Association Between BRAF V600E Allele Frequency and Aggressive Behavior in Papillary Thyroid Microcarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analyses
Variables
2.2. Analysis Methods
3. Results
3.1. General Findings
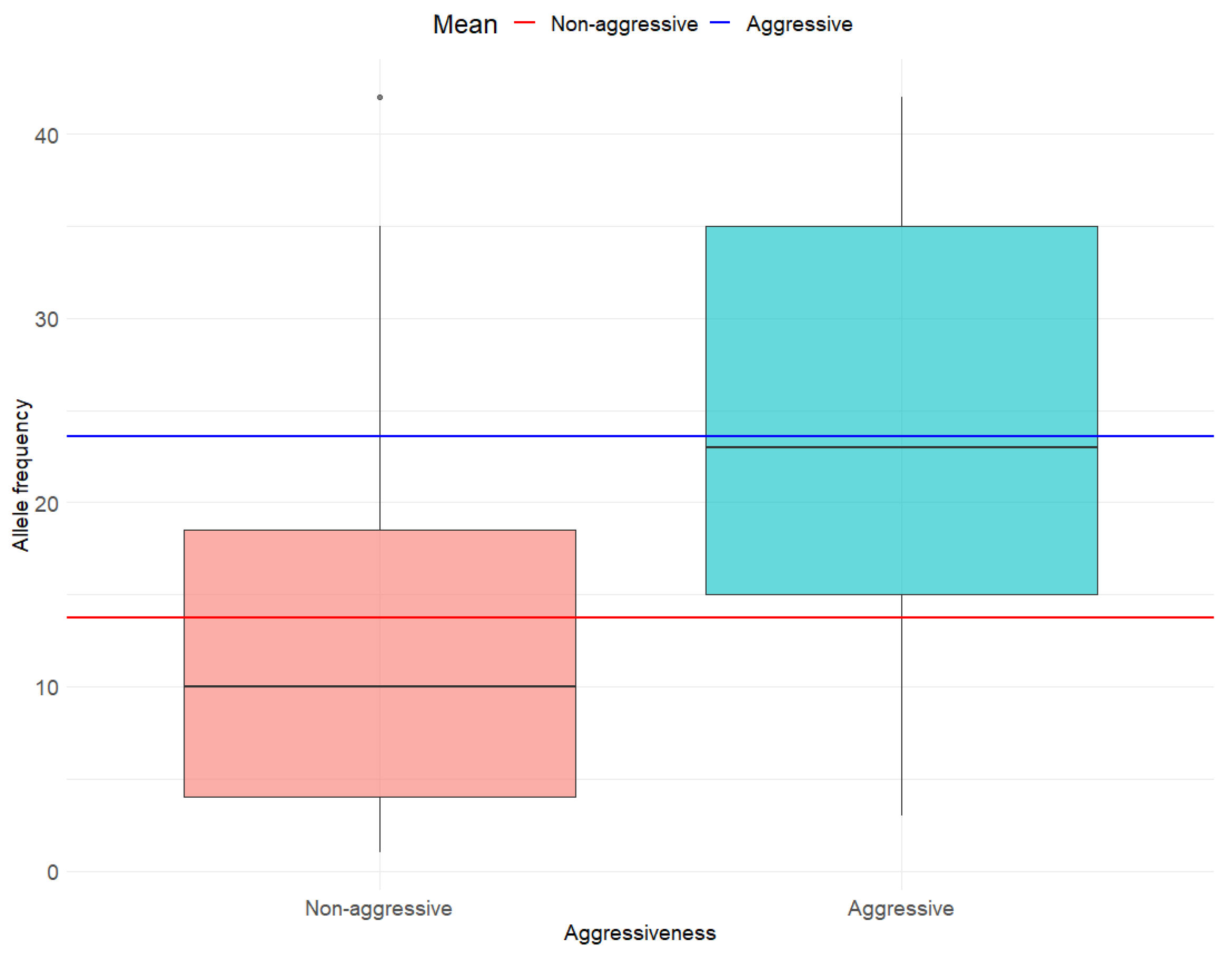
3.2. Association Between BRAF V600E AF and Any Histo-Pathological Determinant of MPTC Tumor Aggressiveness
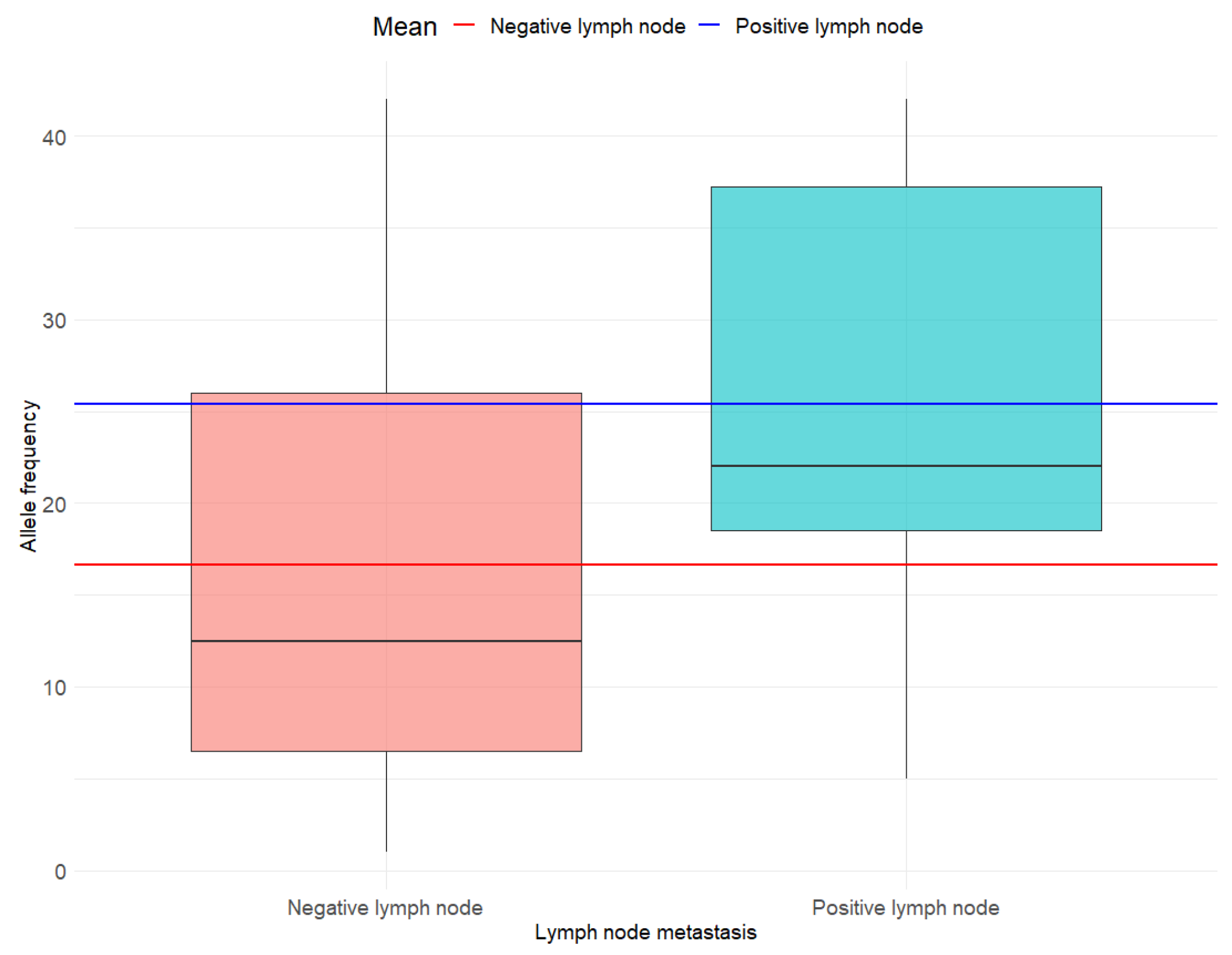
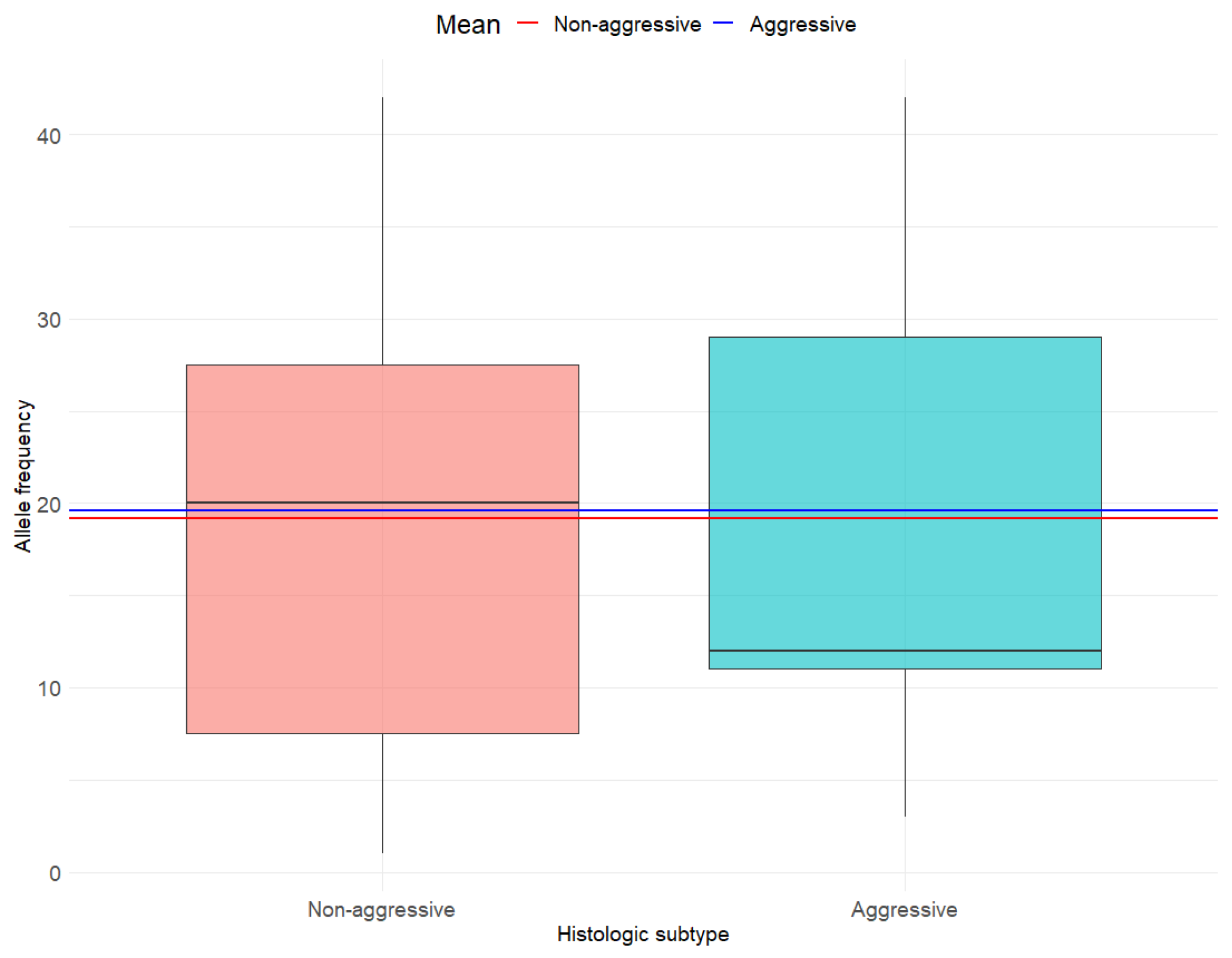
3.3. Associations Between BRAF V600E AF and Individual Histo-Pathological Determinants of MPTC Tumor Aggressiveness
3.4. Supplementary Analyses
4. Discussion
4.1. Aggressivity
4.2. Lymph Node Metastasis
4.3. ETE
4.4. Histological Subtype and Extensive LVI
4.5. Prognosis
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Kartal, K.; Aygun, N.; Uludag, M. Clinicopathologic Differences Between Micropapillary and Papillary Thyroid Carcinoma. Sisli. Etfal. Hastan. Tıp Bul. 2019, 53, 120–124. [Google Scholar] [CrossRef]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef]
- Zalzali, M.; Debreuve, A.; Richard, C.; Filieri, C.; Schvartz, C. Micropapillary carcinoma: Description and rise in incidence in the French Marne-Ardennes thyroid cancer registry. Ann. Endocrinol. 2019, 80, 229–233. [Google Scholar] [CrossRef]
- Lam, A.K. Papillary Thyroid Carcinoma: Current Position in Epidemiology, Genomics, and Classification. Methods Mol. Biol. 2022, 2534, 1–15. [Google Scholar] [CrossRef]
- Chen, X.; Lin, S.; Lin, Y.; Wu, S.; Zhuo, M.; Zhang, A.; Zheng, J.; You, Z. BRAF-activated WT1 contributes to cancer growth and regulates autophagy and apoptosis in papillary thyroid carcinoma. J. Transl. Med. 2022, 20, 79. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, B.; Theurer, S.; Staniszewska, M.; Heupel, J.; Koch, A.; Mergener, S.; Walk, F.; Fischer, C.; Kutritz, A.; Schmid, K.W.; et al. An Animal Model Further Uncovers the Role of Mutant BRAF V600E during Papillary Thyroid Cancer Development. Am. J. Pathol. 2020, 190, 702–710. [Google Scholar] [CrossRef]
- Attia, A.S.; Hussein, M.; Issa, P.P.; Elnahla, A.; Farhoud, A.; Magazine, B.M.; Youssef, M.R.; Aboueisha, M.; Shama, M.; Toraih, E.; et al. Association of BRAF V600E Mutation with the Aggressive Behavior of Papillary Thyroid Microcarcinoma: A Meta-Analysis of 33 Studies. Int. J. Mol. Sci. 2022, 23, 15626. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.A.; Bogatchenko, M.; Pusztaszeri, M.; Forest, V.I.; Hier, M.P.; Yang, J.W.; Tamilia, M.; Payne, R.J. BRAF V600E mutation is associated with aggressive features in papillary thyroid carcinomas ≤ 1.5 cm. J. Otolaryngol. Head Neck Surg. 2021, 50, 63. [Google Scholar] [CrossRef]
- Spyroglou, A.; Kostopoulos, G.; Tseleni, S.; Toulis, K.; Bramis, K.; Mastorakos, G.; Konstadoulakis, M.; Vamvakidis, K.; Alexandraki, K.I. Hobnail Papillary Thyroid Carcinoma, A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2785. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Xiong, J.; Li, C.; Liao, Y.; Zhu, Y.; Mao, J. Risk and Prognostic Factors for BRAF V600E Mutations in Papillary Thyroid Carcinoma. Biomed. Res. Int. 2022, 2022, 9959649. [Google Scholar] [CrossRef]
- Shan, K.S.; Rehman, T.U.; Ivanov, S.; Domingo, G.; Raez, L.E. Molecular Targeting of the BRAF Proto-Oncogene/Mitogen-Activated Protein Kinase (MAPK) Pathway across Cancers. Int. J. Mol. Sci. 2024, 25, 624. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, G.; Sheng, C.; Gusdon, A.M.; Huang, Y.; Lv, Z.; Xu, H.; Xing, M.; Qu, S. BRAF V600E mutation in papillary thyroid microcarcinoma: A meta-analysis. Endocr. Relat. Cancer 2015, 22, 159–168. [Google Scholar] [CrossRef]
- Ren, H.; Shen, Y.; Hu, D.; He, W.; Zhou, J.; Cao, Y.; Mao, Y.; Dou, Y.; Xiong, W.; Xiao, Q.; et al. Co-existence of BRAF V600E and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag. Res. 2018, 10, 1005–1013. [Google Scholar] [CrossRef]
- Abdulhaleem, M.; Bandargal, S.; Pusztaszeri, M.P.; Rajab, M.; Greenspoon, H.; Krasner, J.R.; Da Silva, S.D.; Forest, V.I.; Payne, R.J. The Impact of BRAF V600E Mutation Allele Frequency on the Histopathological Characteristics of Thyroid Cancer. Cancers 2023, 16, 113. [Google Scholar] [CrossRef]
- Tabriz, N.; Grone, J.; Uslar, V.; Tannapfel, A.; Weyhe, D. BRAF V600E mutation correlates with aggressive clinico-pathological features but does not influence tumor recurrence in papillary thyroid carcinoma-10-year single-center results. Gland. Surg. 2020, 9, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Bandoh, N.; Goto, T.; Kato, Y.; Kubota, A.; Sakaue, S.; Takeda, R.; Hayashi, S.; Hayashi, M.; Baba, S.; Yamaguchi-Isochi, T.; et al. BRAF V600E mutation co-existing with oncogenic mutations is associated with aggressive clinicopathologic features and poor prognosis in papillary thyroid carcinoma. Asian J. Surg. 2024, 47, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Macerola, E.; Poma, A.M.; Vignali, P.; Basolo, A.; Ugolini, C.; Torregrossa, L.; Santini, F.; Basolo, F. Molecular Genetics of Follicular-Derived Thyroid Cancer. Cancers 2021, 13, 1139. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Xv, J.; Wang, J.; Wang, G.; Zhao, Y. Genetic alterations and allele frequency of BRAF V600E and TERT mutation in papillary thyroid carcinoma with intermediate-to-high recurrence risk: A retrospective study. Clin. Exp. Med. 2024, 24, 76. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Liu, Z. Associations between BRAF V600E and prognostic factors and poor outcomes in papillary thyroid carcinoma: A meta-analysis. World J. Surg. Oncol. 2016, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, P.; Hua, S.; Gao, J.; Zhang, B.; Wan, H.; Wu, Q.; Zhang, J.; Chen, G. Genetic and Clinicopathologic Characteristics of Papillary Thyroid Carcinoma in the Chinese Population: High BRAF Mutation Allele Frequency, Multiple Driver Gene Mutations, and RET Fusion May Indicate More Advanced TN Stage. Onco Targets Ther. 2022, 15, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer-Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef]
- Lubitz, C.C.; Economopoulos, K.P.; Pawlak, A.C.; Lynch, K.; Dias-Santagata, D.; Faquin, W.C.; Sadow, P.M. Hobnail variant of papillary thyroid carcinoma: An institutional case series and molecular profile. Thyroid 2014, 24, 958–965. [Google Scholar] [CrossRef]
- Oh, W.J.; Lee, Y.S.; Cho, U.; Bae, J.S.; Lee, S.; Kim, M.H.; Lim, D.J.; Park, G.S.; Lee, Y.S.; Jung, C.K. Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean J. Pathol. 2014, 48, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Xie, H.; Wei, B.; Shen, H.; Liu, A.; Gao, Y.; Wang, L. Relationship between BRAF V600E gene mutation and the clinical and pathologic characteristics of papillary thyroid microcarcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 3492–3499. [Google Scholar]
- Chen, P.; Pan, L.; Huang, W.; Feng, H.; Ouyang, W.; Wu, J.; Wang, J.; Deng, Y.; Luo, J.; Chen, Y. BRAF V600E and lymph node metastases in papillary thyroid cancer. Endocr. Connect. 2020, 9, 999–1008. [Google Scholar] [CrossRef]
- Lai, Y.; Gu, Y.; Yu, M.; Deng, J. Younger Than 55 Years Old and BRAF V600E Mutation are Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinomas ≤ 1.0 cm but Not in >1.0 cm. Int. J. Gen. Med. 2023, 16, 1403–1414. [Google Scholar] [CrossRef]
- Paja Fano, M.; Ugalde Olano, A.; Fuertes Thomas, E.; Oleaga Alday, A. Immunohistochemical detection of the BRAF V600E mutation in papillary thyroid carcinoma. Evaluation against real-time polymerase chain reaction. Endocrinol. Diabetes Nutr. Engl. Ed. 2017, 64, 75–81. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, X.; Zhang, H.; Sun, W. Clinical and molecular features of progressive papillary thyroid microcarcinoma. Int. J. Surg. 2024, 110, 2313–2322. [Google Scholar] [CrossRef]
- Tallini, G.; De Leo, A.; Repaci, A.; de Biase, D.; Bacchi Reggiani, M.L.; Di Nanni, D.; Ambrosi, F.; Di Gioia, C.; Grani, G.; Rhoden, K.J.; et al. Does the Site of Origin of the Microcarcinoma with Respect to the Thyroid Surface Matter? A Multicenter Pathologic and Clinical Study for Risk Stratification. Cancers 2020, 12, 246. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Fagin, J.A.; Minkowitz, G.; Wong, R.J.; Roman, B.; Patel, S.; Untch, B.; Ganly, I.; Shaha, A.R.; Shah, J.P.; et al. Natural History and Tumor Volume Kinetics of Papillary Thyroid Cancers During Active Surveillance. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Higashiyama, T.; Kobayashi, K.; Miya, A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Toda, K.; Yamada, K.; Yamamoto, N.; Ikenaga, M.; Fujimoto, Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: Our treatment strategies and outcomes. World J. Surg. 2010, 34, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Schumm, M.A.; Nikiforov, Y.E.; Nikiforova, M.N.; Wald, A.I.; Tseng, C.H.; Smooke-Praw, S.; Wu, J.X.; Yeh, M.W.; Livhits, M.J. Association of BRAF V600E allele frequency with clinicopathologic outcomes in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2024, 14, dgae774. [Google Scholar] [CrossRef]
- Nechifor-Boila, A.; Zahan, A.; Banescu, C.; Moldovan, V.; Piciu, D.; Voidazan, S.; Borda, A. Impact of BRAF V600E Mutation on Event-Free Survival in Patients with Papillary Thyroid Carcinoma: A Retrospective Study in a Romanian Population. Cancers 2023, 15, 4053. [Google Scholar] [CrossRef]
- Gandolfi, G.; Sancisi, V.; Torricelli, F.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A. Allele percentage of the BRAF V600E mutation in papillary thyroid carcinomas and corresponding lymph node metastases: No evidence for a role in tumor progression. J. Clin. Endocrinol. Metab. 2013, 98, E934–E942. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, B.; Zhao, Y.; Chen, P.; Ji, M.; Hou, P.; Shi, B. Association of BRAF V600E mutation with clinicopathological features of papillary thyroid carcinoma: A study on a Chinese population. Int. J. Clin. Exp. Pathol. 2014, 7, 6922–6928. [Google Scholar]
| Variable | Mean (SD) or N (%) |
|---|---|
| Age | 49.6 (12.6) |
| Biological sex | |
| Female | 31 (91.2%) |
| Male | 3 (8.8%) |
| Allele frequency | 19.2 (13.1) |
| Aggressiveness | |
| Non-aggressive | 15 (44.1%) |
| Aggressive | 19 (55.9%) |
| Lymph node metastasis | |
| No metastasis | 24 (70.6%) |
| Metastasis | 10 (29.4%) |
| Aggressive histology | |
| Not aggressive | 27 (79.4%) |
| Aggressive | 7 (20.6%) |
| Extrathyroidal extension | |
| No | 31 (91.2%) |
| Yes | 3 (8.8%) |
| Lymphovascular invasion | |
| No | 33 (97.1%) |
| Yes | 1 (2.9%) |
| Bethesda score | |
| B3 | 4 (11.8%) |
| B4 | 1 (2.9%) |
| B5 | 6 (17.6%) |
| B6 | 23 (67.7%) |
| Histological subtype | |
| Classical | 19 (55.9%) |
| Follicular | 6 (17.6%) |
| Oncocytic follicular | 2 (5.9%) |
| Tall cell | 7 (20.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatar, L.; Bandargal, S.; Pusztaszeri, M.P.; Forest, V.-I.; Hier, M.P.; Kouz, J.; Chowdhury, R.; Payne, R.J. Association Between BRAF V600E Allele Frequency and Aggressive Behavior in Papillary Thyroid Microcarcinoma. Cancers 2025, 17, 2553. https://doi.org/10.3390/cancers17152553
Tatar L, Bandargal S, Pusztaszeri MP, Forest V-I, Hier MP, Kouz J, Chowdhury R, Payne RJ. Association Between BRAF V600E Allele Frequency and Aggressive Behavior in Papillary Thyroid Microcarcinoma. Cancers. 2025; 17(15):2553. https://doi.org/10.3390/cancers17152553
Chicago/Turabian StyleTatar, Luiza, Saruchi Bandargal, Marc P. Pusztaszeri, Véronique-Isabelle Forest, Michael P. Hier, Jasmine Kouz, Raisa Chowdhury, and Richard J. Payne. 2025. "Association Between BRAF V600E Allele Frequency and Aggressive Behavior in Papillary Thyroid Microcarcinoma" Cancers 17, no. 15: 2553. https://doi.org/10.3390/cancers17152553
APA StyleTatar, L., Bandargal, S., Pusztaszeri, M. P., Forest, V.-I., Hier, M. P., Kouz, J., Chowdhury, R., & Payne, R. J. (2025). Association Between BRAF V600E Allele Frequency and Aggressive Behavior in Papillary Thyroid Microcarcinoma. Cancers, 17(15), 2553. https://doi.org/10.3390/cancers17152553








