Modeling the Bone Marrow Microenvironment to Better Understand the Pathogenesis, Progression, and Treatment of Hematological Cancers
Simple Summary
Abstract
1. Hematological Malignancies
2. The Bone Marrow Niche Is a Complex and Heterogeneous Organ
3. Modeling the Bone Marrow Niche
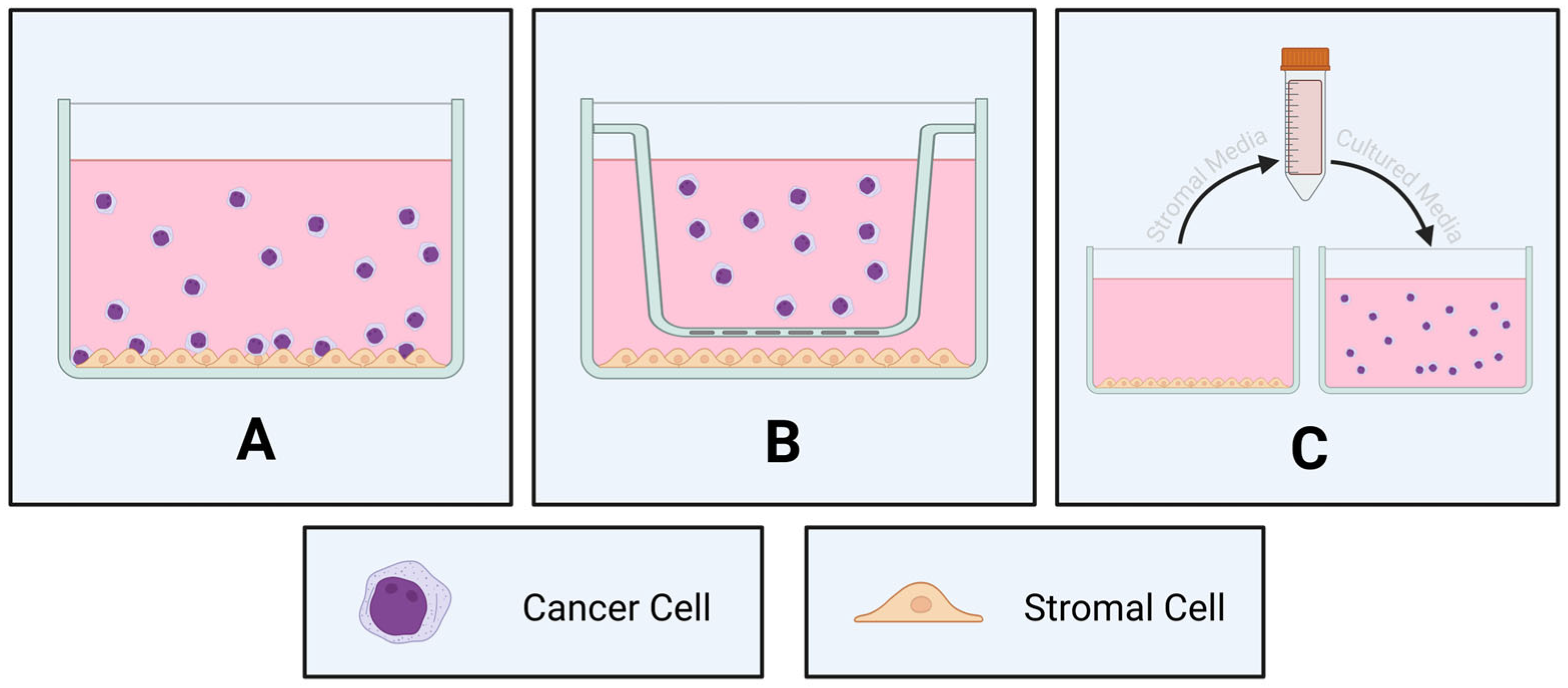
3.1. Two-Dimensional Co-Cultures
3.2. Three-Dimensional Co-Cultures
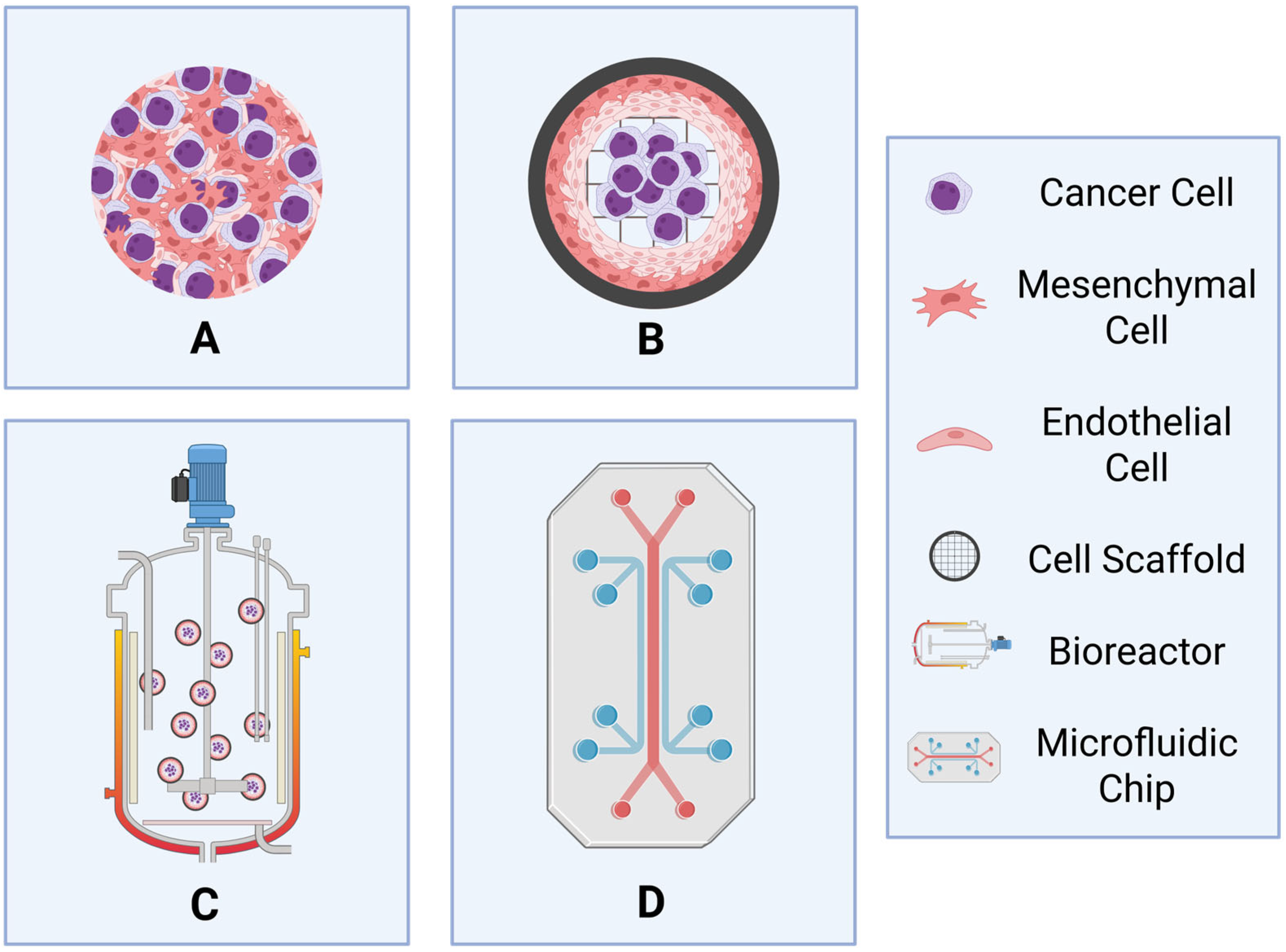
3.2.1. Spheroids and Organoids
3.2.2. Importance of Scaffold Choice
3.2.3. Dynamic 3D Models
4. Important Considerations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| ALL | Acute Lymphoblastic Leukemia |
| AML | Acute Myeloid Leukemia |
| B-ALL | B-cell Acute Lymphoblastic Leukemia |
| CLL | Chronic Lymphocytic Leukemia |
| CML | Chronic Myeloid Leukemia |
| DLBCL | Diffuse Large B-cell Lymphoma |
| ECM | Extracellular Matrix |
| HSC | Hematopoietic Stem Cell |
| iPSC-BMO | Induced Pluripotent Stem-Cell-Derived Bone Marrow Organoids |
| LSC | Leukemia Stem Cell |
| MDS | Myelodysplastic Syndromes |
| MM | Multiple Myeloma |
| MPN | Myeloproliferative Neoplasms |
| MSC | Mesenchymal Stem Cell |
| PDMS | Polydimethylsiloxane |
| T-ALL | T-cell Acute Lymphoblastic Leukemia |
References
- Tietsche de Moraes Hungria, V.; Chiattone, C.; Pavlovsky, M.; Abenoza, L.M.; Agreda, G.P.; Armenta, J.; Arrais, C.; Flores, O.A.; Barroso, F.; Basquiera, A.L.; et al. Epidemiology of Hematologic Malignancies in Real-World Settings: Findings from the Hemato-Oncology Latin America Observational Registry Study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- An, Z.Y.; Fu, H.X.; He, Y.; Zhu, X.L.; Huang, Q.S.; Wu, J.; Liu, K.Y.; Zhang, X.H. Projected Global Trends in Hematological Malignancies: Incidence, Mortality, and Disability-Adjusted Life Years from 2020 to 2030. Blood 2023, 142 (Suppl. S1), 3810. [Google Scholar] [CrossRef]
- Skelding, K.A.; Barry, D.L.; Theron, D.Z.; Lincz, L.F. Bone Marrow Microenvironment as a Source of New Drug Targets for the Treatment of Acute Myeloid Leukaemia. Int. J. Mol. Sci. 2022, 24, 563. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, S.; Nemutlu, E.; Uckan Cetinkaya, D.; Kir, S.; Ozgul, R.K. Characterization of Human Bone Marrow Niches with Metabolome and Transcriptome Profiling. J. Cell Sci. 2021, 134, jcs25072. [Google Scholar] [CrossRef]
- Lo Celso, C.; Fleming, H.E.; Wu, J.W.; Zhao, C.X.; Miake-Lye, S.; Fujisaki, J.; Cote, D.; Rowe, D.W.; Lin, C.P.; Scadden, D.T. Live-Animal Tracking of Individual Haematopoietic Stem/Progenitor Cells in Their Niche. Nature 2009, 457, 92–96. [Google Scholar] [CrossRef]
- Ding, L.; Morrison, S.J. Haematopoietic Stem Cells and Early Lymphoid Progenitors Occupy Distinct Bone Marrow Niches. Nature 2013, 495, 231–235. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct Measurement of Local Oxygen Concentration in the Bone Marrow of Live Animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Alvarez-Martins, I.; Remedio, L.; Matias, I.; Diogo, L.N.; Monteiro, E.C.; Dias, S. The Impact of Chronic Intermittent Hypoxia on Hematopoiesis and the Bone Marrow Microenvironment. Pflugers. Arch. 2016, 468, 919–932. [Google Scholar] [CrossRef]
- Ren, J.; Szombath, G.; Vitale-Cross, L.; Stroncek, D.F.; Robey, P.G.; Hajdara, A.; Szalayova, I.; Mayer, B.; Martin, D.; Mezey, E.; et al. The Potential Use of Thp-1, a Monocytic Leukemia Cell Line, to Predict Immune-Suppressive Potency of Human Bone-Marrow Stromal Cells (Bmscs) in Vitro: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 13258. [Google Scholar] [CrossRef]
- Gonzalez-Lugo, J.D.; Verma, A. Targeting Inflammation in Lower-Risk Mds. Hematology Am. Soc. Hematol. Educ. Program. 2022, 2022, 382–387. [Google Scholar] [CrossRef]
- Mei, Y.; Ren, K.; Liu, Y.; Ma, A.; Xia, Z.; Han, X.; Li, E.; Tariq, H.; Bao, H.; Xie, X.; et al. Bone Marrow-Confined Il-6 Signaling Mediates the Progression of Myelodysplastic Syndromes to Acute Myeloid Leukemia. J. Clin. Investig. 2022, 132, e152673. [Google Scholar] [CrossRef]
- Zavidij, O.; Haradhvala, N.J.; Mouhieddine, T.H.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.K.; et al. Single-Cell Rna Sequencing Reveals Compromised Immune Microenvironment in Precursor Stages of Multiple Myeloma. Nat. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef]
- Dufva, O.; Polonen, P.; Bruck, O.; Keranen, M.A.I.; Klievink, J.; Mehtonen, J.; Huuhtanen, J.; Kumar, A.; Malani, D.; Siitonen, S.; et al. Immunogenomic Landscape of Hematological Malignancies. Cancer Cell 2020, 38, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Aasebo, E.; Brenner, A.K.; Birkeland, E.; Tvedt, T.H.A.; Selheim, F.; Berven, F.S.; Bruserud, O. The Constitutive Extracellular Protein Release by Acute Myeloid Leukemia Cells-a Proteomic Study of Patient Heterogeneity and Its Modulation by Mesenchymal Stromal Cells. Cancers 2021, 13, 1509. [Google Scholar] [CrossRef] [PubMed]
- Bobyleva, P.; Gornostaeva, A.; Andreeva, E.; Ezdakova, M.; Gogiya, B.; Buravkova, L. Reciprocal Modulation of Cell Functions Upon Direct Interaction of Adipose Mesenchymal Stromal and Activated Immune Cells. Cell Biochem. Funct. 2019, 37, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, N.P.; Ruiz-Aparicio, P.F.; Uribe, G.I.; Linares-Ballesteros, A.; Vernot, J.P. Leukemia-Induced Cellular Senescence and Stemness Alterations in Mesenchymal Stem Cells Are Reversible Upon Withdrawal of B-Cell Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 8166. [Google Scholar] [CrossRef]
- Bonilla, X.; Vanegas, N.P.; Vernot, J.P. Acute Leukemia Induces Senescence and Impaired Osteogenic Differentiation in Mesenchymal Stem Cells Endowing Leukemic Cells with Functional Advantages. Stem Cells Int. 2019, 2019, 3864948. [Google Scholar] [CrossRef]
- Abdul-Aziz, A.M.; Sun, Y.; Hellmich, C.; Marlein, C.R.; Mistry, J.; Forde, E.; Piddock, R.E.; Shafat, M.S.; Morfakis, A.; Mehta, T.; et al. Acute Myeloid Leukemia Induces Protumoral P16ink4a-Driven Senescence in the Bone Marrow Microenvironment. Blood 2019, 133, 446–456. [Google Scholar] [CrossRef]
- de Oliveira, T.D.; Vom Stein, A.; Rebollido-Rios, R.; Lobastova, L.; Lettau, M.; Janssen, O.; Wagle, P.; Nguyen, P.H.; Hallek, M.; Hansen, H.P. Stromal Cells Support the Survival of Human Primary Chronic Lymphocytic Leukemia (Cll) Cells through Lyn-Driven Extracellular Vesicles. Front. Med. 2022, 9, 1059028. [Google Scholar] [CrossRef]
- Pan, C.; Hu, T.; Liu, P.; Ma, D.; Cao, S.; Shang, Q.; Zhang, L.; Chen, Q.; Fang, Q.; Wang, J. Bm-Mscs Display Altered Gene Expression Profiles in B-Cell Acute Lymphoblastic Leukemia Niches and Exert Pro-Proliferative Effects Via Overexpression of Ifi6. J. Transl. Med. 2023, 21, 593. [Google Scholar] [CrossRef]
- Vom Stein, A.F.; Rebollido-Rios, R.; Lukas, A.; Koch, M.; von Lom, A.; Reinartz, S.; Bachurski, D.; Rose, F.; Bozek, K.; Abdallah, A.T.; et al. Lyn Kinase Programs Stromal Fibroblasts to Facilitate Leukemic Survival Via Regulation of C-Jun and Thbs1. Nat. Commun. 2023, 14, 1330. [Google Scholar] [CrossRef]
- Haga, C.L.; Boregowda, S.V.; Booker, C.N.; Krishnappa, V.; Strivelli, J.; Cappelli, E.; Phinney, D.G. Mesenchymal Stem/Stromal Cells from a Transplanted, Asymptomatic Patient with Fanconi Anemia Exhibit an Aging-Like Phenotype and Dysregulated Expression of Genes Implicated in Hematopoiesis and Myelodysplasia. Cytotherapy 2023, 25, 362–368. [Google Scholar] [CrossRef]
- Kawano, Y.; Kawano, H.; LaMere, M.W.; LaMere, E.A.; Byun, D.K.; McGrath, K.E.; Palis, J.; Bajaj, J.; Liesveld, J.L.; Katayama, Y.; et al. Il-1r1 and Il-18 Signals Regulate Mesenchymal Stromal Cells in an Aged Murine Model of Myelodysplastic Syndromes. Blood 2025, 145, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Passaro, D.; Garcia-Albornoz, M.; Diana, G.; Chakravarty, P.; Ariza-McNaughton, L.; Batsivari, A.; Borras-Eroles, C.; Abarrategi, A.; Waclawiczek, A.; Ombrato, L.; et al. Integrated Omics Unveil the Bone-Marrow Microenvironment in Human Leukemia. Cell Rep. 2021, 35, 109119. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Williams, D.S.; Bartlett, D.B.; Essex, S.J.; McNee, G.; Allwood, J.W.; Jewell, E.; Barkhuisen, A.; Parry, H.; Anandram, S.; et al. Alterations in Bone Marrow Metabolism Are an Early and Consistent Feature during the Development of Mgus and Multiple Myeloma. Blood Cancer J. 2015, 5, e359. [Google Scholar] [CrossRef] [PubMed]
- Mancek-Keber, M.; Lainscek, D.; Bencina, M.; Chen, J.G.; Romih, R.; Hunter, Z.R.; Treon, S.P.; Jerala, R. Extracellular Vesicle-Mediated Transfer of Constitutively Active Myd88(L265p) Engages Myd88(Wt) and Activates Signaling. Blood 2018, 131, 1720–1729. [Google Scholar] [CrossRef]
- Kfoury, Y.S.; Ji, F.; Jain, E.; Mazzola, M.; Schiroli, G.; Papazian, A.; Mercier, F.; Sykes, D.B.; Kiem, A.; Randolph, M.; et al. The Bone Marrow Stroma in Human Myelodysplastic Syndrome Reveals Alterations That Regulate Disease Progression. Blood Adv. 2023, 7, 6608–6623. [Google Scholar] [CrossRef]
- Mead, A.J.; Mullally, A. Myeloproliferative Neoplasm Stem Cells. Blood 2017, 129, 1607–1616. [Google Scholar] [CrossRef]
- Sandberg, R.; Ernberg, I. The Molecular Portrait of in Vitro Growth by Meta-Analysis of Gene-Expression Profiles. Genome Biol. 2005, 6, R65. [Google Scholar] [CrossRef]
- Ross, D.T.; Scherf, U.; Eisen, M.B.; Perou, C.M.; Rees, C.; Spellman, P.; Iyer, V.; Jeffrey, S.S.; Van de Rijn, M.; Waltham, M.; et al. Systematic Variation in Gene Expression Patterns in Human Cancer Cell Lines. Nat. Genet. 2000, 24, 227–235. [Google Scholar] [CrossRef]
- Le, V.H.; Lee, S.; Lee, S.; Wang, T.; Jang, W.H.; Yoon, Y.; Kwon, S.; Kim, H.; Lee, S.W.; Kim, K.H. In Vivo Longitudinal Visualization of Bone Marrow Engraftment Process in Mouse Calvaria Using Two-Photon Microscopy. Sci. Rep. 2017, 7, 44097. [Google Scholar] [CrossRef]
- Gomariz, A.; Helbling, P.M.; Isringhausen, S.; Suessbier, U.; Becker, A.; Boss, A.; Nagasawa, T.; Paul, G.; Goksel, O.; Szekely, G.; et al. Quantitative Spatial Analysis of Haematopoiesis-Regulating Stromal Cells in the Bone Marrow Microenvironment by 3d Microscopy. Nat. Commun. 2018, 9, 2532. [Google Scholar] [CrossRef] [PubMed]
- Hasenberg, A.; Otto, L.; Gunzer, M. Intravital 2-Photon Microscopy of Diverse Cell Types in the Murine Tibia. Methods Mol. Biol. 2021, 2236, 189–201. [Google Scholar] [PubMed]
- Reinisch, A.; Thomas, D.; Corces, M.R.; Zhang, X.; Gratzinger, D.; Hong, W.J.; Schallmoser, K.; Strunk, D.; Majeti, R. A Humanized Bone Marrow Ossicle Xenotransplantation Model Enables Improved Engraftment of Healthy and Leukemic Human Hematopoietic Cells. Nat. Med. 2016, 22, 812–821. [Google Scholar] [CrossRef]
- Antonelli, A.; Noort, W.A.; Jaques, J.; de Boer, B.; de Jong-Korlaar, R.; Brouwers-Vos, A.Z.; Lubbers-Aalders, L.; van Velzen, J.F.; Bloem, A.C.; Yuan, H.; et al. Establishing Human Leukemia Xenograft Mouse Models by Implanting Human Bone Marrow-Like Scaffold-Based Niches. Blood 2016, 128, 2949–2959. [Google Scholar] [CrossRef]
- Abarrategi, A.; Foster, K.; Hamilton, A.; Mian, S.A.; Passaro, D.; Gribben, J.; Mufti, G.; Bonnet, D. Versatile Humanized Niche Model Enables Study of Normal and Malignant Human Hematopoiesis. J. Clin. Investig. 2017, 127, 543–548. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Klein, T.; Born, G.; Hilpert, M.; Scherberich, A.; Lengerke, C.; Skoda, R.C.; Bourgine, P.E.; Martin, I. Culturing Patient-Derived Malignant Hematopoietic Stem Cells in Engineered and Fully Humanized 3d Niches. Proc. Natl. Acad. Sci. USA 2021, 118, e2114227118. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Dominguez, A.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The Bone Marrow Microenvironment at Single-Cell Resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef]
- Grisolano, J.L.; Wesselschmidt, R.L.; Pelicci, P.G.; Ley, T.J. Altered Myeloid Development and Acute Leukemia in Transgenic Mice Expressing Pml-Rar Alpha under Control of Cathepsin G Regulatory Sequences. Blood 1997, 89, 376–387. [Google Scholar] [CrossRef]
- Tomasson, M.H.; Williams, I.R.; Hasserjian, R.; Udomsakdi, C.; McGrath, S.M.; Schwaller, J.; Druker, B.; Gilliland, D.G. Tel/Pdgfbetar Induces Hematologic Malignancies in Mice That Respond to a Specific Tyrosine Kinase Inhibitor. Blood 1999, 93, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, K.L.; Hetherington, C.J.; Harakawa, N.; Yergeau, D.A.; Zhou, L.; Liu, L.Q.; Little, M.T.; Tenen, D.G.; Zhang, D.E. Analysis of the Role of Aml1-Eto in Leukemogenesis, Using an Inducible Transgenic Mouse Model. Blood 2000, 96, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, R.; Chen, J. Co-Culture Systems of Drug-Treated Acute Myeloid Leukemia Cells and T Cells for in Vitro and in Vivo Study. STAR Protoc. 2020, 1, 100097. [Google Scholar] [CrossRef] [PubMed]
- Farinello, D.; Wozinska, M.; Lenti, E.; Genovese, L.; Bianchessi, S.; Migliori, E.; Sacchetti, N.; di Lillo, A.; Bertilaccio, M.T.S.; de Lalla, C.; et al. A Retinoic Acid-Dependent Stroma-Leukemia Crosstalk Promotes Chronic Lymphocytic Leukemia Progression. Nat. Commun. 2018, 9, 1787. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, W.; Tsao, T.; Qiu, Y.; Zhao, Y.; Samudio, I.; Sarbassov, D.D.; Kornblau, S.M.; Baggerly, K.A.; Kantarjian, H.M.; et al. High-Throughput Profiling of Signaling Networks Identifies Mechanism-Based Combination Therapy to Eliminate Microenvironmental Resistance in Acute Myeloid Leukemia. Haematologica 2017, 102, 1537–1548. [Google Scholar] [CrossRef]
- Smith, A.M.; Dun, M.D.; Lee, E.M.; Harrison, C.; Kahl, R.; Flanagan, H.; Panicker, N.; Mashkani, B.; Don, A.S.; Morris, J.; et al. Activation of Protein Phosphatase 2a in Flt3+ Acute Myeloid Leukemia Cells Enhances the Cytotoxicity of Flt3 Tyrosine Kinase Inhibitors. Oncotarget 2016, 7, 47465–47478. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Li, S.; Li, N.; Chen, Y.; Zhang, B.; Qu, C.; Ding, H.; Huang, J.; Dai, M. Direct Comparison of Five Serum Biomarkers in Early Diagnosis of Hepatocellular Carcinoma. Cancer Manag. Res. 2018, 10, 1947–1958. [Google Scholar] [CrossRef]
- Hou, L.; Liu, T.; Tan, J.; Meng, W.; Deng, L.; Yu, H.; Zou, X.; Wang, Y. Long-Term Culture of Leukemic Bone Marrow Primary Cells in Biomimetic Osteoblast Niche. Int. J. Hematol. 2009, 90, 281–291. [Google Scholar] [CrossRef]
- Bam, R.; Khan, S.; Ling, W.; Randal, S.S.; Li, X.; Barlogie, B.; Edmondson, R.; Yaccoby, S. Primary Myeloma Interaction and Growth in Coculture with Healthy Donor Hematopoietic Bone Marrow. BMC Cancer 2015, 15, 864. [Google Scholar] [CrossRef]
- Binder, M.; Szalat, R.E.; Talluri, S.; Fulciniti, M.; Avet-Loiseau, H.; Parmigiani, G.; Samur, M.K.; Munshi, N.C. Bone Marrow Stromal Cells Induce Chromatin Remodeling in Multiple Myeloma Cells Leading to Transcriptional Changes. Nat. Commun. 2024, 15, 4139. [Google Scholar] [CrossRef]
- Hassan, E.M.; Walker, G.C.; Wang, C.; Zou, S. Anti-Leukemia Effect Associated with down-Regulated Cd47 and up-Regulated Calreticulin by Stimulated Macrophages in Co-Culture. Cancer Immunol. Immunother. 2021, 70, 787–801. [Google Scholar] [CrossRef]
- Nishi, M.; Tateishi, K.; Sundararaj, J.S.; Ino, Y.; Nakai, Y.; Hatayama, Y.; Yamaoka, Y.; Mihana, Y.; Miyakawa, K.; Kimura, H.; et al. Development of a Contacting Transwell Co-Culture System for the in Vitro Propagation of Primary Central Nervous System Lymphoma. Front. Cell Dev. Biol. 2023, 11, 1275519. [Google Scholar] [CrossRef] [PubMed]
- Miari, K.E.; Williams, M.T.S. Stromal Bone Marrow Fibroblasts and Mesenchymal Stem Cells Support Acute Myeloid Leukaemia Cells and Promote Therapy Resistance. Br. J. Pharmacol. 2024, 181, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Delfino, P.; Gatti, A.; Bonato, A.; Kamga, P.T.; Bazzoni, R.; Ugel, S.; Mercuri, A.; Caligola, S.; Krampera, M. Hs-5 and Hs-27a Stromal Cell Lines to Study Bone Marrow Mesenchymal Stromal Cell-Mediated Support to Cancer Development. Front. Cell Dev. Biol. 2020, 8, 584232. [Google Scholar] [CrossRef]
- Narazaki, A.; Shimizu, R.; Yoshihara, T.; Kikuta, J.; Sakaguchi, R.; Tobita, S.; Mori, Y.; Ishii, M.; Nishikawa, K. Determination of the Physiological Range of Oxygen Tension in Bone Marrow Monocytes Using Two-Photon Phosphorescence Lifetime Imaging Microscopy. Sci. Rep. 2022, 12, 3497. [Google Scholar] [CrossRef]
- Zhu, J.; Guerineau, H.; Lefebvre-Fortane, A.M.; Largeaud, L.; Lambert, J.; Rousselot, P.; Boudouin, M.; Calvo, J.; Prost, S.; Clauser, S.; et al. The Axl Inhibitor Bemcentinib Overcomes Microenvironment-Mediated Resistance to Pioglitazone in Acute Myeloid Leukemia. FEBS J. 2025, 292, 115–128. [Google Scholar] [CrossRef]
- Garrido, S.M.; Appelbaum, F.R.; Willman, C.L.; Banker, D.E. Acute Myeloid Leukemia Cells Are Protected from Spontaneous and Drug-Induced Apoptosis by Direct Contact with a Human Bone Marrow Stromal Cell Line (Hs-5). Exp. Hematol. 2001, 29, 448–457. [Google Scholar] [CrossRef]
- Konopleva, M.; Konoplev, S.; Hu, W.; Zaritskey, A.Y.; Afanasiev, B.V.; Andreeff, M. Stromal Cells Prevent Apoptosis of Aml Cells by up-Regulation of Anti-Apoptotic Proteins. Leukemia 2002, 16, 1713–1724. [Google Scholar] [CrossRef]
- Liu, H.; Radisky, D.C.; Wang, F.; Bissell, M.J. Polarity and Proliferation Are Controlled by Distinct Signaling Pathways Downstream of Pi3-Kinase in Breast Epithelial Tumor Cells. J. Cell Biol. 2004, 164, 603–612. [Google Scholar] [CrossRef]
- Jaroscak, J.; Goltry, K.; Smith, A.; Waters-Pick, B.; Martin, P.L.; Driscoll, T.A.; Howrey, R.; Chao, N.; Douville, J.; Burhop, S.; et al. Augmentation of Umbilical Cord Blood (Ucb) Transplantation with Ex Vivo-Expanded Ucb Cells: Results of a Phase 1 Trial Using the Aastromreplicell System. Blood 2003, 101, 5061–5067. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found in Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A New Cell-Laden 3d Alginate-Matrigel Hydrogel Resembles Human Breast Cancer Cell Malignant Morphology, Spread and Invasion Capability Observed “in Vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef]
- Qin, L.; Moreno Rueda, L.Y.; Ray, U.; Mahmud, I.; Tan, L.; Lorenzi, P.L.; Liu, S.; Lin, H.; Mery, D.E.; Zhan, F.; et al. Targeting Caseinolytic Mitochondrial Matrix Peptidase, a Novel Contributor to the Pathobiology of High-Risk Multiple Myeloma. Blood 2025, 145, 2614–2629. [Google Scholar] [CrossRef]
- Frenz-Wiessner, S.; Fairley, S.D.; Buser, M.; Goek, I.; Salewskij, K.; Jonsson, G.; Illig, D.; Putlitz, B.Z.; Petersheim, D.; Li, Y.; et al. Generation of Complex Bone Marrow Organoids from Human Induced Pluripotent Stem Cells. Nat. Methods 2024, 21, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.O.; Rodriguez-Romera, A.; Reyat, J.S.; Olijnik, A.A.; Colombo, M.; Wang, G.; Wen, W.X.; Sousos, N.; Murphy, L.C.; Grygielska, B.; et al. Human Bone Marrow Organoids for Disease Modeling, Discovery, and Validation of Therapeutic Targets in Hematologic Malignancies. Cancer Discov. 2023, 13, 364–385. [Google Scholar] [CrossRef] [PubMed]
- Braham, M.V.J.; Minnema, M.C.; Aarts, T.; Sebestyen, Z.; Straetemans, T.; Vyborova, A.; Kuball, J.; Oner, F.C.; Robin, C.; Alblas, J. Cellular Immunotherapy on Primary Multiple Myeloma Expanded in a 3d Bone Marrow Niche Model. Oncoimmunology 2018, 7, e1434465. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, P.; Muz, B.; Gilson, R.C.; Azab, F.; Luderer, M.; King, J.; Achilefu, S.; Vij, R.; Azab, A.K. 3d Tissue-Engineered Bone Marrow as a Novel Model to Study Pathophysiology and Drug Resistance in Multiple Myeloma. Biomaterials 2015, 73, 70–84. [Google Scholar] [CrossRef]
- Britto, L.S.; Balasubramani, D.; Desai, S.; Phillips, P.; Trehan, N.; Cesarman, E.; Koff, J.L.; Singh, A. T Cells Spatially Regulate B Cell Receptor Signaling in Lymphomas through H3k9me3 Modifications. Adv. Healthc. Mater. 2025, 14, e2401192. [Google Scholar] [CrossRef]
- Shah, S.B.; Carlson, C.R.; Lai, K.; Zhong, Z.; Marsico, G.; Lee, K.M.; Velez, N.E.F.; Abeles, E.B.; Allam, M.; Hu, T.; et al. Combinatorial Treatment Rescues Tumour-Microenvironment-Mediated Attenuation of Malt1 Inhibitors in B-Cell Lymphomas. Nat. Mater. 2023, 22, 511–523. [Google Scholar] [CrossRef]
- Vidal-Crespo, A.; Matas-Cespedes, A.; Rodriguez, V.; Rossi, C.; Valero, J.G.; Serrat, N.; Sanjuan-Pla, A.; Menendez, P.; Roue, G.; Lopez-Guillermo, A.; et al. Daratumumab Displays in Vitro and in Vivo Anti-Tumor Activity in Models of B-Cell Non-Hodgkin Lymphoma and Improves Responses to Standard Chemo-Immunotherapy Regimens. Haematologica 2020, 105, 1032–1041. [Google Scholar] [CrossRef]
- Kastenschmidt, J.M.; Schroers-Martin, J.G.; Sworder, B.J.; Sureshchandra, S.; Khodadoust, M.S.; Liu, C.L.; Olsen, M.; Kurtz, D.M.; Diehn, M.; Wagar, L.E.; et al. A Human Lymphoma Organoid Model for Evaluating and Targeting the Follicular Lymphoma Tumor Immune Microenvironment. Cell Stem Cell 2024, 31, 410–420.e4. [Google Scholar] [CrossRef] [PubMed]
- Faria, C.; Gava, F.; Gravelle, P.; Valero, J.G.; Dobano-Lopez, C.; Van Acker, N.; Quelen, C.; Jalowicki, G.; Morin, R.; Rossi, C.; et al. Patient-Derived Lymphoma Spheroids Integrating Immune Tumor Microenvironment as Preclinical Follicular Lymphoma Models for Personalized Medicine. J. Immunother. Cancer 2023, 11, e007156. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Quinones-Perez, M.; Dai, Z.; Juarez, V.M.; Bhatia, E.; Carlson, C.R.; Shah, S.B.; Patel, A.; Fang, Z.; Hu, T.; et al. Human Immune Organoids to Decode B Cell Response in Healthy Donors and Patients with Lymphoma. Nat. Mater. 2025, 24, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, J.; Piazza, M.; Pizzi, M.; Manni, S.; Piazza, F.; Caputo, I.; Cinetto, F.; Pisoni, L.; Trojan, D.; Scarpa, R.; et al. A Bone-Based 3D Scaffold as an in-Vitro Model of Microenvironment-Dlbcl Lymphoma Cell Interaction. Front. Oncol. 2022, 12, 947823. [Google Scholar] [CrossRef]
- Balandran, J.C.; Davila-Velderrain, J.; Sandoval-Cabrera, A.; Zamora-Herrera, G.; Teran-Cerqueda, V.; Garcia-Stivalet, L.A.; Limon-Flores, J.A.; Armenta-Castro, E.; Rodriguez-Martinez, A.; Leon-Chavez, B.A.; et al. Patient-Derived Bone Marrow Spheroids Reveal Leukemia-Initiating Cells Supported by Mesenchymal Hypoxic Niches in Pediatric B-All. Front. Immunol. 2021, 12, 746492. [Google Scholar] [CrossRef]
- Rivera, M.; Lim, C.E.; Jiang, Q. Protocol for in Vitro Co-Culture Assay for Rapid Expansion of Human T Cell Acute Lymphoblastic Leukemia. STAR Protoc. 2024, 5, 103103. [Google Scholar] [CrossRef]
- Barbaglio, F.; Belloni, D.; Scarfo, L.; Sbrana, F.V.; Ponzoni, M.; Bongiovanni, L.; Pavesi, L.; Zambroni, D.; Stamatopoulos, K.; Caiolfa, V.R.; et al. Three-Dimensional Co-Culture Model of Chronic Lymphocytic Leukemia Bone Marrow Microenvironment Predicts Patient-Specific Response to Mobilizing Agents. Haematologica 2021, 106, 2334–2344. [Google Scholar] [CrossRef]
- Ferrarini, M.; Steimberg, N.; Ponzoni, M.; Belloni, D.; Berenzi, A.; Girlanda, S.; Caligaris-Cappio, F.; Mazzoleni, G.; Ferrero, E. Ex-Vivo Dynamic 3-D Culture of Human Tissues in the Rccs Bioreactor Allows the Study of Multiple Myeloma Biology and Response to Therapy. PLoS ONE 2013, 8, e71613. [Google Scholar] [CrossRef]
- Santos Rosalem, G.; Torres, L.A.G.; de Las Casas, E.B.; Mathias, F.A.S.; Ruiz, J.C.; Carvalho, M.G.R. Microfluidics and Organ-on-a-Chip Technologies: A Systematic Review of the Methods Used to Mimic Bone Marrow. PLoS ONE 2020, 15, e0243840. [Google Scholar] [CrossRef]
- David, R.; Gee, S.; Khan, K.; Wilson, A.; Doherty, A. Three Dimensional and Microphysiological Bone Marrow Models Detect in Vivo Positive Compounds. Sci. Rep. 2021, 11, 21959. [Google Scholar] [CrossRef]
- Ma, C.; Witkowski, M.T.; Harris, J.; Dolgalev, I.; Sreeram, S.; Qian, W.; Tong, J.; Chen, X.; Aifantis, I.; Chen, W. Leukemia-on-a-Chip: Dissecting the Chemoresistance Mechanisms in B Cell Acute Lymphoblastic Leukemia Bone Marrow Niche. Sci. Adv. 2020, 6, eaba5536. [Google Scholar] [CrossRef]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas. J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar]
- Fairfield, H.; Falank, C.; Farrell, M.; Vary, C.; Boucher, J.M.; Driscoll, H.; Liaw, L.; Rosen, C.J.; Reagan, M.R. Development of a 3D Bone Marrow Adipose Tissue Model. Bone 2019, 118, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.J.; Kolaja, K.; Cottrell, J.A. A Functional Three-Dimensional Microphysiological Human Model of Myeloma Bone Disease. J. Bone Miner. Res. 2021, 36, 1914–1930. [Google Scholar] [CrossRef] [PubMed]
- Braham, M.V.; Deshantri, A.K.; Minnema, M.C.; Oner, F.C.; Schiffelers, R.M.; Fens, M.H.; Alblas, J. Liposomal Drug Delivery in an in Vitro 3d Bone Marrow Model for Multiple Myeloma. Int. J. Nanomedicine 2018, 13, 8105–8118. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Li, E.; Aydemir, I.; Liu, Y.; Han, X.; Bi, H.; Wang, P.; Tao, K.; Ji, A.; Chen, Y.H.; et al. Development of Ipsc-Derived Human Bone Marrow Organoid for Autonomous Hematopoiesis and Patient-Derived Hspc Engraftment. Blood Adv. 2025, 9, 54–65. [Google Scholar] [CrossRef]
- Rellick, S.L.; Hu, G.; Piktel, D.; Martin, K.H.; Geldenhuys, W.J.; Nair, R.R.; Gibson, L.F. Co-Culture Model of B-Cell Acute Lymphoblastic Leukemia Recapitulates a Transcription Signature of Chemotherapy-Refractory Minimal Residual Disease. Sci. Rep. 2021, 11, 15840. [Google Scholar] [CrossRef]
- Aljitawi, O.S.; Li, D.; Xiao, Y.; Zhang, D.; Ramachandran, K.; Stehno-Bittel, L.; Van Veldhuizen, P.; Lin, T.L.; Kambhampati, S.; Garimella, R. A Novel Three-Dimensional Stromal-Based Model for in Vitro Chemotherapy Sensitivity Testing of Leukemia Cells. Leuk Lymphoma 2014, 55, 378–391. [Google Scholar] [CrossRef]
- Houshmand, M.; Soleimani, M.; Atashi, A.; Saglio, G.; Abdollahi, M.; Zarif, M.N. Mimicking the Acute Myeloid Leukemia Niche for Molecular Study and Drug Screening. Tissue Eng. Part C Methods 2017, 23, 72–85. [Google Scholar] [CrossRef]
- Bray, L.J.; Binner, M.; Korner, Y.; von Bonin, M.; Bornhauser, M.; Werner, C. A Three-Dimensional Ex Vivo Tri-Culture Model Mimics Cell-Cell Interactions between Acute Myeloid Leukemia and the Vascular Niche. Haematologica 2017, 102, 1215–1226. [Google Scholar] [CrossRef]
- Cheung, H.L.; Wong, Y.H.; Li, Y.Y.; Yang, X.; Ko, L.H.; Kabigting, J.E.T.; Chan, K.C.; Leung, A.Y.H.; Chan, B.P. Microenvironment Matters: In Vitro 3D Bone Marrow Niches Differentially Modulate Survival, Phenotype and Drug Responses of Acute Myeloid Leukemia (Aml) Cells. Biomaterials 2025, 312, 122719. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Tian, Y.F.; Ahn, H.; Schneider, R.S.; Yang, S.N.; Roman-Gonzalez, L.; Melnick, A.M.; Cerchietti, L.; Singh, A. Integrin-Specific Hydrogels as Adaptable Tumor Organoids for Malignant B and T Cells. Biomaterials 2015, 73, 110–119. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Jahnen-Dechent, W.; Labude, N.; Bovi, M.; Hieronymus, T.; Zenke, M.; Schneider, R.K.; Neuss, S. Cord Blood-Hematopoietic Stem Cell Expansion in 3D Fibrin Scaffolds with Stromal Support. Biomaterials 2012, 33, 6987–6997. [Google Scholar] [CrossRef]
- Severn, C.E.; Macedo, H.; Eagle, M.J.; Rooney, P.; Mantalaris, A.; Toye, A.M. Polyurethane Scaffolds Seeded with Cd34(+) Cells Maintain Early Stem Cells Whilst Also Facilitating Prolonged Egress of Haematopoietic Progenitors. Sci. Rep. 2016, 6, 32149. [Google Scholar] [CrossRef] [PubMed]
- Tomimori, Y.; Takagi, M.; Yoshida, T. The Construction of an in Vitro Three-Dimensional Hematopoietic Microenvironment for Mouse Bone Marrow Cells Employing Porous Carriers. Cytotechnology 2000, 34, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Raic, A.; Rodling, L.; Kalbacher, H.; Lee-Thedieck, C. Biomimetic Macroporous Peg Hydrogels as 3d Scaffolds for the Multiplication of Human Hematopoietic Stem and Progenitor Cells. Biomaterials 2014, 35, 929–940. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, B.; Wang, X.; Xiao, R.; Wang, C. Three-Dimensional Co-Culture of Mesenchymal Stromal Cells and Differentiated Osteoblasts on Human Bio-Derived Bone Scaffolds Supports Active Multi-Lineage Hematopoiesis in Vitro: Functional Implication of the Biomimetic Hsc Niche. Int. J. Mol. Med. 2016, 38, 1141–1151. [Google Scholar] [CrossRef]
- Leisten, I.; Kramann, R.; Ferreira, M.S.V.; Bovi, M.; Neuss, S.; Ziegler, P.; Wagner, W.; Knuchel, R.; Schneider, R.K. 3D Co-Culture of Hematopoietic Stem and Progenitor Cells and Mesenchymal Stem Cells in Collagen Scaffolds as a Model of the Hematopoietic Niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef]
- Bai, T.; Li, J.; Sinclair, A.; Imren, S.; Merriam, F.; Sun, F.; O’Kelly, M.B.; Nourigat, C.; Jain, P.; Delrow, J.J.; et al. Expansion of Primitive Human Hematopoietic Stem Cells by Culture in a Zwitterionic Hydrogel. Nat. Med. 2019, 25, 1566–1575. [Google Scholar] [CrossRef]
- Bourgine, P.E.; Klein, T.; Paczulla, A.M.; Shimizu, T.; Kunz, L.; Kokkaliaris, K.D.; Coutu, D.L.; Lengerke, C.; Skoda, R.; Schroeder, T.; et al. In Vitro Biomimetic Engineering of a Human Hematopoietic Niche with Functional Properties. Proc. Natl. Acad. Sci. USA 2018, 115, E5688–E5695. [Google Scholar] [CrossRef] [PubMed]
- Krater, M.; Jacobi, A.; Otto, O.; Tietze, S.; Muller, K.; Poitz, D.M.; Palm, S.; Zinna, V.M.; Biehain, U.; Wobus, M.; et al. Bone Marrow Niche-Mimetics Modulate Hspc Function Via Integrin Signaling. Sci. Rep. 2017, 7, 2549. [Google Scholar] [CrossRef] [PubMed]
- Bianco, J.E.R.; Rosa, R.G.; Congrains-Castillo, A.; Joazeiro, P.P.; Waldman, S.D.; Weber, J.F.; Saad, S.T.O. Characterization of a Novel Decellularized Bone Marrow Scaffold as an Inductive Environment for Hematopoietic Stem Cells. Biomater. Sci. 2019, 7, 1516–1528. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Funamoto, S.; Kimura, T.; Nam, K.; Fujisato, T.; Kishida, A. The Effect of Decellularized Bone/Bone Marrow Produced by High-Hydrostatic Pressurization on the Osteogenic Differentiation of Mesenchymal Stem Cells. Biomaterials 2011, 32, 7060–7067. [Google Scholar] [CrossRef]
- Li, D.; Lin, T.L.; Lipe, B.; Hopkins, R.A.; Shinogle, H.; Aljitawi, O.S. A Novel Extracellular Matrix-Based Leukemia Model Supports Leukemia Cells with Stem Cell-Like Characteristics. Leuk Res. 2018, 72, 105–112. [Google Scholar] [CrossRef]
- Zhang, W.; Lee, W.Y.; Siegel, D.S.; Tolias, P.; Zilberberg, J. Patient-Specific 3D Microfluidic Tissue Model for Multiple Myeloma. Tissue Eng. Part C Methods 2014, 20, 663–670. [Google Scholar] [CrossRef]


| Summary of Model | Advantages | Limitations | Uses | References |
|---|---|---|---|---|
| Genetically modified | Physiologically relevant; same species; can examine the role of the immune system and native components in hematological malignancies | Expensive; not necessarily relevant to humans; not suitable for high-throughput drug screening | Pathogenesis; drug sensitivity | [32,39,40,41,42] |
| Xenograft/implantation | May be physiologically relevant (if orthotopic or involving humanized ossicles); can examine role of multiple cell types simultaneously in physiologically relevant situations | Expensive; not necessarily equivalent to humans; cannot examine the role of the immune system; not suitable for high-throughput drug screening | Drug sensitivity; progression | [31,34,35,36,37,43] |
| Summary of Model | Advantages | Limitations | Uses | References |
|---|---|---|---|---|
| Direct co-culture: cancer cells are grown directly on bone marrow stromal cell monolayers | Easy and inexpensive to establish; can study the effect of stromal cells on cancer cells and vice versa; can examine the impact of cell–cell contact; more relevant than 2D single-cell culture; allows the study of homogeneous populations | Does not contain multiple cell types; lacks 3D or anatomical factors associated with the niche; unable to perform downstream assays separately for each cell type; not possible to perform high-throughput assays for drug treatments | Examines cell biology and signaling processes involved in relapse and drug sensitivity (adhesion, migration, proliferation); allows the examination of drug sensitivity | [43,45,46,47,48,49,50] |
| Indirect co-culture: cancer cells are grown indirectly with microenvironment cells separated by a permeable membrane or insert | Easy and inexpensive to establish; can study the effect of microenvironment cells on cancer cells and vice versa; allows the sharing of secreted factors through a permeable membrane; allows the study of homogeneous populations | Lacks cell–cell contact; does not contain multiple cell types; lacks 3D or anatomical factors associated with the niche; not possible to perform high-throughput assays for drug treatments | Examines signaling processes involved in bone marrow microenvironment; allows the examination of drug sensitivity | [51,52] |
| Indirect co-culture (media): cancer or microenvironment cells are grown in conditioned media from another cell type | Easy and inexpensive to establish; can study the effect of secreted factors on different cell types; allows the study of homogeneous populations | Lacks cell–cell contact; does not contain multiple cell types; lacks 3D or anatomical factors associated with the niche | Allows the examination of drug sensitivity; examines the effect of secreted biomolecules on other cell types | [47] |
| Model | Summary of Model | Advantages | Limitations | Uses | References |
|---|---|---|---|---|---|
| Static 3D co-culture | Scaffold-free: cancer and microenvironment cells are grown without a scaffold and are allowed to morph into spheroids/organoids in the absence of an anchor | More physiologically relevant than the 2D model; does not require specialized equipment; can study cell–cell interactions | Does not contain multiple cell types; lacks ECM–cancer cell interactions; time-consuming; grown under static conditions; not suitable for high-throughput drug screening | Examines signaling processes; drug sensitivity | [44,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] |
| Scaffold-based: cancer and microenvironment cells are grown in the presence of a synthetic or biological scaffold and are allowed to grow as spheroids/organoids | More accurately recapitulates the bone marrow microenvironment; can study cell–cell and cell–ECM interactions | Time-consuming; expensive; grown under static conditions; not suitable for high-throughput drug screening | Examines signaling processes; drug sensitivity | ||
| Dynamic 3D co-culture | Bioreactor: uses a 3D bioreactor to grow cancer cells, microenvironment cells, and scaffolds | Can examine multiple cell types; can study cell–cell and cell–ECM interactions; grown under dynamic conditions | Expensive and requires specialized equipment; time-consuming; dynamic growth conditions can disrupt cells or the scaffold architecture; not suitable for high-throughput drug screening | Examines signaling processes; drug sensitivity | [77,78] |
| Microfluidics: uses a 3D bioreactor to grow cancer cells, microenvironment cells, and scaffolds (mimics osteoblastic and vascular niches) | Can examine multiple cell types; can examine multiple niches simultaneously; can study cell–cell and cell–ECM interactions; grown under dynamic conditions | Expensive and requires specialized equipment; time-consuming; dynamic growth conditions can disrupt cells or the scaffold architecture; not suitable for high-throughput drug screening | Model processes involved in progression and relapse; drug sensitivity | [79,80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skelding, K.A.; Barry, D.L.; Lincz, L.F. Modeling the Bone Marrow Microenvironment to Better Understand the Pathogenesis, Progression, and Treatment of Hematological Cancers. Cancers 2025, 17, 2571. https://doi.org/10.3390/cancers17152571
Skelding KA, Barry DL, Lincz LF. Modeling the Bone Marrow Microenvironment to Better Understand the Pathogenesis, Progression, and Treatment of Hematological Cancers. Cancers. 2025; 17(15):2571. https://doi.org/10.3390/cancers17152571
Chicago/Turabian StyleSkelding, Kathryn A., Daniel L. Barry, and Lisa F. Lincz. 2025. "Modeling the Bone Marrow Microenvironment to Better Understand the Pathogenesis, Progression, and Treatment of Hematological Cancers" Cancers 17, no. 15: 2571. https://doi.org/10.3390/cancers17152571
APA StyleSkelding, K. A., Barry, D. L., & Lincz, L. F. (2025). Modeling the Bone Marrow Microenvironment to Better Understand the Pathogenesis, Progression, and Treatment of Hematological Cancers. Cancers, 17(15), 2571. https://doi.org/10.3390/cancers17152571








