Recent Advances in PET and Radioligand Therapy for Lung Cancer: FDG and FAP
Simple Summary
Abstract
1. Introduction
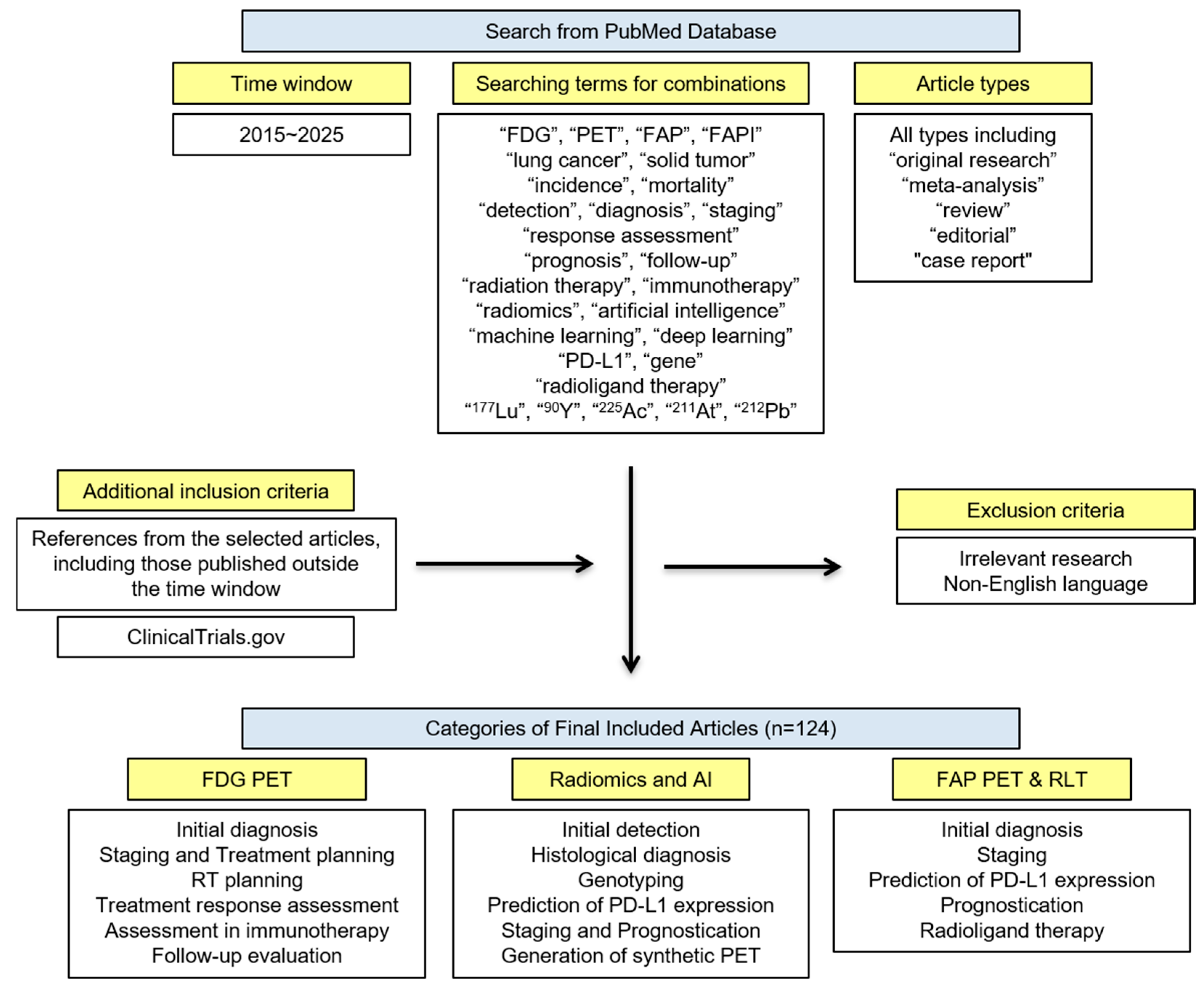
2. Method—Search Strategy
3. FDG PET
3.1. Initial Diagnosis
- –
- FDG PET complements CT in differentiating malignant from benign lung nodules.
- –
- FDG uptake is low in sub-centimeter or sub-solid nodules, as well as in small nodules near the diaphragm due to respiratory motion.
- –
- Relative FDG uptake analysis is recommended for the diagnosis of lung cancer.
- –
- In endemic areas for infectious diseases, the diagnostic reliability of FDG PET/CT for lung cancer is reduced.
- –
- DTP PET imaging is not recommended for routine clinical practice.
3.2. Staging and Treatment Planning
- –
- FDG PET/CT is recommended for staging both NSCLC and SCLC, particularly for N and M staging.
- –
- Combining EBUS-FNA with FDG PET/CT significantly improves the specificity of N staging.
- –
- The cost-effectiveness of FDG PET/CT in NSCLC largely depends on reducing futile thoracotomies.
- –
- FDG PET/CT significantly affects binary staging decisions in SCLC.
3.3. RT Planning
- –
- FDG PET-guided RT can deliver successful definitive treatment for both NSCLC and SCLC.
3.4. Treatment Response Assessment
- –
- PERCIST may provide a more accurate assessment of treatment response than RECIST. However, its clinical adoption remains limited due to several challenges.
- –
- Accurate assessment of treatment response depends on the appropriate timing of FDG PET/CT after treatment.
3.5. Response Assessment in Immunotherapy
- –
- Follow-up FDG PET/CT is recommended during immunotherapy to distinguish atypical treatment responses.
3.6. Follow-Up Evaluation
- –
- FDG PET/CT has demonstrated diagnostic value for routine follow-up imaging in lung cancer.
4. Radiomics and AI
4.1. Initial Detection
- –
- AI approaches have been applied to PET/CT for lung nodule detection and whole-body tumor segmentation.
4.2. Histological Diagnosis
- –
- FDG PET/CT-based models can differentiate benign from malignant lung nodules, as well as ADC from SqCC.
4.3. Genotyping
- –
- FDG PET/CT-based models can assist in identifying driver gene mutations in NSCLC.
4.4. Prediction of PD-L1 Expression
- –
- FDG PET/CT–based models can help predict PD-L1 expression in NSCLC.
4.5. Staging and Prognostication
- –
- FDG PET/CT–based models can support staging and prognostication in NSCLC.
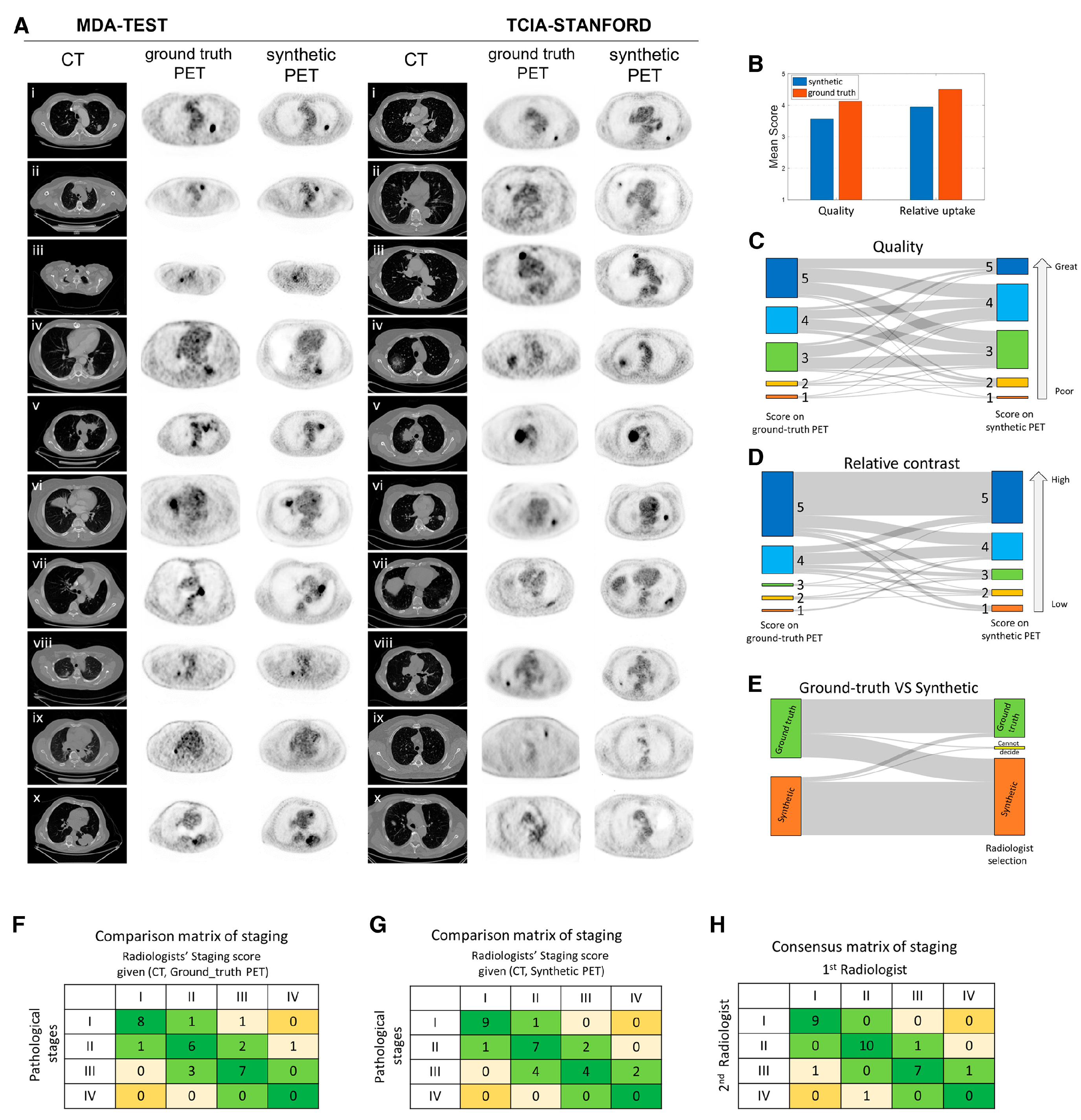
4.6. Generation of Synthetic PET Image
- –
- Generative DL models can synthesize PET images from CT data.
5. FAP PET and RLT
5.1. Initial Diagnosis and Staging
- –
- Non-quinoline-based FAP-targeted peptides and peptidomimetics have recently been developed.
- –
- FAP PET offers a high TBR and low physiological uptake in normal organs such as the brain and liver.
- –
- FAP PET demonstrates superior sensitivity to FDG PET in detecting nodal and distant metastases, particularly in NSCLC.
- –
- FAP PET outperforms FDG PET in identifying non-solid nodular lung ADC.
5.2. Prediction of PD-L1 Expression and Prognostication
- –
- FAP uptake appears to be positively correlated with PD-L1 expression in lung cancer.
- –
- Elevated FAP uptake may be associated with poorer prognosis in lung cancer.
5.3. FAP RLT
- –
- Ongoing clinical trials are evaluating FAP RLT in FAP-positive solid tumors.
- –
- FAP RLT combined with immune checkpoint inhibitors may enhance therapeutic efficacy.
6. Limitations and Future Directions
6.1. FDG PET/CT with Radiomics and AI
6.2. FAP PET
6.3. FAP RLT
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | adenocarcinoma |
| AI | artificial intelligence |
| AJCC | American joint committee on cancer |
| AUC | area under the receiver operating characteristic curve |
| CAF | cancer-associated fibroblast |
| CI | confidence intervals |
| CMR | complete metabolic response |
| CNN | convolutional neural network |
| CR | complete response |
| DDPM | denoising diffusion probabilistic model |
| DL | deep learning |
| DT | decision tree |
| DTP | dual-time-point |
| EBUS-FNA | endobronchial ultrasound-guided fine needle aspiration |
| ES | extensive stage |
| EGFR | epidermal growth factor receptor gene |
| FAP | fibroblast activation protein |
| FAPI | fibroblast activation protein inhibitor |
| FDG PET/CT | 18F-fluorodeoxyglucose positron emission tomography/computed tomography |
| FN | false-negative |
| FP | false-positive |
| GAN | generative adversarial network |
| HRNet | high-resolution representation learning model |
| IHC | immunohistochemistry |
| imPERCIST | immunotherapy-modified PERCIST |
| iRECIST | immune RECIST |
| imRECIST | immune-modified RECIST |
| KRAS | Kirsten rat sarcoma |
| LS | limited stage |
| MIP | maximum-intensity projection |
| ML | machine learning |
| NSCLC | non-small cell lung cancer |
| ODA | overall diagnostic accuracy |
| OS | overall survival |
| PD | progressive disease |
| PD-1 | programmed death 1 |
| PD-L1 | programmed death ligand 1 |
| PERCIST | PET response criteria in solid tumors |
| PFS | progression-free survival |
| PMD | progressive metabolic disease |
| PMR | partial metabolic response |
| PPV | positive predictive value |
| PR | partial response |
| RECIST | response evaluation criteria in solid tumors |
| ResNet | residual network |
| RF | radiomic feature |
| RFS | recurrence-free survival |
| RLT | radioligand therapy |
| RT | radiation therapy |
| SBRT | stereotactic body radiation therapy |
| SCLC | small cell lung cancer |
| SD | stable disease |
| Sens | sensitivity |
| SMD | stable metabolic disease |
| Spec | specificity |
| SqCC | squamous cell carcinoma |
| STP | single-time-point |
| SUL | SUV corrected for lean body mass |
| SUV | standardized uptake value |
| SVM | support vector machine |
| TBR | tumor-to-background ratio |
| TP | true-positive |
| TP53 | tumor protein p53 |
| TME | tumor microenvironment |
| TNM | tumor, node, and metastasis |
| VOI | volume of interest |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients with Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Quere, G.; Blanc, E.; Lemarignier, C.; Vercellino, L.; de Margerie-Mellon, C.; Merlet, P.; Querellou, S. FDG PET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn. Interv. Imaging 2016, 97, 1003–1017. [Google Scholar] [CrossRef]
- Grimm, J.; Kiessling, F.; Pichler, B.J. Quo Vadis, Molecular Imaging? J. Nucl. Med. 2020, 61, 1428–1434. [Google Scholar] [CrossRef]
- Schwenck, J.; Sonanini, D.; Cotton, J.M.; Rammensee, H.G.; la Fougère, C.; Zender, L.; Pichler, B.J. Advances in PET imaging of cancer. Nat. Rev. Cancer 2023, 23, 474–490. [Google Scholar] [CrossRef]
- Wahl, R.L.; Chareonthaitawee, P.; Clarke, B.; Drzezga, A.; Lindenberg, L.; Rahmim, A.; Thackeray, J.; Ulaner, G.A.; Weber, W.; Zukotynski, K.; et al. Mars Shot for Nuclear Medicine, Molecular Imaging, and Molecularly Targeted Radiopharmaceutical Therapy. J. Nucl. Med. 2021, 62, 6–14. [Google Scholar] [CrossRef]
- Mori, Y.; Dendl, K.; Cardinale, J.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U. FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease. Radiology 2023, 306, e220749. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Zhu, T.; Hsu, J.C.; Guo, J.; Chen, W.; Cai, W.; Wang, K. Radionuclide-based theranostics—a promising strategy for lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2353–2374. [Google Scholar] [CrossRef] [PubMed]
- Zukotynski, K.A.; Gerbaudo, V.H. Understanding the Value of FAPI versus FDG PET/CT in Primary and Metastatic Lung Cancer. Radiology 2023, 308, e231768. [Google Scholar] [CrossRef] [PubMed]
- Ost, D.; Fein, A.M.; Feinsilver, S.H. Clinical practice. The solitary pulmonary nodule. N. Engl. J. Med. 2003, 348, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Nasim, F.; Ost, D.E. Management of the solitary pulmonary nodule. Curr. Opin. Pulm. Med. 2019, 25, 344–353. [Google Scholar] [CrossRef]
- Greenspan, B.S. Role of PET/CT for precision medicine in lung cancer: Perspective of the Society of Nuclear Medicine and Molecular Imaging. Transl. Lung Cancer Res. 2017, 6, 617–620. [Google Scholar] [CrossRef]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef]
- Weir-McCall, J.R.; Harris, S.; Miles, K.A.; Qureshi, N.R.; Rintoul, R.C.; Dizdarevic, S.; Pike, L.; Cheow, H.K.; Gilbert, F.J. Impact of solitary pulmonary nodule size on qualitative and quantitative assessment using 18F-fluorodeoxyglucose PET/CT: The SPUTNIK trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1560–1569. [Google Scholar] [CrossRef]
- Deppen, S.A.; Blume, J.D.; Kensinger, C.D.; Morgan, A.M.; Aldrich, M.C.; Massion, P.P.; Walker, R.C.; McPheeters, M.L.; Putnam, J.B., Jr.; Grogan, E.L. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: A meta-analysis. JAMA 2014, 312, 1227–1236. [Google Scholar] [CrossRef]
- Niyonkuru, A.; Chen, X.; Bakari, K.H.; Wimalarathne, D.N.; Bouhari, A.; Arnous, M.M.R.; Lan, X. Evaluation of the diagnostic efficacy of 18F-Fluorine-2-Deoxy-D-Glucose PET/CT for lung cancer and pulmonary tuberculosis in a Tuberculosis-endemic Country. Cancer Med. 2020, 9, 931–942. [Google Scholar] [CrossRef]
- Houshmand, S.; Salavati, A.; Segtnan, E.A.; Grupe, P.; Høilund-Carlsen, P.F.; Alavi, A. Dual-time-point Imaging and Delayed-time-point Fluorodeoxyglucose-PET/Computed Tomography Imaging in Various Clinical Settings. PET Clin. 2016, 11, 65–84. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Lei, J.; Tian, J.; Zhai, Y. Dual time point 18FDG-PET/CT versus single time point 18FDG-PET/CT for the differential diagnosis of pulmonary nodules: A meta-analysis. Acta Radiol. 2013, 54, 770–777. [Google Scholar] [CrossRef]
- Dingemans, A.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lee, K.Y.; Kim, H.J.; Kim, W.S.; So, Y. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Le Pechoux, C.; Faivre-Finn, C.; Ramella, S.; McDonald, F.; Manapov, F.; Putora, P.M.; Slotman, B.; De Ruysscher, D.; Ricardi, U.; Geets, X.; et al. ESTRO ACROP guidelines for target volume definition in the thoracic radiation treatment of small cell lung cancer. Radiother. Oncol. 2020, 152, 89–95. [Google Scholar] [CrossRef]
- Kirchner, J.; Sawicki, L.M.; Nensa, F.; Schaarschmidt, B.M.; Reis, H.; Ingenwerth, M.; Bogner, S.; Aigner, C.; Buchbender, C.; Umutlu, L.; et al. Prospective comparison of 18F-FDG PET/MRI and 18F-FDG PET/CT for thoracic staging of non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 437–445. [Google Scholar] [CrossRef]
- Owens, C.; Hindocha, S.; Lee, R.; Millard, T.; Sharma, B. The lung cancers: Staging and response, CT, 18F-FDG PET/CT, MRI, DWI: Review and new perspectives. Br. J. Radiol. 2023, 96, 20220339. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef]
- Schmidt-Hansen, M.; Baldwin, D.R.; Hasler, E.; Zamora, J.; Abraira, V.; Roqué, I.F.M. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst. Rev. 2014, 2014, CD009519. [Google Scholar] [CrossRef]
- Divisi, D.; Barone, M.; Crisci, R. Current role of standardized uptake value(max)-derived ratios in N2 fluorine-18 fluorodeoxyglucose positron-emission tomography non-small cell lung cancer. J. Thorac. Dis. 2018, 10, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Park, H.L.; Kang, H.S.; Lee, H.Y.; Yoo, I.R.; Lee, S.H.; Yeo, C.D. Clinical Characteristics and Outcome of Pathologic N0 Non-small Cell Lung Cancer Patients With False Positive Mediastinal Lymph Node Metastasis on FDG PET-CT. In Vivo 2021, 35, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, Q.; Ma, Y.; Wang, Y.; Feng, Y.; Zhao, B.; Yang, Y. Implications of false negative and false positive diagnosis in lymph node staging of NSCLC by means of 18F-FDG PET/CT. PLoS ONE 2013, 8, e78552. [Google Scholar] [CrossRef] [PubMed]
- Dyas, A.R.; King, R.W.; Ghanim, A.F.; Cerfolio, R.J. Clinical Misstagings and Risk Factors of Occult Nodal Disease in Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2018, 106, 1492–1498. [Google Scholar] [CrossRef]
- Feng, M.; Yang, X.; Ma, Q.; He, Y. Retrospective analysis for the false positive diagnosis of PET-CT scan in lung cancer patients. Medicine 2017, 96, e7415. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Gong, F.; Xu, S.; Wu, J.; Wang, H.; He, T. The diagnostic value of PET/CT for the lymph node metastasis in Asian patients with non-small cell lung cancer: A meta-analysis. Hell. J. Nucl. Med. 2022, 25, 196–204. [Google Scholar] [CrossRef]
- Dunne, E.G.; Fick, C.N.; Jones, D.R. Mediastinal Staging in Non-Small-Cell Lung Cancer: Saying Goodbye to Mediastinoscopy. J. Clin. Oncol. 2023, 41, 3785–3790. [Google Scholar] [CrossRef]
- Kalade, A.V.; Eddie Lau, W.F.; Conron, M.; Wright, G.M.; Desmond, P.V.; Hicks, R.J.; Chen, R. Endoscopic ultrasound-guided fine-needle aspiration when combined with positron emission tomography improves specificity and overall diagnostic accuracy in unexplained mediastinal lymphadenopathy and staging of non-small-cell lung cancer. Intern. Med. J. 2008, 38, 837–844. [Google Scholar] [CrossRef]
- Peeters, S.T.; Dooms, C.; Van Baardwijk, A.; Dingemans, A.M.; Martinussen, H.; Vansteenkiste, J.; Decaluwé, H.; De Leyn, P.; Yserbyt, J.; Nackaerts, K.; et al. Selective mediastinal node irradiation in non-small cell lung cancer in the IMRT/VMAT era: How to use E(B)US-NA information in addition to PET-CT for delineation? Radiother. Oncol. 2016, 120, 273–278. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Zhang, H.; Shi, Y.; Wu, H.; Zhang, J.; Qian, Y.; Li, C.; Yang, J. Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients. Int. J. Cancer 2013, 132, E37–E47. [Google Scholar] [CrossRef]
- Taira, A.V.; Herfkens, R.J.; Gambhir, S.S.; Quon, A. Detection of bone metastases: Assessment of integrated FDG PET/CT imaging. Radiology 2007, 243, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Luo, W.; Zhao, Y.; Xu, F.; Zhou, Q. The utility of 18F-FDG PET/CT for the diagnosis of adrenal metastasis in lung cancer: A PRISMA-compliant meta-analysis. Nucl. Med. Commun. 2017, 38, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.; Subramaniam, R.M. FDG PET/CT for Primary Staging of Lung Cancer and Mesothelioma. Semin. Nucl. Med. 2022, 52, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Viney, R.C.; Boyer, M.J.; King, M.T.; Kenny, P.M.; Pollicino, C.A.; McLean, J.M.; McCaughan, B.C.; Fulham, M.J. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 2357–2362. [Google Scholar] [CrossRef]
- MacManus, M.P.; Hicks, R.J.; Matthews, J.P.; Hogg, A.; McKenzie, A.F.; Wirth, A.; Ware, R.E.; Ball, D.L. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: Implications for radical radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 287–293. [Google Scholar] [CrossRef]
- de Groot, P.M.; Chung, J.H.; Ackman, J.B.; Berry, M.F.; Carter, B.W.; Colletti, P.M.; Hobbs, S.B.; McComb, B.L.; Movsas, B.; Tong, B.C.; et al. ACR Appropriateness Criteria® Noninvasive Clinical Staging of Primary Lung Cancer. J. Am. Coll. Radiol. 2019, 16, S184–S195. [Google Scholar] [CrossRef]
- Fischer, B.; Lassen, U.; Mortensen, J.; Larsen, S.; Loft, A.; Bertelsen, A.; Ravn, J.; Clementsen, P.; Høgholm, A.; Larsen, K.; et al. Preoperative staging of lung cancer with combined PET-CT. N. Engl. J. Med. 2009, 361, 32–39. [Google Scholar] [CrossRef]
- Søgaard, R.; Fischer, B.M.; Mortensen, J.; Højgaard, L.; Lassen, U. Preoperative staging of lung cancer with PET/CT: Cost-effectiveness evaluation alongside a randomized controlled trial. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 802–809. [Google Scholar] [CrossRef]
- Ganti, A.K.P.; Loo, B.W.; Bassetti, M.; Blakely, C.; Chiang, A.; D’Amico, T.A.; D’Avella, C.; Dowlati, A.; Downey, R.J.; Edelman, M.; et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1441–1464. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Chen, J.H.; Liang, J.A.; Chu, S.; Lin, W.Y.; Kao, C.H. 18F-FDG PET or PET/CT for detecting extensive disease in small-cell lung cancer: A systematic review and meta-analysis. Nucl. Med. Commun. 2014, 35, 697–703. [Google Scholar] [CrossRef]
- Martucci, F.; Pascale, M.; Valli, M.C.; Pesce, G.A.; Froesch, P.; Giovanella, L.; Richetti, A.; Treglia, G. Impact of 18F-FDG PET/CT in Staging Patients With Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Med. 2019, 6, 336. [Google Scholar] [CrossRef]
- Vaz, S.C.; Adam, J.A.; Delgado Bolton, R.C.; Vera, P.; van Elmpt, W.; Herrmann, K.; Hicks, R.J.; Lievens, Y.; Santos, A.; Schöder, H.; et al. Joint EANM/SNMMI/ESTRO practice recommendations for the use of 2-[18F]FDG PET/CT external beam radiation treatment planning in lung cancer V1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1386–1406. [Google Scholar] [CrossRef] [PubMed]
- Hallqvist, A.; Alverbratt, C.; Strandell, A.; Samuelsson, O.; Björkander, E.; Liljegren, A.; Albertsson, P. Positron emission tomography and computed tomographic imaging (PET/CT) for dose planning purposes of thoracic radiation with curative intent in lung cancer patients: A systematic review and meta-analysis. Radiother. Oncol. 2017, 123, 71–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nestle, U.; Schimek-Jasch, T.; Kremp, S.; Schaefer-Schuler, A.; Mix, M.; Küsters, A.; Tosch, M.; Hehr, T.; Eschmann, S.M.; Bultel, Y.P.; et al. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): A multicentre, open-label, randomised, controlled trial. Lancet Oncol. 2020, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, P.; Salem, A.; Mistry, H.; Gornall, M.; Harden, S.; Julyan, P.; Locke, I.; McAleese, J.; McMenemin, R.; Mohammed, N.; et al. 18F-Fludeoxyglucose PET/CT in SCLC: Analysis of the CONVERT Randomized Controlled Trial. J. Thorac. Oncol. 2019, 14, 1296–1305. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Mena, E.; Yanamadala, A.; Reddy, S.; Solnes, L.B.; Wachsmann, J.; Subramaniam, R.M. The Value of FDG PET/CT in Treatment Response Assessment, Follow-Up, and Surveillance of Lung Cancer. AJR Am. J. Roentgenol. 2017, 208, 420–433. [Google Scholar] [CrossRef]
- Coche, E. Evaluation of lung tumor response to therapy: Current and emerging techniques. Diagn. Interv. Imaging 2016, 97, 1053–1065. [Google Scholar] [CrossRef]
- William, W.N., Jr.; Pataer, A.; Kalhor, N.; Correa, A.M.; Rice, D.C.; Wistuba, I.I.; Heymach, J.; Lee, J.J.; Kim, E.S.; Munden, R.; et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. 2013, 8, 222–228. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), 122S–150S. [Google Scholar] [CrossRef]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ling, X.; Zhang, L.; Tang, Y.; Xiao, Z.; Cheng, Y.; Guo, B.; Gong, J.; Huang, L.; Xu, H. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Tajmir, S.H.; Rowe, S.P.; Sheikhbahaei, S.; Solnes, L.B. 18F-FDG PET/CT for Response Assessment in Lung Cancer. Semin. Nucl. Med. 2022, 52, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.; Sibley, R.C., III; Subramaniam, R.M. Lung Cancer Recurrence: 18F-FDG PET/CT in Clinical Practice. AJR Am. J. Roentgenol. 2019, 213, 1136–1144. [Google Scholar] [CrossRef]
- Borcoman, E.; Kanjanapan, Y.; Champiat, S.; Kato, S.; Servois, V.; Kurzrock, R.; Goel, S.; Bedard, P.; Le Tourneau, C. Novel patterns of response under immunotherapy. Ann. Oncol. 2019, 30, 385–396. [Google Scholar] [CrossRef]
- Hodi, F.S.; Ballinger, M.; Lyons, B.; Soria, J.C.; Nishino, M.; Tabernero, J.; Powles, T.; Smith, D.; Hoos, A.; McKenna, C.; et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J. Clin. Oncol. 2018, 36, 850–858. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Humbert, O.; Cadour, N.; Paquet, M.; Schiappa, R.; Poudenx, M.; Chardin, D.; Borchiellini, D.; Benisvy, D.; Ouvrier, M.J.; Zwarthoed, C.; et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: Frequency and clinical significance of atypical evolutive patterns. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1158–1167. [Google Scholar] [CrossRef]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.; et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef]
- Rossi, G.; Bauckneht, M.; Genova, C.; Rijavec, E.; Biello, F.; Mennella, S.; Dal Bello, M.G.; Cittadini, G.; Bruzzi, P.; Piva, R.; et al. Comparison Between 18F-FDG PET-Based and CT-Based Criteria in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Nucl. Med. 2020, 61, 990–998. [Google Scholar] [CrossRef]
- Remon, J.; Soria, J.C.; Peters, S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Gong, H.L.; Deng, Y.F.; Li, W.M. Diagnostic efficacy of PET and PET/CT for recurrent lung cancer: A meta-analysis. Acta Radiol. 2014, 55, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.J.; Marcus, C.; Tahari, A.K.; Wahl, R.L.; Subramaniam, R.M. Follow-up or Surveillance 18F-FDG PET/CT and Survival Outcome in Lung Cancer Patients. J. Nucl. Med. 2014, 55, 1062–1068. [Google Scholar] [CrossRef]
- Marcus, C.; Paidpally, V.; Antoniou, A.; Zaheer, A.; Wahl, R.L.; Subramaniam, R.M. 18F-FDG PET/CT and lung cancer: Value of fourth and subsequent posttherapy follow-up scans for patient management. J. Nucl. Med. 2015, 56, 204–208. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Chen, M.; Copley, S.J.; Viola, P.; Lu, H.; Aboagye, E.O. Radiomics and artificial intelligence for precision medicine in lung cancer treatment. Semin. Cancer Biol. 2023, 93, 97–113. [Google Scholar] [CrossRef]
- Kotoulas, S.C.; Spyratos, D.; Porpodis, K.; Domvri, K.; Boutou, A.; Kaimakamis, E.; Mouratidou, C.; Alevroudis, I.; Dourliou, V.; Tsakiri, K.; et al. A Thorough Review of the Clinical Applications of Artificial Intelligence in Lung Cancer. Cancers 2025, 17, 882. [Google Scholar] [CrossRef]
- Protonotarios, N.E.; Katsamenis, I.; Sykiotis, S.; Dikaios, N.; Kastis, G.A.; Chatziioannou, S.N.; Metaxas, M.; Doulamis, N.; Doulamis, A. A few-shot U-Net deep learning model for lung cancer lesion segmentation via PET/CT imaging. Biomed. Phys. Eng. Express 2022, 8, 025019. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, S.K.; Hwang, D.; Choi, H.; Ha, S.; Seo, J.M.; Eo, J.S.; Lee, J.S. Automatic Lung Cancer Segmentation in [18F]FDG PET/CT Using a Two-Stage Deep Learning Approach. Nucl. Med. Mol. Imaging 2023, 57, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, F.; Onishi, Y.; Ote, K.; Tashima, H.; Reader, A.J.; Yamaya, T. Deep learning-based PET image denoising and reconstruction: A review. Radiol. Phys. Technol. 2024, 17, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Azimi, M.S.; Kamali-Asl, A.; Ay, M.R.; Zeraatkar, N.; Hosseini, M.S.; Sanaat, A.; Dadgar, H.; Arabi, H. Deep learning-based partial volume correction in standard and low-dose positron emission tomography-computed tomography imaging. Quant. Imaging Med. Surg. 2024, 14, 2146–2164. [Google Scholar] [CrossRef]
- Schwyzer, M.; Martini, K.; Benz, D.C.; Burger, I.A.; Ferraro, D.A.; Kudura, K.; Treyer, V.; von Schulthess, G.K.; Kaufmann, P.A.; Huellner, M.W.; et al. Artificial intelligence for detecting small FDG-positive lung nodules in digital PET/CT: Impact of image reconstructions on diagnostic performance. Eur. Radiol. 2020, 30, 2031–2040. [Google Scholar] [CrossRef]
- Leung, K.H.; Rowe, S.P.; Sadaghiani, M.S.; Leal, J.P.; Mena, E.; Choyke, P.L.; Du, Y.; Pomper, M.G. Deep Semisupervised Transfer Learning for Fully Automated Whole-Body Tumor Quantification and Prognosis of Cancer on PET/CT. J. Nucl. Med. 2024, 65, 643–650. [Google Scholar] [CrossRef]
- Constantino, C.S.; Oliveira, F.P.M.; Machado, M.; Vinga, S.; Costa, D.C. The Use of Maximum-Intensity Projections and Deep Learning Adds Value to the Fully Automatic Segmentation of Lesions Avid for [18F]FDG and [68Ga]Ga-PSMA in PET/CT. J. Nucl. Med. 2025, 66, 795–801. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Han, J.; Lin, Q.; Zhao, L.; Li, Q.; Zhao, J.; Li, H.; Wang, Y.; Hu, C. A machine learning-based PET/CT model for automatic diagnosis of early-stage lung cancer. Front. Oncol. 2023, 13, 1192908. [Google Scholar] [CrossRef]
- Lai, Y.C.; Wu, K.C.; Tseng, N.C.; Chen, Y.J.; Chang, C.J.; Yen, K.Y.; Kao, C.H. Differentiation Between Malignant and Benign Pulmonary Nodules by Using Automated Three-Dimensional High-Resolution Representation Learning With Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography. Front. Med. 2022, 9, 773041. [Google Scholar] [CrossRef]
- Shen, H.; Chen, L.; Liu, K.; Zhao, K.; Li, J.; Yu, L.; Ye, H.; Zhu, W. A subregion-based positron emission tomography/computed tomography (PET/CT) radiomics model for the classification of non-small cell lung cancer histopathological subtypes. Quant. Imaging Med. Surg. 2021, 11, 2918–2932. [Google Scholar] [CrossRef]
- Zhao, H.; Su, Y.; Lyu, Z.; Tian, L.; Xu, P.; Lin, L.; Han, W.; Fu, P. Non-invasively Discriminating the Pathological Subtypes of Non-small Cell Lung Cancer with Pretreatment 18F-FDG PET/CT Using Deep Learning. Acad. Radiol. 2024, 31, 35–45. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Yang, L.; Xu, P.; Li, M.; Wang, M.; Peng, M.; Zhang, Y.; Wu, T.; Chu, W.; Wang, K.; Meng, H.; et al. PET/CT Radiomic Features: A Potential Biomarker for EGFR Mutation Status and Survival Outcome Prediction in NSCLC Patients Treated With TKIs. Front. Oncol. 2022, 12, 894323. [Google Scholar] [CrossRef]
- Hinzpeter, R.; Kulanthaivelu, R.; Kohan, A.; Murad, V.; Mirshahvalad, S.A.; Avery, L.; Ortega, C.; Metser, U.; Hope, A.; Yeung, J.; et al. Predictive [18F]-FDG PET/CT-Based Radiogenomics Modelling of Driver Gene Mutations in Non-small Cell Lung Cancer. Acad. Radiol. 2024, 31, 5314–5323. [Google Scholar] [CrossRef]
- Li, T.; Ma, W.; Al-Obeidi, E. Evolving Precision First-Line Systemic Treatment for Patients with Unresectable Non-Small Cell Lung Cancer. Cancers 2024, 16, 2350. [Google Scholar] [CrossRef]
- Monaco, L.; De Bernardi, E.; Bono, F.; Cortinovis, D.; Crivellaro, C.; Elisei, F.; L’Imperio, V.; Landoni, C.; Mathoux, G.; Musarra, M.; et al. The “digital biopsy” in non-small cell lung cancer (NSCLC): A pilot study to predict the PD-L1 status from radiomics features of [18F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3401–3411. [Google Scholar] [CrossRef]
- Mu, W.; Jiang, L.; Shi, Y.; Tunali, I.; Gray, J.E.; Katsoulakis, E.; Tian, J.; Gillies, R.J.; Schabath, M.B. Non-invasive measurement of PD-L1 status and prediction of immunotherapy response using deep learning of PET/CT images. J. Immunother. Cancer 2021, 9, e002118. [Google Scholar] [CrossRef] [PubMed]
- Da-Ano, R.; Andrade-Miranda, G.; Tankyevych, O.; Visvikis, D.; Conze, P.H.; Rest, C.C.L. Automated PD-L1 status prediction in lung cancer with multi-modal PET/CT fusion. Sci. Rep. 2024, 14, 16720. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Lan, J.; Chen, S.; Wang, L.; Xin, E.; Xie, J.; Zheng, X.; Wang, L.G.; Tang, K. Explainable PET-based intratumoral and peritumoral machine learning model for predicting visceral pleural invasion in clinical-stage IA non-small cell lung cancer: A two-center study. Clin. Radiol. 2025, 85, 106903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cai, C.; Chen, T.; Gui, H.; Deng, J.; Yang, M.; Yu, B.; Song, Y.; Wang, T.; Sun, X.; et al. PET/CT based cross-modal deep learning signature to predict occult nodal metastasis in lung cancer. Nat. Commun. 2023, 14, 7513. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, C.; Chen, T.; Gui, H.; Chen, C.; Deng, J.; Yang, M.; Yu, B.; Song, Y.; Wang, T.; et al. PET/CT-based deep learning grading signature to optimize surgical decisions for clinical stage I invasive lung adenocarcinoma and biologic basis under its prediction: A multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Lucia, F.; Louis, T.; Cousin, F.; Bourbonne, V.; Visvikis, D.; Mievis, C.; Jansen, N.; Duysinx, B.; Le Pennec, R.; Nebbache, M.; et al. Multicentric development and evaluation of [18F]FDG PET/CT and CT radiomic models to predict regional and/or distant recurrence in early-stage non-small cell lung cancer treated by stereotactic body radiation therapy. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Spadea, M.F.; Maspero, M.; Zaffino, P.; Seco, J. Deep learning based synthetic-CT generation in radiotherapy and PET: A review. Med. Phys. 2021, 48, 6537–6566. [Google Scholar] [CrossRef] [PubMed]
- Salehjahromi, M.; Karpinets, T.V.; Sujit, S.J.; Qayati, M.; Chen, P.; Aminu, M.; Saad, M.B.; Bandyopadhyay, R.; Hong, L.; Sheshadri, A.; et al. Synthetic PET from CT improves diagnosis and prognosis for lung cancer: Proof of concept. Cell Rep. Med. 2024, 5, 101463. [Google Scholar] [CrossRef]
- Müller-Franzes, G.; Niehues, J.M.; Khader, F.; Arasteh, S.T.; Haarburger, C.; Kuhl, C.; Wang, T.; Han, T.; Nolte, T.; Nebelung, S.; et al. A multimodal comparison of latent denoising diffusion probabilistic models and generative adversarial networks for medical image synthesis. Sci. Rep. 2023, 13, 12098. [Google Scholar] [CrossRef]
- Hope, T.A.; Calais, J.; Goenka, A.H.; Haberkorn, U.; Konijnenberg, M.; McConathy, J.; Oprea-Lager, D.E.; Trimnal, L.; Zan, E.; Herrmann, K.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Fibroblast Activation Protein (FAP) PET. J. Nucl. Med. 2025, 66, 26–33. [Google Scholar] [CrossRef]
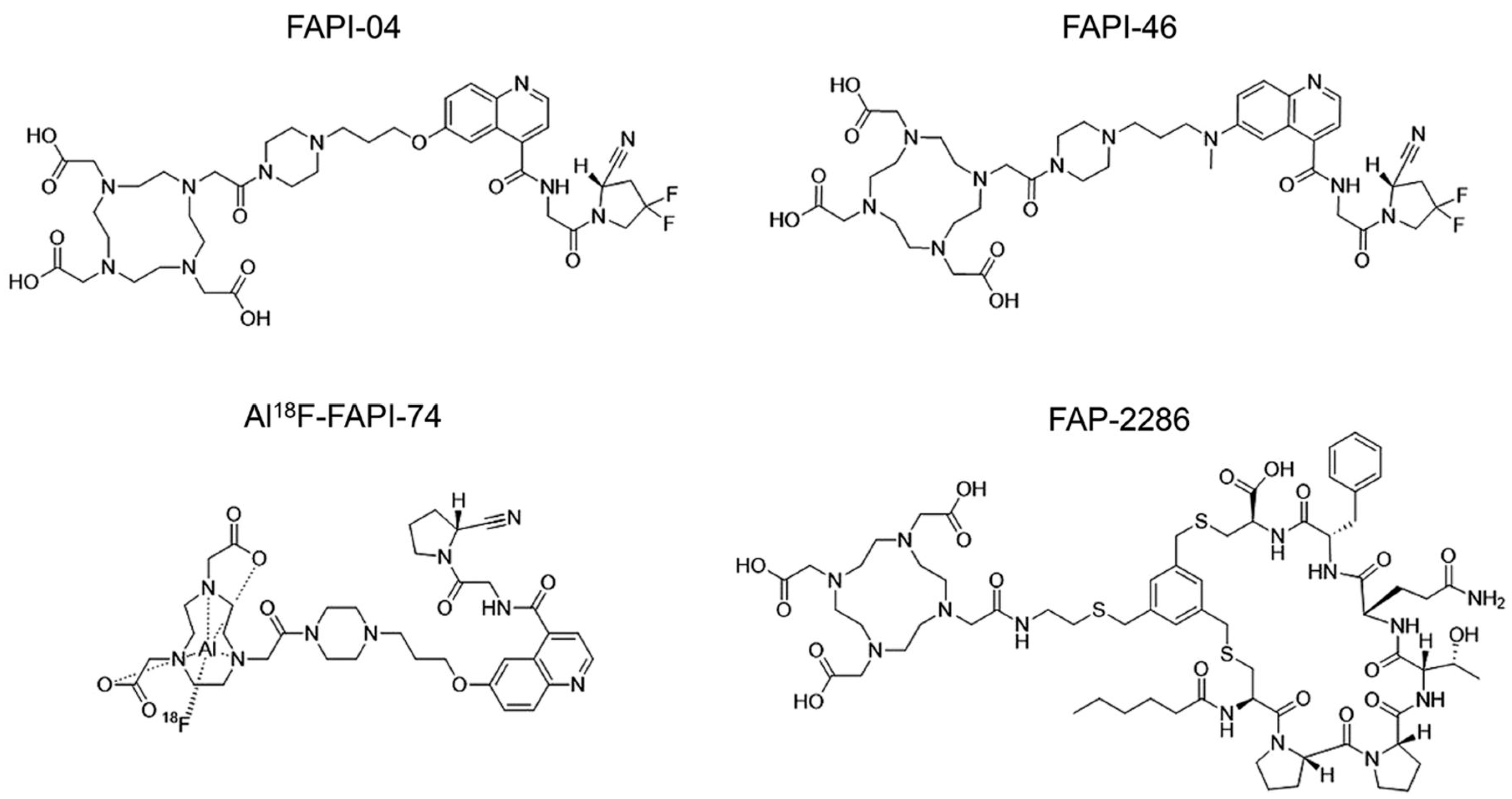
- Martin, M.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; De Loose, J.; Verhulst, E.; De Meester, I.; Van Der Veken, P.; Roesch, F. Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics. Cancers 2023, 15, 1889. [Google Scholar] [CrossRef]
- Chandekar, K.R.; Prashanth, A.; Vinjamuri, S.; Kumar, R. FAPI PET/CT Imaging-An Updated Review. Diagnostics 2023, 13, 2018. [Google Scholar] [CrossRef]
- Wu, J.; Deng, H.; Zhong, H.; Wang, T.; Rao, Z.; Wang, Y.; Chen, Y.; Zhang, C. Comparison of 68Ga-FAPI and 18F-FDG PET/CT in the Evaluation of Patients With Newly Diagnosed Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 924223. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, D.; Wu, J.; Zhong, H.; Han, Y.; Jiang, H.; Chen, Y.; Chen, G.; Zhan, X.; Zhou, P. Performance of [18F]FDG PET/CT versus FAPI PET/CT for lung cancer assessment: A systematic review and meta-analysis. Eur. Radiol. 2024, 34, 1077–1085. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Xu, X.; Meng, X.; Zhang, H.; Zhang, A.; Song, Y.; Zhu, H.; Yang, Z.; Li, N. Higher accuracy of [68Ga]Ga-DOTA-FAPI-04 PET/CT comparing with 2-[18F]FDG PET/CT in clinical staging of NSCLC. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ma, L.; Li, P.; Lu, J.; Ren, J.; Yan, S.; Wu, H.; Yuan, S.; Fu, Z.; Yu, J. FAPI Compared with FDG PET/CT for Diagnosis of Primary and Metastatic Lung Cancer. Radiology 2023, 308, e222785. [Google Scholar] [CrossRef]
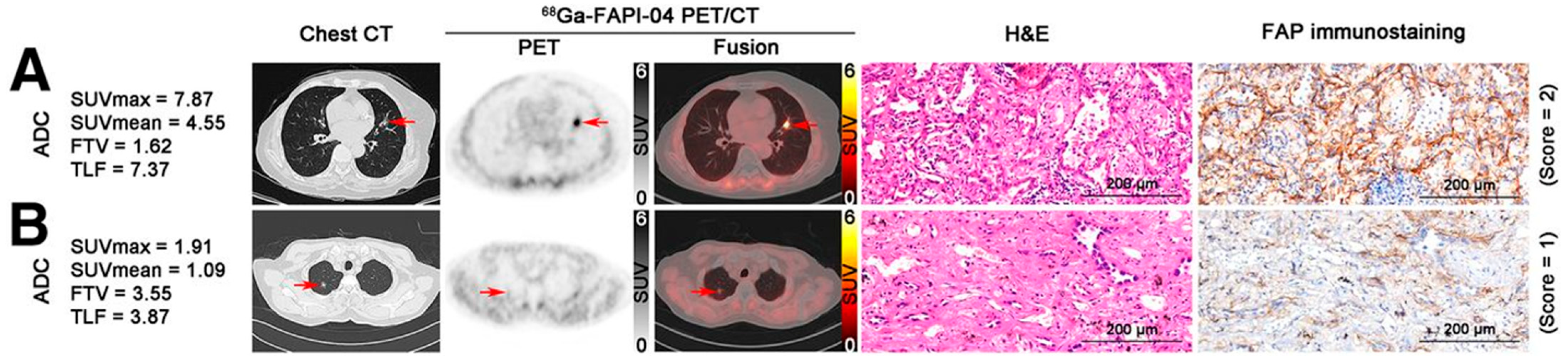
- Chen, X.; Liu, X.; Wang, L.; Zhou, W.; Zhang, Y.; Tian, Y.; Tan, J.; Dong, Y.; Fu, L.; Wu, H. Expression of fibroblast activation protein in lung cancer and its correlation with tumor glucose metabolism and histopathology. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2938–2948. [Google Scholar] [CrossRef]
- Liang, H.X.; Huang, Q.W.; He, Y.M.; Mai, Y.Q.; Chen, Z.L.; Wang, B.P.; Fang, N.; Hu, J.F.; Li, X.; Zhang, N.; et al. Comparison of the diagnostic accuracy between 18F-FAPI-04 PET/CT and 18F-FDG PET/CT in the clinical stage IA of lung adenocarcinoma. J. Thorac. Dis. 2025, 17, 661–675. [Google Scholar] [CrossRef]
- Li, C.; Chen, Q.; Tian, Y.; Chen, J.; Xu, K.; Xiao, Z.; Zhong, J.; Wu, J.; Wen, B.; He, Y. 68Ga-FAPI-04 PET/CT in Non-Small Cell Lung Cancer: Accurate Evaluation of Lymph Node Metastasis and Correlation with Fibroblast Activation Protein Expression. J. Nucl. Med. 2024, 65, 527–532. [Google Scholar] [CrossRef]
- Qin, J.; Han, C.; Li, H.; Wang, Z.; Hu, X.; Liu, L.; Zhu, S.; Zhao, J.; Sun, Y.; Wei, Y. Relationship between PD-L1 expression and [18F]FAPI versus [18F]FDG uptake on PET/CT in lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 3211–3222. [Google Scholar] [CrossRef]
- Liao, Y.; Ni, Y.; He, R.; Liu, W.; Du, J. Clinical implications of fibroblast activation protein-α in non-small cell lung cancer after curative resection: A new predictor for prognosis. J. Cancer Res. Clin. Oncol. 2013, 139, 1523–1528. [Google Scholar] [CrossRef]
- Watanabe, M.; Fendler, W.P.; Grafe, H.; Hirmas, N.; Hamacher, R.; Lanzafame, H.; Pabst, K.M.; Hautzel, H.; Aigner, C.; Kasper, S.; et al. Prognostic Implications of 68Ga-FAPI-46 PET/CT-Derived Parameters on Overall Survival in Various Types of Solid Tumors. J. Nucl. Med. 2024, 65, 1027–1034. [Google Scholar] [CrossRef]
- Altmann, A.; Haberkorn, U.; Siveke, J. The Latest Developments in Imaging of Fibroblast Activation Protein. J. Nucl. Med. 2021, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3651–3667. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, J.; Tang, W.; Zhang, Y.; Zhang, C.; Chen, Y. Efficacy and Safety Evaluation of 177Lu-FAP-2286 in the Treatment of Advanced Lung Cancer. Clin. Nucl. Med. 2024, 49, 830–837. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, X.; Xu, W.; Pang, Y.; Sun, L.; Wu, X.; Xu, P.; Zhang, J.; Guo, Z.; Lin, Q.; et al. Clinical Evaluation of 68Ga-FAPI-RGD for Imaging of Fibroblast Activation Protein and Integrin αvβ3 in Various Cancer Types. J. Nucl. Med. 2023, 64, 1210–1217. [Google Scholar] [CrossRef]
- Wang, R.; Jakobsson, V.; Wang, J.; Zhao, T.; Peng, X.; Li, B.; Xue, J.; Liang, N.; Zhu, Z.; Chen, X.; et al. Dual targeting PET tracer [68Ga]Ga-FAPI-RGD in patients with lung neoplasms: A pilot exploratory study. Theranostics 2023, 13, 2979–2992. [Google Scholar] [CrossRef]
- Ceuppens, H.; Pombo Antunes, A.R.; Navarro, L.; Ertveldt, T.; Berdal, M.; Nagachinta, S.; De Ridder, K.; Lahoutte, T.; Keyaerts, M.; Devoogdt, N.; et al. Efficient α and β− radionuclide therapy targeting fibroblast activation protein-α in an aggressive preclinical mouse tumour model. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 444–457. [Google Scholar] [CrossRef]



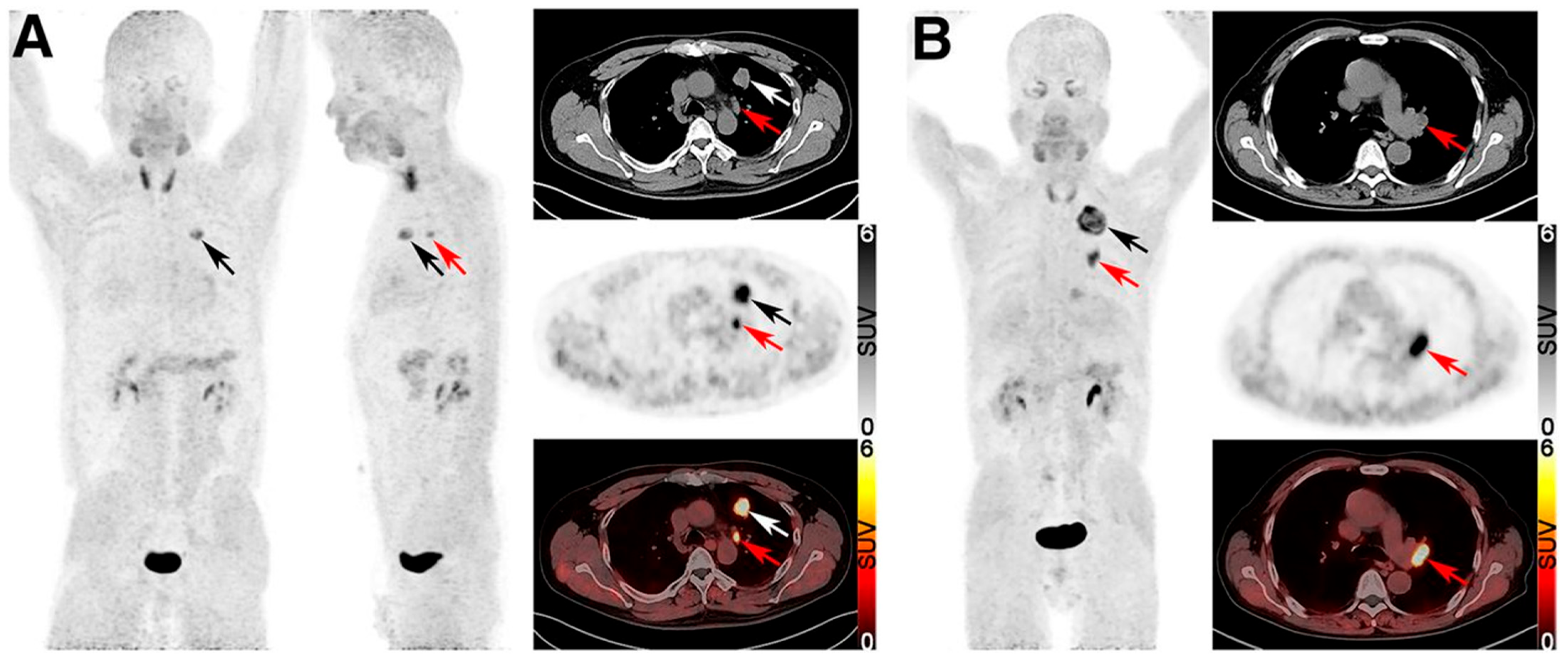
| Treatment | Time Interval |
|---|---|
| Chemotherapy | 1–4 weeks |
| Surgery | 6 weeks |
| Radiofrequency ablation | 6–8 weeks |
| Radiation therapy | 12 weeks |
| Immunotherapy | 8–9 weeks |
| ID | Official Title | Summary | Start Date |
|---|---|---|---|
| NCT04939610 | LuMIERE: A Phase 1/2, Multicenter, Open-label, Non-randomized Study to Investigate Safety and Tolerability, Pharmacokinetics, Dosimetry, and Preliminary Activity of 177Lu-FAP-2286 in Patients With an Advanced Solid Tumor | Phase 1 of this study is designed to evaluate the safety and establish the recommended intravenous Phase 2 dose for 177Lu FAP 2286 monotherapy. Phase 2 is designed to evaluate the safety and efficacy of 177Lu FAP 2286 as monotherapy. Participants in both Phase 1 and 2 will be selected for treatment with 177Lu-FAP-2286 based on 68Ga-FAP-2286 PET to determine tumor FAP expression (pancreas ductal adenocarcinoma, NSCLC, and breast cancer). | 30 July 2021 |
| NCT05723640 | Phase I, Open-Label Study of the Safety and Dosimetry of a 4-Dose Regimen of Escalating Doses of 177Lu-LNC1004 Injection in Adult Patients With Advanced FAP-Positive Solid Tumors | This study aims to determine the safe and tolerable dose of 177Lu-LNC1004 injection. The treatment regimen will consist of a single dose intravenous administration of 177Lu-LNC1004 Injection per 6-week cycle, for a total of 2 cycles. The dose per cycle will be fixed for each patient and will be escalated in 4 different dose levels, from 30 mCi to 100 mCi (1.11–3.7 GBq). | 3 October 2023 |
| NCT06211647 | A Clinical Study to Evaluate the Safety, Tolerability, Dosimetry and Preliminary Efficacy of 177Lu-XT117 Injection in FAP-positive Patients With Advanced Solid Tumors | This is a single-center, single-arm clinical study to evaluate the safety, tolerability, dosimetry and preliminary efficacy of 177Lu-XT117 injection in patients with FAP-positive advanced solid tumors. Dose escalation will be conducted to determine the dose limiting toxicity, maximum tolerated dose, recommended Phase 2 dose, and to assess dosimetry characteristics. | January 2024 |
| NCT06638034 | Diagnosis of Metastatic Tumors on 68Ga-FAPI-RGD PET-CT and Radioligand Therapy | This study conducts preliminary clinical transformation and internal irradiation dosimetry research on 177Lu-FAPI-RGD—a new dual-targeted 177Lu therapeutic drug for the first time in human. | 1 May 2024 |
| NCT06636617 | Safety, Dosimetry and Treatment Response of 177Lu-JH04 in Patients with FAP-Positive Tumors | This is a pilot study to assess the dosimetry, toxicity and response of 177Lu-JH04 in patients with FAP-positive tumors. All patients underwent 68Ga-FAPI PET/CT for selection and were successively divided into three groups of 3 people each. The three groups received successively an approximately 3.70 GBq (100 mCi), 5.55 GBq (150 mCi) and 7.40 GBq (200 mCi) of 177Lu-JH04 up to 4 cycles. | 21 August 2024 |
| NCT06710756 | A Phase I/IIa Image-Guided, Alpha-Particle Therapy Study of 203Pb-PSV359 and 212Pb-PSV359 in Patients With Solid Tumors That Are Known to be FAP-Positive | This is a prospective, multi-center open label dose finding, dose expansion study of 212Pb-PSV359 in subjects with a positive 203Pb-PSV359 SPECT/CT. | 28 April 2025 |
| NCT06640413 | A Phase I Study to Evaluate the Safety and Preliminary Signs of Efficacy of 177Lu-OncoFAP-23 Alone or in Combination With L19-IL2 as a Treatment of Metastatic FAP-positive Solid Tumors | This study is a prospective phase I, open-label, multiple ascending, multi-center dose escalation study to evaluate the safety and preliminary signs of efficacy of 177Lu-OncoFAP-23 alone and in combination with the antibody-cytokine conjugate L19-IL2 for the treatment of advanced/metastatic FAP-positive solid tumors. | May 2025 (estimated) |
| NCT06911489 | A Phase 1 Clinical Trial to Evaluate the Safety and Tolerability of 68Ga-NRT6020 Injection and 177Lu-NRT6020 Injection in FAP-Positive Participants With Advanced Solid Tumors | This study is designed to evaluate the preliminary efficacy, biodistribution, radiation dosimetry, and pharmacokinetics of 68Ga/177Lu-NRT6020 in FAP-positive patients with advanced solid tumors who have failed or have no available standard therapy. | July 2025 (estimated) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.J.; Chung, H.W.; So, Y.; Kim, I.A.; Kim, H.J.; Lee, K.Y. Recent Advances in PET and Radioligand Therapy for Lung Cancer: FDG and FAP. Cancers 2025, 17, 2549. https://doi.org/10.3390/cancers17152549
Lee EJ, Chung HW, So Y, Kim IA, Kim HJ, Lee KY. Recent Advances in PET and Radioligand Therapy for Lung Cancer: FDG and FAP. Cancers. 2025; 17(15):2549. https://doi.org/10.3390/cancers17152549
Chicago/Turabian StyleLee, Eun Jeong, Hyun Woo Chung, Young So, In Ae Kim, Hee Joung Kim, and Kye Young Lee. 2025. "Recent Advances in PET and Radioligand Therapy for Lung Cancer: FDG and FAP" Cancers 17, no. 15: 2549. https://doi.org/10.3390/cancers17152549
APA StyleLee, E. J., Chung, H. W., So, Y., Kim, I. A., Kim, H. J., & Lee, K. Y. (2025). Recent Advances in PET and Radioligand Therapy for Lung Cancer: FDG and FAP. Cancers, 17(15), 2549. https://doi.org/10.3390/cancers17152549





