Bertè, R.; Sansoni, E.; Amaducci, E.; Broglia, C.M.; Alquati, S.; Garetto, F.; et al. Early Palliative Care Italian Study Group (EPCISG) Systematic vs. on-demand early palliative care in gastric cancer patients: A randomized clinical trial assessing patient and healthcare service outcomes. Support. Care Cancer 2019, 27, 2425–2434. [Google Scholar] [CrossRef]
- Tattersall, M.H.N.; Martin, A.; Devine, R.; Ryan, J.; Jansen, J.; Hastings, L.; Boyer, M.; Glare, P.; Stockler, M.; Butow, P. Early Contact with Palliative Care Services: A Randomized Trial in Patients with Newly Detected Incurable Metastatic Cancer. J. Palliat. Care Med. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Temel, J.S.; Sloan, J.; Zemla, T.; Greer, J.A.; Jackson, V.A.; El-Jawahri, A.; Kamdar, M.; Kamal, A.; Blinderman, C.D.; Strand, J.; et al. Multisite, Randomized Trial of Early Integrated Palliative and Oncology Care in Patients with Advanced Lung and Gastrointestinal Cancer: Alliance A221303. J. Palliat. Med. 2020, 23, 922–929. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; El-Jawahri, A.; Pirl, W.F.; Park, E.R.; Jackson, V.A.; Back, A.L.; Kamdar, M.; Jacobsen, J.; Chittenden, E.H.; et al. Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J. Clin. Oncol. 2017, 35, 834–841. [Google Scholar] [CrossRef]
- Vanbutsele, G.; Pardon, K.; Van Belle, S.; Surmont, V.; De Laat, M.; Colman, R.; Eecloo, K.; Cocquyt, V.; Geboes, K.; Deliens, L. Effect of early and systematic integration of palliative care in patients with advanced cancer: A randomised controlled trial. Lancet Oncol. 2018, 19, 394–404. [Google Scholar] [CrossRef]
- Vanbutsele, G.; Van Belle, S.; Surmont, V.; De Laat, M.; Colman, R.; Eecloo, K.; Naert, E.; De Man, M.; Geboes, K.; Deliens, L.; et al. The effect of early and systematic integration of palliative care in oncology on quality of life and health care use near the end of life: A randomised controlled trial. Eur. J. Cancer 2020, 124, 186–193. [Google Scholar] [CrossRef]
- Wallen, G.R.; Baker, K.; Stolar, M.; Miller-Davis, C.; Ames, N.; Yates, J.; Bolle, J.; Pereira, D.; St Germain, D.; Handel, D.; et al. Palliative care outcomes in surgical oncology patients with advanced malignancies: A mixed methods approach. Qual. Life Res. 2012, 21, 405–415. [Google Scholar] [CrossRef]
- Woo, S.M.; Song, M.K.; Lee, M.; Joo, J.; Kim, D.H.; Kim, J.-H.; Han, S.-S.; Park, S.-J.; Kim, T.H.; Lee, W.J. Effect of Early Management on Pain and Depression in Patients with Pancreatobiliary Cancer: A Randomized Clinical Trial. Cancers 2019, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Schenker, Y.; Bahary, N.; Claxton, R.; Childers, J.; Chu, E.; Kavalieratos, D.; King, L.; Lembersky, B.; Tiver, G.; Arnold, R.M. A Pilot Trial of Early Specialty Palliative Care for Patients with Advanced Pancreatic Cancer: Challenges Encountered and Lessons Learned. J. Palliat. Med. 2018, 21, 28–36. [Google Scholar] [CrossRef]
- Zhuang, H.; Ma, Y.; Wang, L.; Zhang, H. Effect of early palliative care on quality of life in patients with non-small-cell lung cancer. Curr. Oncol. 2018, 25, e54–e58. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Hannon, B.; Leighl, N.; Oza, A.; Moore, M.; Rydall, A.; Rodin, G.; Tannock, I.; et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 2014, 383, 1721–1730. [Google Scholar] [CrossRef]
- Zimmermann, C.; Pope, A.; Hannon, B.; Bedard, P.L.; Rodin, G.; Dhani, N.; Li, M.; Herx, L.; Krzyzanowska, M.K.; Howell, D.; et al. Symptom screening with Targeted Early Palliative care (STEP) versus usual care for patients with advanced cancer: A mixed methods study. Support. Care Cancer 2023, 31, 404. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, C.-S.; Chen, X.-Z.; Hu, G.-Q.; Cheng, Z.-B.; Sun, Y.; Li, W.-X.; Chen, Y.-Y.; Xie, F.-Y.; Liang, S.-B.; et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase 3 multicentre randomised controlled trial. Eur. J. Cancer 2017, 75, 150–158. [Google Scholar] [CrossRef]
- Blome, C.; Augustin, M. Measuring change in quality of life: Bias in prospective and retrospective evaluation. Value Health 2015, 18, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, L.A.; Jones, K.F.; El-Jawahri, A.; Sanders, J.; Greer, J.A.; Temel, J.S. Why and How to Integrate Early Palliative Care Into Cutting-Edge Personalized Cancer Care. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e100038. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, D.; Cherny, N.I.; ESMO Guidelines Working Group. ESMO Clinical Practice Guidelines on palliative care: Advanced care planning. Ann. Oncol. 2014, 25 (Suppl. 3), iii138–iii142. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Cancer Type | Stage | Definition of Early PC | Definition of Delayed PC | Assessed Symptoms | Method for Symptom Assessment | Follow-Up | Effective Intervention? | Results of Findings |
|---|---|---|---|---|---|---|---|---|---|
| Adenis et al., 2024 [11] | Upper GI cancers | Metastatic | PC integrated with oncology for 24 weeks | SOC alone | QoL, anxiety, depression | EORTC QLQ-C30, HADS | 24 weeks | No | No difference in QoL or survival |
| Allende et al., 2024 [12] | NSCLC | Metastatic | Programmed visits to meet with the palliative, nutrition, and psychological care specialists at baseline and after the 2nd, 4th, and 6th cycles | PC assessment on request | QoL, physical, functional, emotional, social, lung symptom, anxiety, depression, survival | EORTC-QLQ-C30, HADS, ESAS | baseline, at 2nd, 4th, and 6th cycles of treatment | Yes | Better survival for baseline QoL values > 70, no significant changes in terms of QoL or symptom burden |
| Bakitas et al., 2009 [13] | GI, lung, genitourinary, breast | Unresectable or metastatic | 4 weekly educational sessions (practical, emotional, spiritual, physical, family) + monthly follow-up | Standard oncology care, with access to PC services as needed | QoL, symptom intensity, mood, hospital/ICU visits | FACIT-Pal, ESAS, CES-D | 1-month post-baseline, then every 3 months | Yes | Higher QOL and mood; no significant difference in symptoms or health care utilization |
| Bakitas et al., 2015 [14] | Lung, GI, breast, genitourinary, hematologic, other | Advanced | Initial PC consultation + 6 weekly APN calls + monthly follow-up | Same intervention initiated 3 months later | QoL, symptom impact, mood, survival, hospital use | FACIT-Pal, QUAL-E, CES-D | 1 year | Yes | Improved 1-year survival, numerically fewer ED/hospital visits, not statistically significant |
| Brims et al., 2019 [15] | Pleural mesothelioma | Advanced | PC physician visit within 3 weeks; follow-up every 4 weeks for 24 weeks | Standard care; PC on request | QoL, symptom burden, mood, satisfaction | EORTC QLQ-C30 | 12 and 24 weeks | No | No difference between groups; preference for intervention noted |
| Carmo et al., 2017 [16] | Various solid tumors | Metastatic | Visits every 3 weeks post-randomization | Usual oncology care only | Depression, anxiety | PHQ-9, HADS | 12 weeks | Yes | Reduced depression and anxiety |
| Celis et al., 2021 [17] | Various solid tumors | Advanced/metastatic | Patient navigation from baseline including multidisciplinary support | Usual oncological care with referral-based access to PC | Pain, fatigue, sleep disturbance, depression/anxiety | FACT-G, PHQ-2, G8, symptom-specific validated tools | 12 weeks | Yes | Increased implementation of supportive care, more AD completion, less pain |
| Chen et al., 2021 [18] | Various solid tumors | Stage IV | Monthly PC and psychosocial support | Usual care | Depression, anxiety, QoL | HADS, FACT-G, ESAS | 12 weeks | Yes | Improved depression, anxiety, QoL |
| Chvetzoff et al., 2022 [19] | Various solid tumors | Advanced/metastatic | Monthly PC clinic + symptom monitoring | Usual care | QoL, mood, satisfaction | EORTC, HADS, satisfaction scale | 12 weeks | Yes | Improved QoL and patient engagement |
| Collins et al., 2022 [20] | Advanced breast, prostate, and brain | Advanced/metastatic | STEP Care: triggered referral at defined disease-specific transition points, min. monthly PC consultations for 3 months | Usual best practice cancer care; PC on referral | QoL, symptom distress, mood, illness understanding, service use | Patient/caregiver-reported outcomes, feasibility metrics, survival follow-up | Up to 36 months (brain); 24 months (breast, prostate) | Feasibility study | Trigger-based early PC was feasible in brain cancer but not breast/prostate; standardized triggers aid equity/access |
| Cusimano et al., 2023 [21] | Various solid tumors | Stage II–IV | PC within 4 weeks of enrollment | Usual care | QoL, symptom burden | FACT-O, PHQ-9 | 8 weeks | Yes | Feasible, acceptable, improved support |
| Dey et al., 2023 [22] | Cervical cancer | Locally advanced (IB2 to IIIB) | PC integrated from the start of chemoradiotherapy | Standard chemoradiotherapy alone | QoL subscales: physical, emotional, social, functional | FACT-G | 3 months after treatment completion | Yes | Improved social and emotional well-being in intervention arm |
| Dyar et al., 2012 [23] | Various solid tumors | Advanced | Structured inpatient PC intervention | Usual care | Pain, fatigue, dyspnea | BPI, ESAS | 6 weeks | Yes | Greater symptom improvement |
| El-Jawahri et al., 2016 [24] | Hematologic malignancies | Advanced | Initiated at hospital admission, frequent PC | Standard transplant care | QoL, depression, anxiety | HADS, FACT-BMT | 100 days | Yes | Improved QOL and depression |
| Eychmüller et al., 2021 [25] | Various solid tumors | Advanced | Single structured PC session after randomization | Standard oncology care only | Distress, health-related QoL | NCCN distress thermometer, FACT-G | 6 months | No | No significant differences in distress or QoL |
| Franciosi et al., 2019 [26] | NSCLC, gastric, pancreatic, biliary tract | Advanced | Systematic PC visits by physician/nurse team from baseline | PC on request | QoL | FACT-G | 12 weeks | No | No significant improvement in QoL |
| Greer et al., 2022 [28] | Breast cancer | Advanced/metastatic | Monthly PC sessions from baseline | Standard oncology care | Depression, anxiety, coping | HADS, PHQ-9, PEACE Scale | 24 weeks | Yes | Improved coping and emotional QOL |
| Greer et al., 2016 [27] | NSCLC | Advanced | Integrated outpatient PC from diagnosis | Standard oncology care only | QoL, healthcare costs | Cost analysis, clinical outcomes | Until death | Yes | Decreased costs |
| Groenvold et al., 2017 [29] | Various solid tumors | Advanced | Specialist PC team referral based on needs screening | Standard care | Primary need symptom score, QoL | EORTC QLQ-C30 | 3 and 8 weeks | No | No significant effect on primary or secondary outcomes |
| Johnsen et al., 2019 [30] | Various solid tumors | Advanced | PC consult + structured multidisciplinary follow-up | Usual oncology care only | QoL, mood, nausea | EORTC QLQ-C30, HADS | 8 weeks | No | Slight nausea improvement only |
| Kang et al., 2024 [31] | Various solid tumors | Stage IV | Monthly PC + telecoaching from baseline | Usual oncology care | QoL, function | EORTC QLQ-C15-PAL, MQOL, SAT-SF | 24 weeks | Yes | Improved emotional and physical functioning |
| Maltoni et al., 2016 [32] | Gastric and pancreatic cancers | Advanced | PC initiated at diagnosis, with monthly follow-up | PC on request | QoL, pain, nausea, anxiety, depression | EORTC QLQ-C15-PAL, HADS | 3 months | Yes | Improved QoL and mood |
| Nottelmann et al., 2021 [33] | Various solid tumors | Advanced | PC within 2 weeks of diagnosis | Standard oncology care | QoL, symptom needs | EORTC QLQ-C30 | 12 weeks | No | No significant difference |
| Odom et al., 2019 [34] | Various solid tumors | Advanced | ENABLE: structured telehealth coaching by nurse for caregivers | Usual support | Caregiver QoL, depression, burden | CES-D, Zarit Burden Interview, CQOLC | 24 weeks | Yes | Improved caregiver QoL and lower depression |
| Patil et al., 2021 [36] | Head and neck cancers | Locally advanced/metastatic | PC initiated before first chemotherapy cycle | Usual care | QoL, symptom burden | EORTC QLQ-C30 | 12 weeks | Yes | Significant improvement in QoL, reduction is hospital visits, not significant |
| Rodin et al., 2022 [37] | Various solid tumors | Advanced | PC from baseline stratified by symptom burden | PC upon request | QoL, symptom distress | ESAS, FAMCARE, FACT-G | 12 weeks | Yes | Improved satisfaction and QOL in high-symptom group |
| Scarpi et al., 2023 [38] | Gastric cancer | Advanced | PC within 2 weeks of enrollment; 2–4 week follow-up | On request | QoL, anxiety, depression | FACT-Ga, HADS | 12 weeks | Yes | Improved psychological outcomes, fewer ED/hospital visits, not significant |
| Slama et al., 2021 [35] | Various solid tumors | Advanced | PC every 6–8 weeks from inclusion | Standard oncology care | QoL, anxiety, depression | EORTC QLQ-C30, HADS | 6 months | No | No significant difference in primary outcomes |
| Tattersall et al., 2014 [39] | Various solid tumors | Advanced | Structured question prompt list and early referral to PC team | Usual care | QoL, communication, unmet needs | POS consultation ratings | 1 month | Yes | Improved communication and satisfaction |
| Temel et al., 2010 [41] | NSCLC | Metastatic | Initial PC within 3 weeks, then monthly | Standard oncology care | QoL, depression, survival | FACT-L, PHQ-9 | 12 weeks | Yes | Improved QoL, less depression, longer survival |
| Temel et al., 2017 [42] | GI and lung cancers | Advanced | PC within 4 weeks, monthly follow-up | Standard oncology care | QoL, mood | HADS, FACT-G, PHQ-9 | 24 weeks | Yes | Improved QOL and mood in lung subgroup, no significant reduction in ED visits or hospitalizations |
| Temel et al., 2020 [40] | GI and lung cancers | Advanced | Monthly structured visits with PC clinician | Usual oncology care only | QoL, coping, communication | FAMCARE, HADS, FACT-G | 24 weeks | Yes | Improved coping, communication |
| Vanbutsele et al., 2018 [43] | Various solid tumors | Advanced | Structured monthly PC from enrollment | Usual care only | QoL, emotional well-being, satisfaction | EORTC QLQ-C30, CANHELP Lite | 12 weeks | Yes | Improved emotional functioning and satisfaction |
| Vanbutsele et al., 2020 [44] | Various solid tumors | Advanced | Same model as 2018: early and systematic integration of PC with monthly visits | PC on request | QoL near end of life, healthcare use | EORTC QLQ-C30, McGill QOL | Until death | Yes | Better QOL scores 6, 3, and 1 month before death |
| Wallen et al., 2012 [45] | Surgical oncology patients | Advanced | Hospital-based PPCS starting post-surgery with interdisciplinary support | Standard surgical/oncologic care | Pain, symptom distress, mood, support, satisfaction | Gracely scales, Symptom Distress Scale, interviews | Up to 12 months | Yes (longer term) | Improved satisfaction, communication, symptom perception |
| Woo et al., 2019 [46] | Pancreatobiliary cancers | Advanced/metastatic | PC initiated within 8 weeks of diagnosis | Phone advice only | Pain, depression | BPI, CES-D | 12 months | Yes | Improved pain control |
| Yael Schenker et al., 2023 [47] | GI, breast, lung | Advanced | Telehealth visits biweekly then monthly | PC on request | QoL, communication, emotional support | Caregiver CANHELP, PHQ-9, BPI | 16 weeks | Yes | Improved caregiver satisfaction |
| Zhuang et al., 2018 [48] | NSCLC | Metastatic | Monthly visits from palliative team using standardized PC framework | Conventional oncology treatment only | QoL, depression, anxiety, pulmonary function | QOL Scale, HADS, PHQ-9, Pulmonary function indices | 12 weeks | Yes | Improved QOL, mood, and lung function in early PC group |
| Zimmermann et al., 2014 [49] | Lung, GI, breast, others | Advanced/metastatic | PC visit within 4 weeks of enrollment; monthly thereafter | Usual care; PC if referred | Depression, anxiety, QoL | FACIT-Sp, QUAL-E, ESAS | 4 months | Yes | Improved QoL, symptoms, patient satisfaction, trend toward fewer ED visits, not significant |
| Zimmermann et al., 2023 [50] | Various solid tumors | Advanced | Scheduled monthly PC visit from baseline | Usual care; no structured PC | Distress, QoL | ESAS-r, FACT-G | 6 months | Yes | Lower symptom distress and better communication, no significant effect on ED visits or hospitalizations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creangă-Murariu, I.; Froicu, E.-M.; Scripcariu, D.V.; Bacoanu, G.; Poroch, M.; Moscalu, M.; Tarniceriu, C.C.; Alexa-Stratulat, T.; Poroch, V. Timing Matters: A Systematic Review of Early Versus Delayed Palliative Care in Advanced Cancer. Cancers 2025, 17, 2598. https://doi.org/10.3390/cancers17152598
Creangă-Murariu I, Froicu E-M, Scripcariu DV, Bacoanu G, Poroch M, Moscalu M, Tarniceriu CC, Alexa-Stratulat T, Poroch V. Timing Matters: A Systematic Review of Early Versus Delayed Palliative Care in Advanced Cancer. Cancers. 2025; 17(15):2598. https://doi.org/10.3390/cancers17152598
Chicago/Turabian StyleCreangă-Murariu, Ioana, Eliza-Maria Froicu, Dragos Viorel Scripcariu, Gema Bacoanu, Mihaela Poroch, Mihaela Moscalu, Claudia Cristina Tarniceriu, Teodora Alexa-Stratulat, and Vladimir Poroch. 2025. "Timing Matters: A Systematic Review of Early Versus Delayed Palliative Care in Advanced Cancer" Cancers 17, no. 15: 2598. https://doi.org/10.3390/cancers17152598
APA StyleCreangă-Murariu, I., Froicu, E.-M., Scripcariu, D. V., Bacoanu, G., Poroch, M., Moscalu, M., Tarniceriu, C. C., Alexa-Stratulat, T., & Poroch, V. (2025). Timing Matters: A Systematic Review of Early Versus Delayed Palliative Care in Advanced Cancer. Cancers, 17(15), 2598. https://doi.org/10.3390/cancers17152598






