Analysis of Risk Factors for High-Risk Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
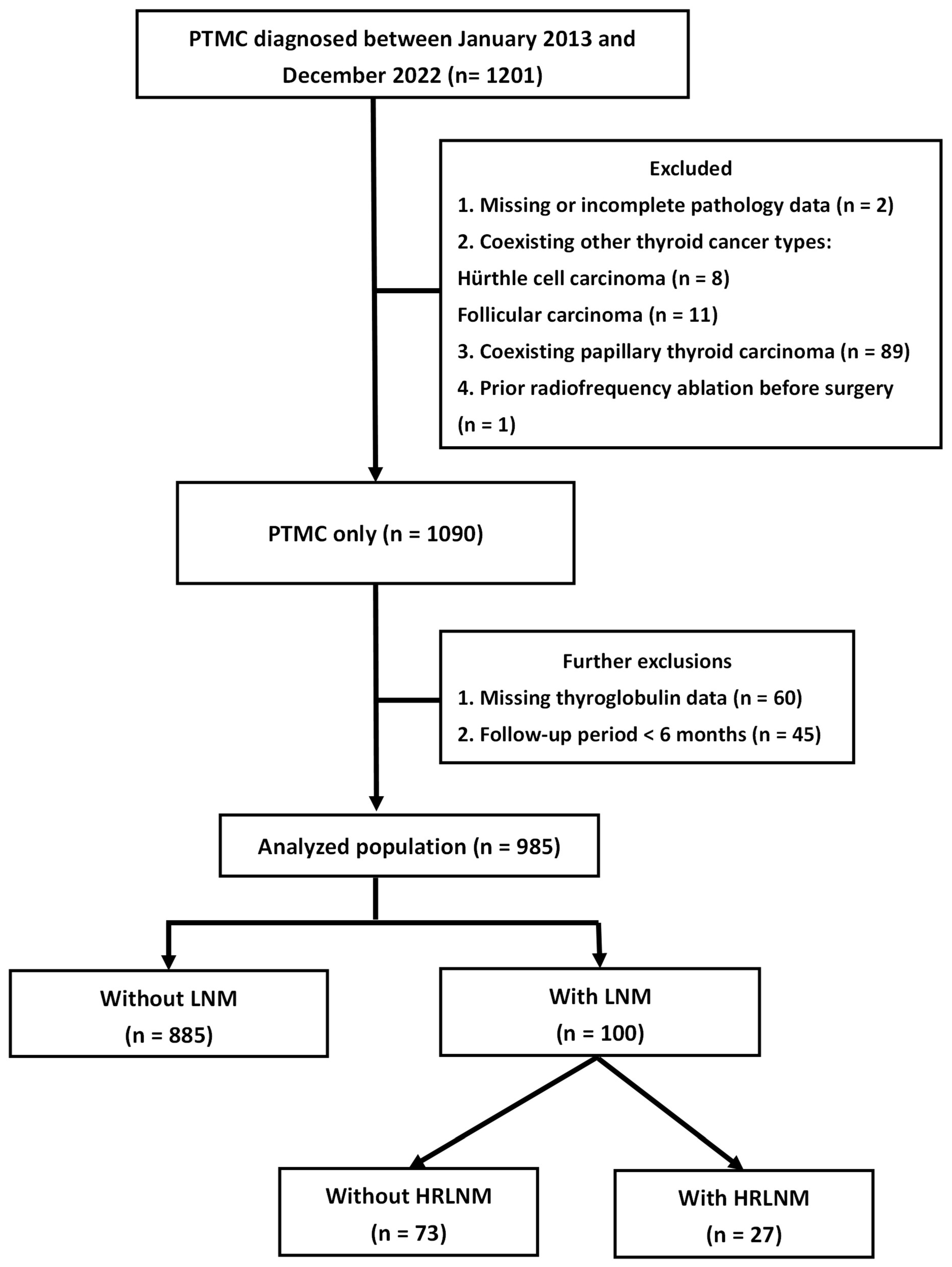
2.1. Patient Recruitment
2.2. Treatment Protocol
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics by Lymph Node Status
3.2. Patient Characteristics by Anatomical Location of the Lymph Node Metastases
3.3. Patient Characteristics by High-Risk Lymph Metastasis
3.4. Factors Associated with High-Risk Lymph Metastasis
3.5. Recurrence-Free Survival Based on Lymph Node Metastasis and High-Risk Lymph Node Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTMC | Papillary thyroid microcarcinoma |
| PTC | Papillary thyroid carcinoma |
| HRLNM | High-risk lymph node metastasis |
| LNM | Lymph node metastasis |
| RFS | Recurrence-free survival |
| SPSS | Statistical Package for the Social Sciences |
| WHO | World Health Organization |
| ETE | Extrathyroidal extension |
| ATA | American Thyroid Association |
| BRAF | V-Raf murine sarcoma viral oncogene homolog B1 |
| OR | Odds ratio |
| ENE | Extranodal extension |
| AJCC-TNM | American Joint Committee on Cancer-Tumor-Node-Metastasis |
| RAI | Radioactive Iodine |
| AS | Active surveillance |
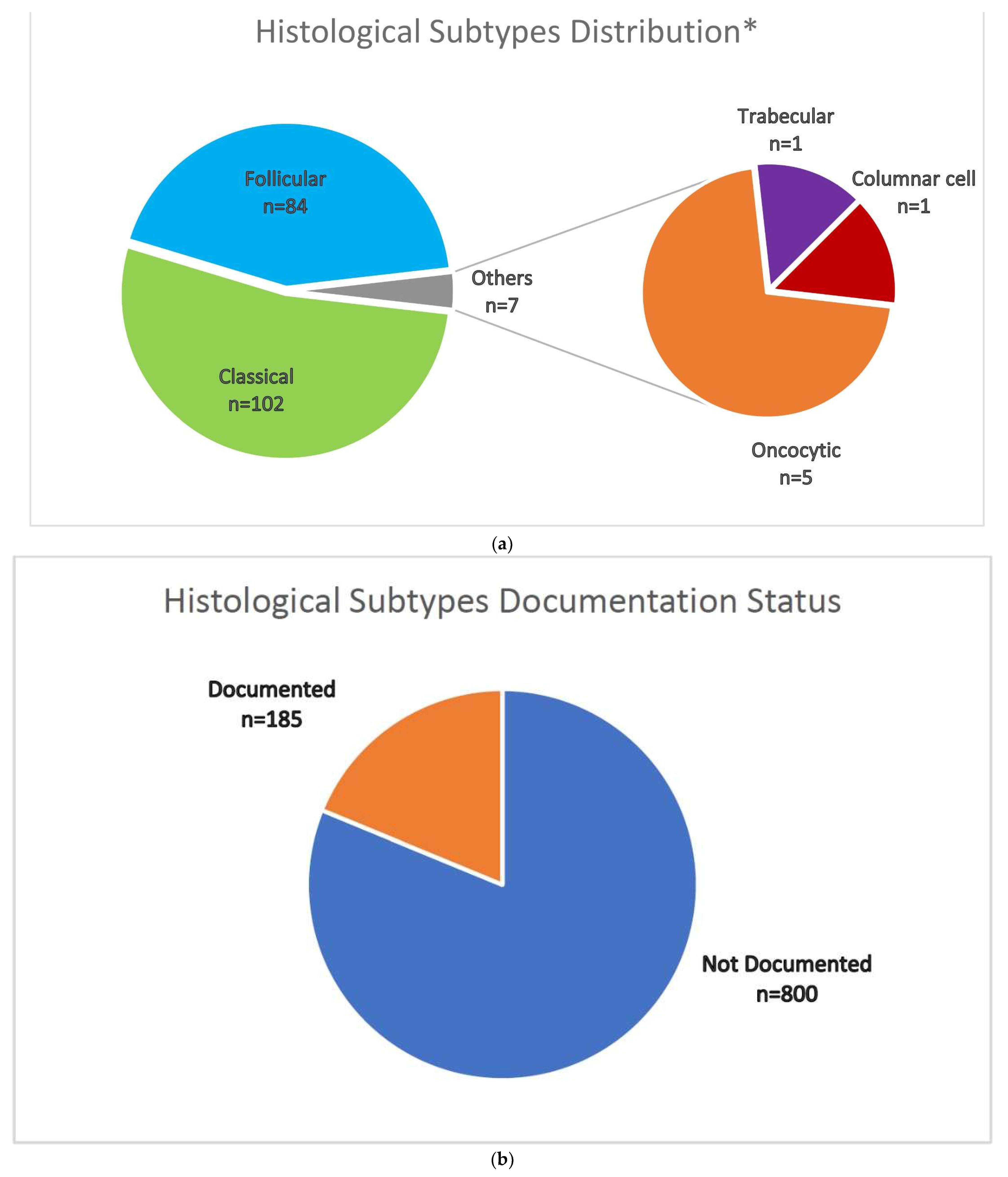
Appendix A
Treatment Protocol
Appendix B
References
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simoes, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Didehban, S.; Abdollahi, A.; Meysamie, A. Evaluation of Etiology, Clinical Manifestations, Diagnosis, Follow-up, Histopathology and Prognosis Factors in Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-analysis. Iran J. Pathol. 2023, 18, 380–391. [Google Scholar] [CrossRef]
- Qian, B.; Hu, L.; Zhang, S.; Zhu, J.; Mei, L.; Huang, T.; Qu, X. Comparison of clinicopathological features and prognosis of papillary thyroid carcinoma and microcarcinoma: A population-based propensity score matching analysis. Front. Endocrinol. 2022, 13, 944758. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Ryu, H.J.; Park, H.; Kim, T.H.; Kim, S.W.; Oh, Y.L.; Chung, J.H. Mortality rate and causes of death in papillary thyroid microcarcinoma. Endocrine 2024, 83, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.M.; Wan, Y.; Sippel, R.S.; Chen, H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann. Surg. 2011, 254, 653–660. [Google Scholar] [CrossRef]
- Siddiqui, S.; White, M.G.; Antic, T.; Grogan, R.H.; Angelos, P.; Kaplan, E.L.; Cipriani, N.A. Clinical and Pathologic Predictors of Lymph Node Metastasis and Recurrence in Papillary Thyroid Microcarcinoma. Thyroid 2016, 26, 807–815. [Google Scholar] [CrossRef]
- So, Y.K.; Son, Y.I.; Hong, S.D.; Seo, M.Y.; Baek, C.H.; Jeong, H.S.; Chung, M.K. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: A study of 551 resections. Surgery 2010, 148, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Povoa, A.A.; Teixeira, E.; Bella-Cueto, M.R.; Melo, M.; Oliveira, M.J.; Sobrinho-Simoes, M.; Maciel, J.; Soares, P. Clinicopathological Features as Prognostic Predictors of Poor Outcome in Papillary Thyroid Carcinoma. Cancers 2020, 12, 3186. [Google Scholar] [CrossRef]
- Medas, F.; Canu, G.L.; Boi, F.; Lai, M.L.; Erdas, E.; Calo, P.G. Predictive Factors of Recurrence in Patients with Differentiated Thyroid Carcinoma: A Retrospective Analysis on 579 Patients. Cancers 2019, 11, 1230. [Google Scholar] [CrossRef]
- Bashir, A.A.; El-Zaheri, M.M.; Bashir, A.A.; Fayyad, L.; Obed, A.H.; Alkam, D.; Bashir, A.Y. Papillary Thyroid Microcarcinoma in Thyroid Surgical Practice: Incidental vs. Non-Incidental: A Ten-Year Comparative Study. Cancers 2025, 17, 2029. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Q.; Wang, X.; Liu, W.; Wang, X. Nomogram for Preoperative Estimation of Cervical Lymph Node Metastasis Risk in Papillary Thyroid Microcarcinoma. Front. Endocrinol. 2021, 12, 613974. [Google Scholar] [CrossRef]
- Lee, J.; Song, Y.; Soh, E.Y. Prognostic significance of the number of metastatic lymph nodes to stratify the risk of recurrence. World J. Surg. 2014, 38, 858–862. [Google Scholar] [CrossRef]
- Randolph, G.W.; Duh, Q.Y.; Heller, K.S.; LiVolsi, V.A.; Mandel, S.J.; Steward, D.L.; Tufano, R.P.; Tuttle, R.M.; American Thyroid Association Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal, S. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012, 22, 1144–1152. [Google Scholar] [CrossRef]
- Wu, S.T.; Chi, S.Y.; Wang, P.W.; Chen, Y.N.; Yang, Y.T.; Chen, W.C.; Chen, J.F.; Chou, C.K. Analysis of overall survival in differentiated thyroid cancer patients with double primary malignancy. Kaohsiung J. Med. Sci. 2021, 37, 63–71. [Google Scholar] [CrossRef]
- De Ywata Carvalho, A.; Kohler, H.F.; Gomes, C.C.; Vartanian, J.G.; Kowalski, L.P. Predictive factors of recurrence of papillary thyroid microcarcinomas: Analysis of 2538 patients. Int. Arch. Otorhinolaryngol. 2021, 25, e585–e593. [Google Scholar]
- Oh, H.S.; Park, S.; Kim, M.; Kwon, H.; Song, E.; Sung, T.Y.; Lee, Y.M.; Kim, W.G.; Kim, T.Y.; Shong, Y.K.; et al. Young Age and Male Sex Are Predictors of Large-Volume Central Neck Lymph Node Metastasis in Clinical N0 Papillary Thyroid Microcarcinomas. Thyroid 2017, 27, 1285–1290. [Google Scholar] [CrossRef]
- Kim, K.; Zheng, X.; Kim, J.K.; Lee, C.R.; Kang, S.W.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y. The contributing factors for lateral neck lymph node metastasis in papillary thyroid microcarcinoma (PTMC). Endocrine 2020, 69, 149–156. [Google Scholar] [CrossRef]
- Ruan, J.; Chen, Z.; Chen, S.; Xu, Z.; Wen, L.; Mao, Z.; Shen, J.; Liu, J.; Wang, W. Lateral lymph node metastasis in papillary thyroid microcarcinoma: A study of 5241 follow-up patients. Endocrine 2024, 83, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ding, Y.; Jiang, W.; Li, X. Can Cervical Lymph Node Metastasis Increase the Risk of Distant Metastasis in Papillary Thyroid Carcinoma? Front. Endocrinol. 2022, 13, 917794. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.W.; Lee, J.; Jung, J.H.; Park, H.Y.; Jeong, J.Y.; Park, J.-Y.; Tufano, R.P. Predictive risk factors for recur-rence or metastasis in papillary thyroid cancer. Int. J. Thyroidol. 2020, 13, 111–117. [Google Scholar] [CrossRef]
- Roh, J.L.; Park, J.W.; Jeong, J.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Extranodal extension of lymph node metastasis as a prognostic indicator of recurrence and survival in papillary thyroid carcinoma. J. Surg. Oncol. 2017, 116, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Denaro, N.; Romano, R.; Alfieri, S.; Dolci, A.; Licitra, L.; Nuzzolese, I.; Ghidini, M.; Bareggi, C.; Bertaglia, V.; Solinas, C.; et al. The Tumor Microenvironment and the Estrogen Loop in Thyroid Cancer. Cancers 2023, 15, 2458. [Google Scholar] [CrossRef]
- Yi, J.W.; Kim, S.J.; Kim, J.K.; Seong, C.Y.; Yu, H.W.; Chai, Y.J.; Choi, J.Y.; Lee, K.E. Upregulation of the ESR1 Gene and ESR Ratio (ESR1/ESR2) is Associated with a Worse Prognosis in Papillary Thyroid Carcinoma: The Impact of the Estrogen Receptor alpha/beta Expression on Clinical Outcomes in Papillary Thyroid Carcinoma Patients. Ann. Surg. Oncol. 2017, 24, 3754–3762. [Google Scholar] [CrossRef]
- Miyauchi, A.; Ito, Y.; Fujishima, M.; Miya, A.; Onoda, N.; Kihara, M.; Higashiyama, T.; Masuoka, H.; Kawano, S.; Sasaki, T.; et al. Long-Term Outcomes of Active Surveillance and Immediate Surgery for Adult Patients with Low-Risk Papillary Thyroid Microcarcinoma: 30-Year Experience. Thyroid 2023, 33, 817–825. [Google Scholar] [CrossRef]
- Choi, S.M.; Kim, J.K.; Lee, C.R.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; Kang, S.W. Completion Total Thyroidectomy Is Not Necessary for Papillary Thyroid Microcarcinoma with Occult Central Lymph Node Metastasis: A Long-Term Serial Follow-Up. Cancers 2020, 12, 3032. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Ito, Y.; Takeuchi, D.; Nakayama, H.; Masaki, C.; Shindo, H.; Teshima, M.; Horiguchi, K.; Yoshida, Y.; Kanai, T.; et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 2021, 31, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Horiuchi, K.; Okamoto, T. Patients’ View on the Management of Papillary Thyroid Microcarcinoma: Active Surveillance or Surgery. Thyroid 2020, 30, 681–687. [Google Scholar] [CrossRef]
- Janjua, N.; Wreesmann, V.B. Aggressive differentiated thyroid cancer. Eur. J. Surg. Oncol. 2018, 44, 367–377. [Google Scholar] [CrossRef]
- Nath, M.C.; Erickson, L.A. Aggressive Variants of Papillary Thyroid Carcinoma: Hobnail, Tall Cell, Columnar, and Solid. Adv. Anat. Pathol. 2018, 25, 172–179. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Hutchinson, M.E.; Gonzalez-Losada, T.; McIver, B.; Reinalda, M.E.; Grant, C.S.; Thompson, G.B.; Sebo, T.J.; Goellner, J.R. Papillary thyroid microcarcinoma: A study of 900 cases observed in a 60-year period. Surgery 2008, 144, 980–987; discussion 987–988. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, Y.; Xu, L.; Wen, J.; Chen, G. Exploring risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: Construction of a novel population-based predictive model. BMC Endocr. Disord. 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]


| Clinical Characteristic | LN Metastasis | Without LN Metastasis | p Value |
|---|---|---|---|
| Patient number (%) | 100 (10.2) | 885 (89.8) | |
| Mean age at diagnosis (y) | 47.62 ± 12.93 | 53.33 ± 11.75 | <0.001 |
| Age < 55 years at diagnosis (%) | 66 (66.0) | 448 (50.6) | 0.004 |
| Female (%) | 67 (67.0) | 737 (83.3) | <0.001 |
| Total thyroidectomy (%) | 95 (95.0) | 688 (77.7) | <0.001 |
| Pathological | |||
| Mean tumor size (cm) | 0.6 ± 0.3 | 0.5 ± 0.3 | <0.001 |
| R2 resection (%) | 17 (17.0) | 26 (2.9) | <0.001 |
| Lympho-vascular invasion (%) | 17 (17.0) | 11 (1.2) | <0.001 |
| Extrathyroidal extension (%) | 22 (22.0) | 53 (6.0) | <0.001 |
| Multiplicity (%) | 46 (46.0) | 301 (34.0) | 0.017 |
| Lymphocytic thyroiditis (%) | 16 (16.0) | 151 (17.1) | 0.785 |
| BRAF mutation (%) * | 34 (75.6) | 181 (58.6) | 0.029 |
| Outcome | |||
| TNM stage (AJCC-8th edition) | <0.001 | ||
| Stage I + II (%) | 77 (77.0) | 866 (97.9) | |
| Stage III + IV (%) | 23 (23.0) | 19 (2.1) | |
| Median follow up period after diagnosis (yr)(IQR) | 4.5 (3.1–7.5) | 5.1 (3.2–7.4) | 0.847 |
| Postoperative thyroglobulin (IQR) | 0.49 (0.2–3.72) | 0.5 (0.2–5.73) | 0.26 |
| Received RAI(%) | 86 (86.0) | 90 (10.2) | <0.001 |
| Recurrence (%) | 9 (9.0) | 5 (0.6) | 0.001 |
| Distant metastasis (%) | 2 (2.0) | 0 | 0.01 |
| Overall mortality (%) | 1 (1.0) | 5 (0.6) | 0.475 |
| Treatment response, excellent (%) | 75 (75.0) | 770 (87.0) | 0.001 |
| Location | Central | Lateral | Both | p Value |
|---|---|---|---|---|
| Patient number | 79 | 9 | 12 | |
| Mean age at diagnosis (y) | 46.91 ± 13.52 | 53.33 ± 9.71 | 48 ± 10.39 | 0.371 |
| Age < 55 years at diagnosis (%) | 53 (67.1) | 5 (55.6) | 8 (66.7) | 0.749 |
| Female (%) | 57 (72.2) | 2 (22.2) † | 8 (66.7) | 0.011 |
| Total thyroidectomy (%) | 75 (94.9) | 8 (88.9) | 12 (100) | 0.461 |
| Pathological | ||||
| Number of metastatic lymph nodes (IQR) | 1 (1–3) | 3 (2–4) † | 6.5(3–12) †‡ | <0.001 |
| Macrometastases (%) | 30 (38) | 5 (55.6) | 10 (83.3) † | 0.009 |
| Mean tumor size (cm) | 0.7 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.2 | 0.125 |
| R2 resection (%) | 12 (15.2) | 2 (22.2) | 3 (25) | 0.548 |
| Lympho-vascular invasion (%) | 11 (13.9) | 3 (33.3) | 3 (25) | 0.178 |
| Extrathyroidal extension (%) | 15 (19) | 3 (33.3) | 4 (33.3) | 0.334 |
| Multiplicity (%) | 35 (44.3) | 4 (44.4) | 7 (58.3) | 0.673 |
| Extranodal extension (%) | 12 (15.4) | 2 (22.2) | 7 (58.3) † | 0.006 |
| Lymphocytic thyroiditis (%) | 13 (16.5) | 1 (11.1) | 2 (16.7) | 1 |
| BRAF mutation (%) * | 29 (76.3) | 2 (100) | 3 (60) | 0.767 |
| Outcome | ||||
| TNM stage (AJCC-8th edition) | 0.046 | |||
| Stage I + II (%) | 64 (81) | 4 (44.4) | 9 (75) | |
| Stage III + IV (%) | 15 (19) | 5 (55.6) | 3 (25) | |
| Median follow up period after diagnosis (yr) (IQR) | 4.3 (3.0–7.2) | 8.1 (2.6–9.7) | 5.3 (4.4–7.3) | 0.355 |
| Postoperative thyroglobulin (IQR) | 0.50 (0.2–3.77) | 8.18 (2.63–9.71) | 5.38 (4.41–7.39) | 0.998 |
| Received RAI(%) | 66 (83.5) | 8 (88.9) | 12 (100) | 0.375 |
| Recurrence (%) | 7 (8.9) | 1 (11.1) | 1 (8.3) | 1 |
| Distant metastasis (%) | 1 (1.3) | 1 (11.1) | 0 | 0.09 |
| Overall mortality (%) | 0 | 1 (11.1) | 0 | 0.27 |
| Treatment response, excellent (%) | 59 (74.7) | 7 (77.8) | 9 (75) | 1 |
| Characteristic | HRLNM | Non-HRLNM | p Value |
|---|---|---|---|
| Patient number (%) | 27 (27.0) | 73 (73.0) | |
| Mean age at diagnosis (y) | 47.48 ± 11.59 | 47.68 ± 13.46 | 0.947 |
| Age < 55 years at diagnosis (%) | 19 (70.4) | 47 (64.4) | 0.575 |
| Female (%) | 14 (51.9) | 53 (72.6) | 0.05 |
| Total thyroidectomy (%) | 26 (96.3) | 69 (94.5) | 0.718 |
| Pathological | |||
| Mean tumor size (cm) | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.374 |
| R2 resection (%) | 7 (25.9) | 10 (13.7) | 0.228 |
| Lympho-vascular invasion (%) | 6 (22.2) | 11 (15.1) | 0.387 |
| Extrathyroidal extension (%) | 10 (37.0) | 12 (16.4) | 0.027 |
| Multiplicity (%) | 13 (48.1) | 33 (45.2) | 0.793 |
| Extranodal extension (%) | 10 (37.0) | 11 (15.3) | 0.018 |
| Lymphocytic thyroiditis (%) | 5 (18.5) | 11 (15.1) | 0.76 |
| BRAF mutation (%) * | 7 (77.8) | 27 (75.0) | 0.862 |
| Outcome | |||
| TNM stage (AJCC-8th edition) | 0.135 | ||
| Stage I + II (%) | 18 (66.7) | 59 (80.8) | |
| Stage III + IV (%) | 9 (33.3) | 14 (19.2) | |
| Median follow up period after diagnosis (yr) (IQR) | 5.6 (3.3–8.6) | 4.3 (3.0–7.1) | 0.094 |
| Postoperative thyroglobulin (IQR) | 0.50 (0.20–2.35) | 0.49 (0.20–3.78) | 0.436 |
| Received RAI | 26 (96.3) | 60 (82.2) | 0.104 |
| Recurrence (%) | 5 (18.5) | 4 (5.5) | 0.043 |
| Distant metastasis (%) | 1 (3.7) | 1 (1.4) | 0.469 |
| Overall mortality, n (%) | 1 (3.7) | 0 | 0.27 |
| Treatment response, excellent (%) | 19 (70.4) | 56 (76.7) | 0.516 |
| Variables | Hazard Ratio (95%CI) | p Value |
|---|---|---|
| Age: | ||
| Age < 55 years at diagnosis | 2.87 (0.82–10.11) | 0.1 |
| Sex: | ||
| Female | Reference | |
| Male | 3.61 (1.06–12.25) | 0.04 |
| Tumor size | 0.34 (0.04–2.77) | 0.313 |
| Extrathyroid extension: | ||
| Absent | Reference | |
| Present | 2.16 (0.57–8.16) | 0.255 |
| Lymphocytic thyroiditis: | ||
| Absent | Reference | |
| Present | 3.83 (0.89–16.42) | 0.071 |
| Extranodal extension: | ||
| Absent | Reference | |
| Present | 3.76 (1.04–13.6) | 0.043 |
| BRAF mutation: | ||
| Absent | Reference | |
| Present | 0.32 (0.06–1.89) | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-H.; Wu, S.-T.; Chen, Y.-N.; Chen, W.-C.; Lim, L.-S.; Chiew, Y.E.W.; Kuo, P.-C.; Yang, Y.-C.; Chi, S.-Y.; Chou, C.-K. Analysis of Risk Factors for High-Risk Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Cancers 2025, 17, 2585. https://doi.org/10.3390/cancers17152585
Chiu Y-H, Wu S-T, Chen Y-N, Chen W-C, Lim L-S, Chiew YEW, Kuo P-C, Yang Y-C, Chi S-Y, Chou C-K. Analysis of Risk Factors for High-Risk Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Cancers. 2025; 17(15):2585. https://doi.org/10.3390/cancers17152585
Chicago/Turabian StyleChiu, Yi-Hsiang, Shu-Ting Wu, Yung-Nien Chen, Wen-Chieh Chen, Lay-San Lim, Yvonne Ee Wern Chiew, Ping-Chen Kuo, Ya-Chen Yang, Shun-Yu Chi, and Chen-Kai Chou. 2025. "Analysis of Risk Factors for High-Risk Lymph Node Metastasis in Papillary Thyroid Microcarcinoma" Cancers 17, no. 15: 2585. https://doi.org/10.3390/cancers17152585
APA StyleChiu, Y.-H., Wu, S.-T., Chen, Y.-N., Chen, W.-C., Lim, L.-S., Chiew, Y. E. W., Kuo, P.-C., Yang, Y.-C., Chi, S.-Y., & Chou, C.-K. (2025). Analysis of Risk Factors for High-Risk Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Cancers, 17(15), 2585. https://doi.org/10.3390/cancers17152585






