IFN γ and the IFN γ Signaling Pathways in Merkel Cell Carcinoma
Simple Summary
Abstract
1. Introduction
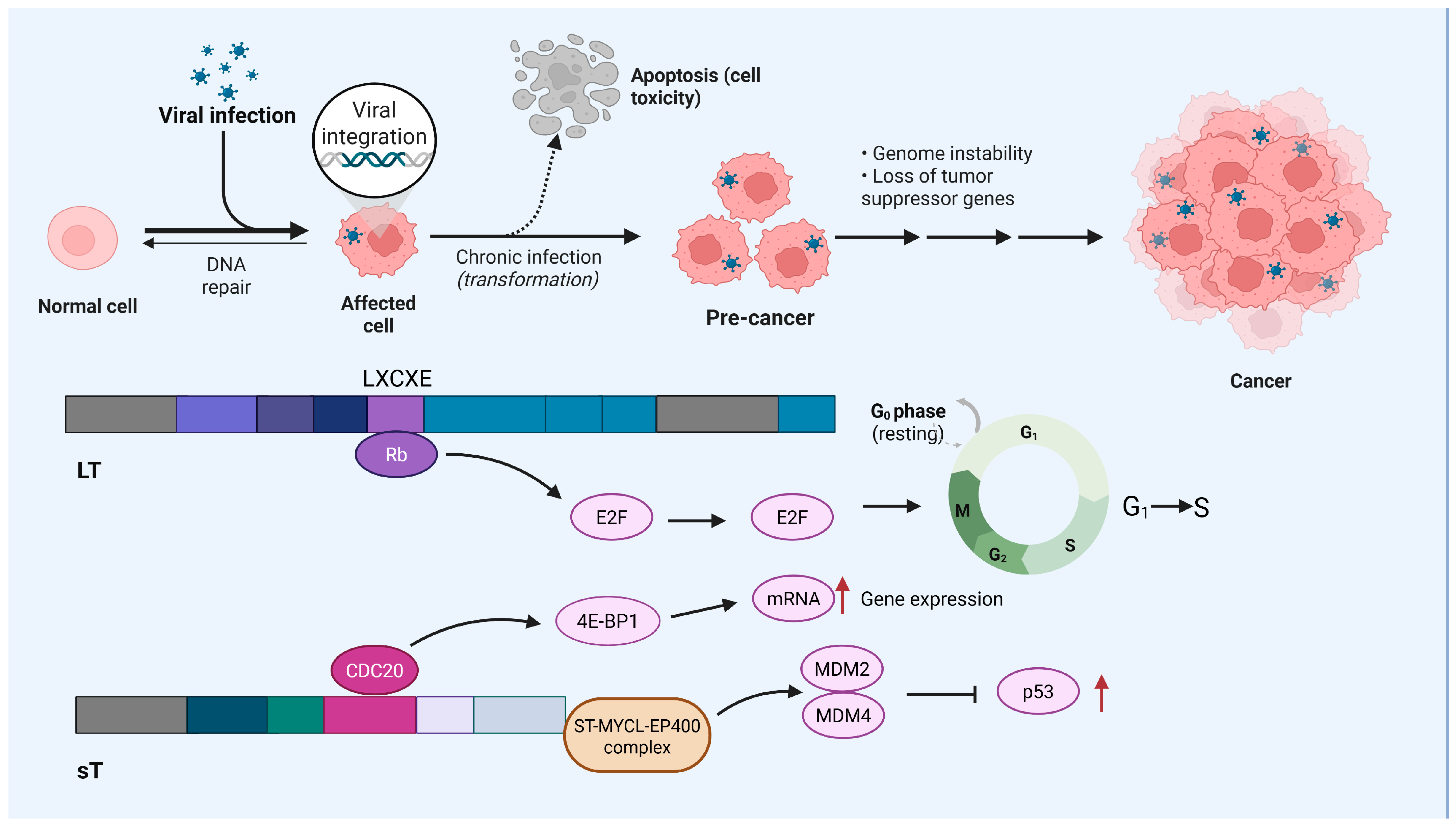
2. MCC and IFN γ
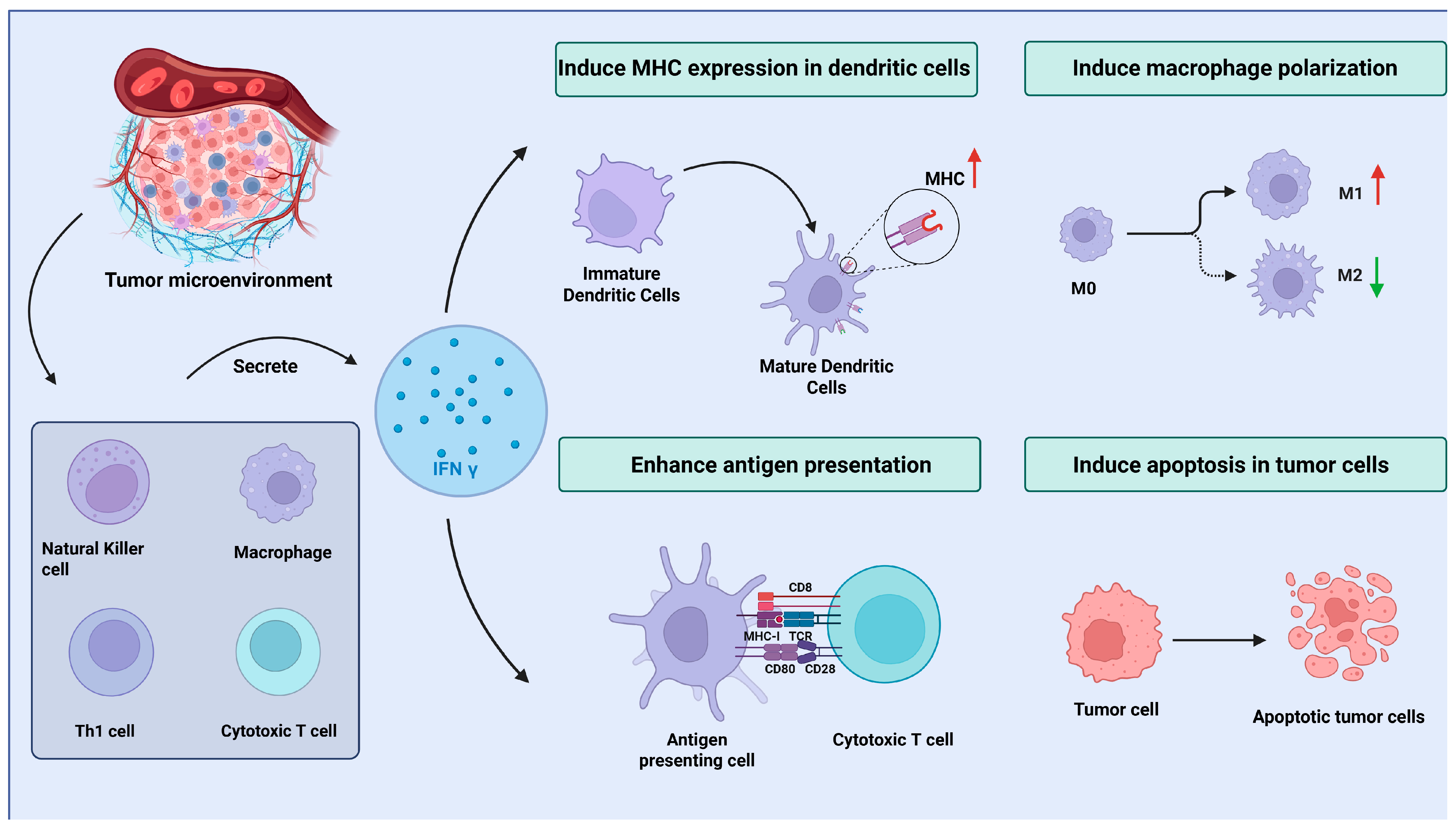
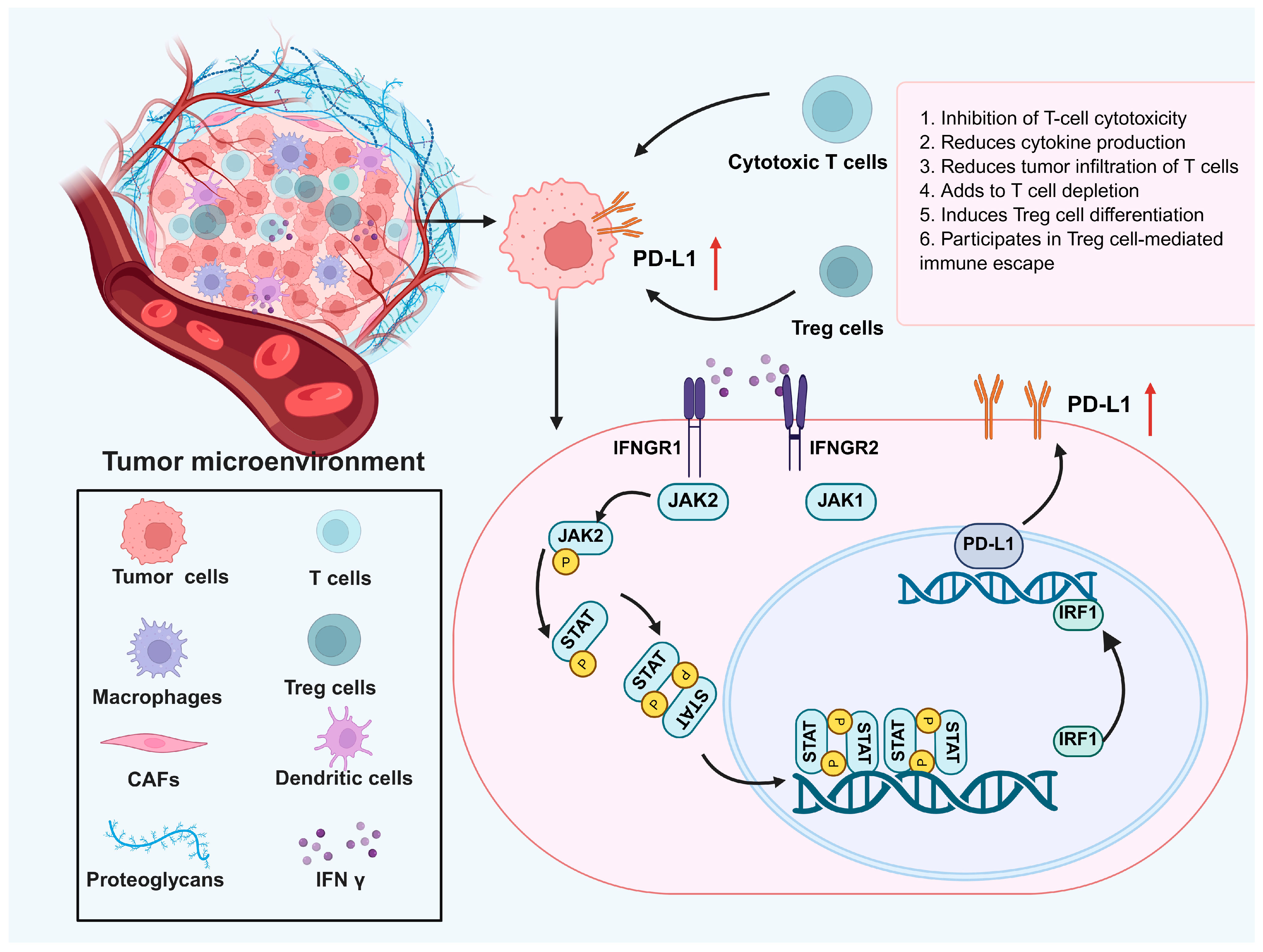
3. The Role of IFN γ in the MCC Microenvironment: Antitumor and Immune Evasion Effects
4. IFN γ and Immunotherapy for MCC
5. Enhancing IFN γ Therapy for MCCs: Overcoming Immune Evasion and Resistance
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APOBEC | Apolipoprotein B mRNA-editing catalytic polypeptide-like |
| CBP | CREB-binding protein |
| CDC20 | Cell division cycle 20 |
| CIITA | Class II transactivator |
| CTLA-4 | Cytotoxic T-lymphocyte associated protein 4 |
| CTL | Cytotoxic T lymphocyte |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DNA | Deoxyribonucleic acid |
| EMT | Epithelial-to-mesenchymal transition |
| ESMO | European Society for Medical Oncology |
| FBXW7 | F-box and WD repeat-domain-containing 7 |
| HDAC | Histone deacetylase |
| ICI | Immune checkpoint inhibitor |
| IDO1 | Indoleamine 2,3-Dioxygenase 1 |
| IFN | Interferon |
| IFN γ | Interferon gamma |
| IFNGR1/2 | Interferon gamma receptor 1/2 |
| IL-2/10/15 | Interleukin-2/10/15 |
| JAK1/2 | Janus kinase 1/2 |
| KDM7A | Lysine demethylase 7A |
| LAG3 | Lymphocyte-activation gene 3 |
| LT | Large T antigen |
| MCC | Merkel cell carcinoma |
| MCPyV | Merkel cell polyomavirus |
| MDSCs | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| MYCL | Myelocytomatosis oncogene-like |
| NF-κB | Nuclear factor Kappa B |
| NK cells | Natural killer cells |
| NCCR | Noncoding control region |
| NCCN | National Comprehensive Cancer Network |
| PD-1 | Programmed death 1 |
| PD-L1 | Programmed death-ligand 1 |
| PP2A | Protein phosphatase 2A |
| Rb | Retinoblastoma protein |
| ROS | Reactive oxygen species |
| sT | Small T antigen |
| STAT1/3/5 | Signal transducer and activator of transcription 1/3/5 |
| STING | Stimulator of interferon genes |
| TAMs | Tumor-associated macrophages |
| TGF-β | Transforming growth factor beta |
| TME | Tumor microenvironment |
| Tregs | Regulatory T Cells |
| UV | Ultraviolet |
References
- Xu, L.; Zou, C.; Zhang, S.; Chu, T.S.M.; Zhang, Y.; Chen, W.; Zhao, C.; Yang, L.; Xu, Z.; Dong, S.; et al. Reshaping the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J. Hematol. Oncol. 2022, 15, 87. [Google Scholar] [CrossRef]
- Fenton, S.E.; Saleiro, D.; Platanias, L.C. Type I and II Interferons in the Anti-Tumor Immune Response. Cancers 2021, 13, 1037. [Google Scholar] [CrossRef]
- Benci, J.L.; Johnson, L.R.; Choa, R.; Xu, Y.; Qiu, J.; Zhou, Z.; Xu, B.; Ye, D.; Nathanson, K.L.; June, C.H.; et al. Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade. Cell 2019, 178, 933–948.e914. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Tsoi, J.; Onyshchenko, M.; Abril-Rodriguez, G.; Ross-Macdonald, P.; Wind-Rotolo, M.; Champhekar, A.; Medina, E.; Torrejon, D.Y.; Shin, D.S.; et al. Conserved Interferon-γ Signaling Drives Clinical Response to Immune Checkpoint Blockade Therapy in Melanoma. Cancer Cell 2020, 38, 500–515.e3. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Deniset, J.F.; Belke, D.; Lee, W.Y.; Jorch, S.K.; Deppermann, C.; Hassanabad, A.F.; Turnbull, J.D.; Teng, G.; Rozich, I.; Hudspeth, K.; et al. Gata6(+) Pericardial Cavity Macrophages Relocate to the Injured Heart and Prevent Cardiac Fibrosis. Immunity 2019, 51, 131–140.e135. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef] [PubMed]
- Spassova, I.; Ugurel, S.; Terheyden, P.; Sucker, A.; Hassel, J.C.; Ritter, C.; Kubat, L.; Habermann, D.; Farahpour, F.; Saeedghalati, M.; et al. Predominance of Central Memory T Cells with High T-Cell Receptor Repertoire Diversity is Associated with Response to PD-1/PD-L1 Inhibition in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 2257–2267. [Google Scholar] [CrossRef]
- Song, L.; Bretz, A.C.; Gravemeyer, J.; Spassova, I.; Muminova, S.; Gambichler, T.; Sriram, A.; Ferrone, S.; Becker, J.C. The HDAC Inhibitor Domatinostat Promotes Cell-Cycle Arrest, Induces Apoptosis, and Increases Immunogenicity of Merkel Cell Carcinoma Cells. J. Investig. Dermatol. 2021, 141, 903–912.e904. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer. 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Kalbasi, A.; Tariveranmoshabad, M.; Hakimi, K.; Kremer, S.; Campbell, K.M.; Funes, J.M.; Vega-Crespo, A.; Parisi, G.; Champekar, A.; Nguyen, C.; et al. Uncoupling interferon signaling and antigen presentation to overcome immunotherapy resistance due to JAK1 loss in melanoma. Sci. Transl. Med. 2020, 12, eabb0152. [Google Scholar] [CrossRef]
- Sauerer, T.; Lischer, C.; Weich, A.; Berking, C.; Vera, J.; Dörrie, J. Single-Molecule RNA Sequencing Reveals IFNγ-Induced Differential Expression of Immune Escape Genes in Merkel Cell Polyomavirus-Positive MCC Cell Lines. Front. Microbiol. 2021, 12, 785662. [Google Scholar] [CrossRef]
- Patel, S.A.; Nilsson, M.B.; Yang, Y.; Le, X.; Tran, H.T.; Elamin, Y.Y.; Yu, X.; Zhang, F.; Poteete, A.; Ren, X.; et al. IL6 Mediates Suppression of T- and NK-cell Function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin. Cancer Res. 2023, 29, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Gerer, K.F.; Erdmann, M.; Hadrup, S.R.; Lyngaa, R.; Martin, L.M.; Voll, R.E.; Schuler-Thurner, B.; Schuler, G.; Schaft, N.; Hoyer, S.; et al. Preclinical evaluation of NF-κB-triggered dendritic cells expressing the viral oncogenic driver of Merkel cell carcinoma for therapeutic vaccination. Ther. Adv. Med. Oncol. 2017, 9, 451–464. [Google Scholar] [CrossRef]
- Alves, A.S.; Scampa, M.; Martineau, J.; Giordano, S.; Kalbermatten, D.F.; Oranges, C.M. Merkel Cell Carcinoma of the External Ear: Population-Based Analysis and Survival Outcomes. Cancers 2022, 14, 5653. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Sindrilaru, A.; Crisan, D.; Thaiss, W.; Steinacker, J.; Beer, M.; Wiegel, T.; Scharffetter-Kochanek, K.; Beer, A.J.; Prasad, V. Short-Interval, Low-Dose Peptide Receptor Radionuclide Therapy in Combination with PD-1 Checkpoint Immunotherapy Induces Remission in Immunocompromised Patients with Metastatic Merkel Cell Carcinoma. Pharmaceutics 2022, 14, 1466. [Google Scholar] [CrossRef]
- Spassova, I.; Ugurel, S.; Kubat, L.; Zimmer, L.; Terheyden, P.; Mohr, A.; Björn Andtback, H.; Villabona, L.; Leiter, U.; Eigentler, T.; et al. Clinical and molecular characteristics associated with response to therapeutic PD-1/PD-L1 inhibition in advanced Merkel cell carcinoma. J. Immunother. Cancer 2022, 10, e003198. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.L.; Zhao, L.; Johnson, B.; Wang, X.; Carskadon, S.; Palanisamy, N.; Rhodes, D.R.; Mannan, R.; Vo, J.N.; Choi, J.E.; et al. Virus-positive Merkel Cell Carcinoma Is an Independent Prognostic Group with Distinct Predictive Biomarkers. Clin. Cancer Res. 2021, 27, 2494–2504. [Google Scholar] [CrossRef]
- Liu, W.; You, J. Molecular Mechanisms of Merkel Cell Polyomavirus Transformation and Replication. Annu. Rev. Virol. 2020, 7, 289–307. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Cheng, J.; Ward-Shaw, E.; Dick, F.A.; DeCaprio, J.A.; Lambert, P.F. Merkel cell polyomavirus large T antigen binding to pRb promotes skin hyperplasia and tumor development. PLoS Pathog. 2022, 18, e1010551. [Google Scholar] [CrossRef]
- Li, Z.; Ji, W.; Hu, Q.; Zhu, P.; Jin, Y.; Duan, G. Current status of Merkel cell carcinoma: Epidemiology, pathogenesis and prognostic factors. Virology 2024, 599, 110186. [Google Scholar] [CrossRef]
- Shuda, M.; Kwun, H.J.; Feng, H.; Chang, Y.; Moore, P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Investig. 2011, 121, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Park, D.E.; Berrios, C.; White, E.A.; Arora, R.; Yoon, R.; Branigan, T.; Xiao, T.; Westerling, T.; Federation, A.; et al. Merkel cell polyomavirus recruits MYCL to the EP400 complex to promote oncogenesis. PLoS Pathog. 2017, 13, e1006668. [Google Scholar] [CrossRef] [PubMed]
- Dimitraki, M.G.; Sourvinos, G. Merkel Cell Polyomavirus (MCPyV) and Cancers: Emergency Bell or False Alarm? Cancers 2022, 14, 5548. [Google Scholar] [CrossRef]
- Tello, T.L.; Coggshall, K.; Yom, S.S.; Yu, S.S. Merkel cell carcinoma: An update and review: Current and future therapy. J. Am. Acad. Dermatol. 2018, 78, 445–454. [Google Scholar] [CrossRef]
- Angeles, C.V.; Sabel, M.S. Immunotherapy for Merkel cell carcinoma. J. Surg. Oncol. 2021, 123, 775–781. [Google Scholar] [CrossRef]
- Truong, K.; Goldinger, S.M.; Chou, S.; Howle, J.R.; Veness, M.J.; Fernandez-Peñas, P.; Varey, A.H.R. Merkel cell carcinoma in situ: A systematic review of prognosis and management. Australas. J. Dermatol. 2022, 63, e6–e12. [Google Scholar] [CrossRef]
- Donizy, P.; Wróblewska, J.P.; Dias-Santagata, D.; Woznica, K.; Biecek, P.; Mochel, M.C.; Wu, C.L.; Kopczynski, J.; Pieniazek, M.; Ryś, J.; et al. Merkel Cell Carcinoma of Unknown Primary: Immunohistochemical and Molecular Analyses Reveal Distinct UV-Signature/MCPyV-Negative and High Immunogenicity/MCPyV-Positive Profiles. Cancers 2021, 13, 1621. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Pernas, S.; Martin, M.; Kaufman, P.A.; Gil-Martin, M.; Gomez Pardo, P.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: A phase 1, single-arm, dose-escalation trial. Lancet Oncol. 2018, 19, 812–824. [Google Scholar] [CrossRef]
- Sergi, M.C.; Lauricella, E.; Porta, C.; Tucci, M.; Cives, M.J. An update on Merkel cell carcinoma. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188880. [Google Scholar] [CrossRef] [PubMed]
- Dellambra, E.; Carbone, M.L.; Ricci, F.; Ricci, F.; Di Pietro, F.R.; Moretta, G.; Verkoskaia, S.; Feudi, E.; Failla, C.M.; Abeni, D.; et al. Merkel Cell Carcinoma. Biomedicines 2021, 9, 718. [Google Scholar] [CrossRef]
- Becker, J.C.; Ugurel, S.; Leiter, U.; Meier, F.; Gutzmer, R.; Haferkamp, S.; Zimmer, L.; Livingstone, E.; Eigentler, T.K.; Hauschild, A.; et al. Adjuvant immunotherapy with nivolumab versus observation in completely resected Merkel cell carcinoma (ADMEC-O): Disease-free survival results from a randomised, open-label, phase 2 trial. Lancet 2023, 402, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Turshudzhyan, A.; Hadfield, M.; Grant-Kels, J. Updates on the diagnosis, current and future therapeutic options in Merkel-cell carcinoma. Melanoma Res. 2021, 31, 421–425. [Google Scholar] [CrossRef]
- Xie, J.; Huppa, J.B.; Newell, E.W.; Huang, J.; Ebert, P.J.; Li, Q.J.; Davis, M.M. Photocrosslinkable pMHC monomers stain T cells specifically and cause ligand-bound TCRs to be ‘preferentially’ transported to the cSMAC. Nat. Immunol. 2012, 13, 674–680. [Google Scholar] [CrossRef]
- Tani, T.; Mathsyaraja, H.; Campisi, M.; Li, Z.H.; Haratani, K.; Fahey, C.G.; Ota, K.; Mahadevan, N.R.; Shi, Y.; Saito, S.; et al. TREX1 Inactivation Unleashes Cancer Cell STING-Interferon Signaling and Promotes Antitumor Immunity. Cancer Discov. 2024, 14, 752–765. [Google Scholar] [CrossRef]
- Bhat, P.; Leggatt, G.; Waterhouse, N.; Frazer, I.H. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017, 8, e2836. [Google Scholar] [CrossRef]
- Xie, C.; Liu, C.; Wu, B.; Lin, Y.; Ma, T.; Xiong, H.; Wang, Q.; Li, Z.; Ma, C.; Tu, Z. Effects of IRF1 and IFN-β interaction on the M1 polarization of macrophages and its antitumor function. Int. J. Mol. Med. 2016, 38, 148–160. [Google Scholar] [CrossRef]
- He, T.; Tang, C.; Xu, S.; Moyana, T.; Xiang, J. Interferon gamma stimulates cellular maturation of dendritic cell line DC2.4 leading to induction of efficient cytotoxic T cell responses and antitumor immunity. Cell. Mol. Immunol. 2007, 4, 105–111. [Google Scholar]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.; Shuda, M.; Weinkam, R.; Schrama, D.; Feng, H.; Chang, Y.; Moore, P.S.; Becker, J.C. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J. Virol. 2010, 84, 7064–7072. [Google Scholar] [CrossRef]
- Kumar, A.; Chen, T.; Pakkanen, S.; Kantele, A.; Söderlund-Venermo, M.; Hedman, K.; Franssila, R. T-helper cell-mediated proliferation and cytokine responses against recombinant Merkel cell polyomavirus-like particles. PLoS ONE 2011, 6, e25751. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.U.; Braunschweig, T.; Ghassemi, A.; Rübben, A. Immunotherapy with imiquimod and interferon alfa for metastasized Merkel cell carcinoma. Curr. Oncol. 2016, 23, e150–e153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonavita, E.; Bromley, C.P.; Jonsson, G.; Pelly, V.S.; Sahoo, S.; Walwyn-Brown, K.; Mensurado, S.; Moeini, A.; Flanagan, E.; Bell, C.R.; et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity 2020, 53, 1215–1229.e8. [Google Scholar] [CrossRef]
- Bhatia, S.; Afanasiev, O.; Nghiem, P. Immunobiology of Merkel cell carcinoma: Implications for immunotherapy of a polyomavirus-associated cancer. Curr. Oncol. Rep. 2011, 13, 488–497. [Google Scholar] [CrossRef]
- Rivas, C.; Aaronson, S.A.; Munoz-Fontela, C. Dual Role of p53 in Innate Antiviral Immunity. Viruses 2010, 2, 298–313. [Google Scholar] [CrossRef]
- Kichev, A.; Rousset, C.I.; Baburamani, A.A.; Levison, S.W.; Wood, T.L.; Gressens, P.; Thornton, C.; Hagberg, H. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling and cell death in the immature central nervous system after hypoxia-ischemia and inflammation. J. Biol. Chem. 2014, 289, 9430–9439. [Google Scholar] [CrossRef]
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ synergy amplifies senescence-associated inflammation and SARS-CoV-2 receptor expression via hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Goyal, A.; Thapa, R.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Singh, M.; Rohilla, S.; Saini, T.K.; Kukreti, N.; et al. Uncovering the complex role of interferon-gamma in suppressing type 2 immunity to cancer. Cytokine 2023, 171, 156376. [Google Scholar] [CrossRef]
- Church, C.; Pulliam, T.; Longino, N.; Park, S.Y.; Smythe, K.S.; Makarov, V.; Riaz, N.; Jing, L.; Amezquita, R.; Campbell, J.S.; et al. Transcriptional and functional analyses of neoantigen-specific CD4 T cells during a profound response to anti-PD-L1 in metastatic Merkel cell carcinoma. J. Immunother. Cancer 2022, 10, e005328. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, T.; Endo, R.; Sasaki, S.; Takahashi, N.; Sato, Y.; Hyodo, M.; Hayakawa, Y.; Harashima, H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 2021, 9, e002852. [Google Scholar] [CrossRef]
- Yang, D.; Tian, R.; Deng, R.; Xue, B.; Liu, S.; Wang, L.; Li, H.; Liu, Q.; Wan, M.; Tang, S.; et al. The dual functions of KDM7A in HBV replication and immune microenvironment. Microbiol. Spectr. 2023, 11, e0164123. [Google Scholar] [CrossRef] [PubMed]
- Hornburg, M.; Desbois, M.; Lu, S.; Guan, Y.; Lo, A.A.; Kaufman, S.; Elrod, A.; Lotstein, A.; DesRochers, T.M.; Munoz-Rodriguez, J.L.; et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell 2021, 39, 928–944.e6. [Google Scholar] [CrossRef]
- Miller, N.J.; Church, C.D.; Fling, S.P.; Kulikauskas, R.; Ramchurren, N.; Shinohara, M.M.; Kluger, H.M.; Bhatia, S.; Lundgren, L.; Cheever, M.A.; et al. Merkel cell polyomavirus-specific immune responses in patients with Merkel cell carcinoma receiving anti-PD-1 therapy. J. Immunother. Cancer 2018, 6, 131. [Google Scholar] [CrossRef]
- Lei, X.; Xiao, R.; Chen, Z.; Ren, J.; Zhao, W.; Tang, W.; Wen, K.; Zhu, Y.; Li, X.; Ouyang, S.; et al. Augmenting antitumor efficacy of Th17-derived Th1 cells through IFN-γ-induced type I interferon response network via IRF7. Proc. Natl. Acad. Sci. USA 2024, 121, e2412120121. [Google Scholar] [CrossRef]
- Du, J.; Zhou, L.; Chen, X.; Yan, S.; Ke, M.; Lu, X.; Wang, Z.; Yu, W.; Xiang, A.P. IFN-γ-primed human bone marrow mesenchymal stem cells induce tumor cell apoptosis in vitro via tumor necrosis factor-related apoptosis-inducing ligand. Int. J. Biochem. Cell Biol. 2012, 44, 1305–1314. [Google Scholar] [CrossRef]
- Imai, D.; Yoshizumi, T.; Okano, S.; Itoh, S.; Ikegami, T.; Harada, N.; Aishima, S.; Oda, Y.; Maehara, Y. IFN-γ Promotes Epithelial-Mesenchymal Transition and the Expression of PD-L1 in Pancreatic Cancer. J. Surg. Res. 2019, 240, 115–123. [Google Scholar] [CrossRef]
- Que, L.; Li, Y.; Dainichi, T.; Kukimoto, I.; Nishiyama, T.; Nakano, Y.; Shima, K.; Suzuki, T.; Sato, Y.; Horike, S.; et al. IFN-γ-Induced APOBEC3B Contributes to Merkel Cell Polyomavirus Genome Mutagenesis in Merkel Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 1793–1803.e11. [Google Scholar] [CrossRef]
- Willmes, C.; Adam, C.; Alb, M.; Völkert, L.; Houben, R.; Becker, J.C.; Schrama, D. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012, 72, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Angelicola, S.; Giunchi, F.; Ruzzi, F.; Frascino, M.; Pitzalis, M.; Scalambra, L.; Semprini, M.S.; Pittino, O.M.; Cappello, C.; Siracusa, I.; et al. PD-L1 and IFN-γ modulate Non-Small Cell Lung Cancer (NSCLC) cell plasticity associated to immune checkpoint inhibitor (ICI)-mediated hyperprogressive disease (HPD). J. Transl. Med. 2025, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Wang, Q.; Wu, G.H.; Liu, W.Z.; Wang, B.; Chen, Y.J. Direct and indirect effects of IFN-α2b in malignancy treatment: Not only an archer but also an arrow. Biomark. Res. 2022, 10, 69. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y. Present and future of cancer nano-immunotherapy: Opportunities, obstacles and challenges. Mol. Cancer 2025, 24, 26. [Google Scholar] [CrossRef]
- Galassi, C.; Galluzzi, L. Cancer stem cell immunoediting by IFNγ. Cell Death Dis. 2023, 14, 538. [Google Scholar] [CrossRef]
- Lau, V.W.C.; Mead, G.J.; Varyova, Z.; Mazet, J.M.; Krishnan, A.; Roberts, E.W.; Prota, G.; Gileadi, U.; Midwood, K.S.; Cerundolo, V.; et al. Remodelling of the immune landscape by IFNγ counteracts IFNγ-dependent tumour escape in mouse tumour models. Nat. Commun. 2025, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, M.; Liu, Z. Dysregulation in IFN-γ signaling and response: The barricade to tumor immunotherapy. Front. Immunol. 2023, 14, 1190333. [Google Scholar] [CrossRef]
- Knopf, P.; Stowbur, D.; Hoffmann, S.H.L.; Hermann, N.; Maurer, A.; Bucher, V.; Poxleitner, M.; Tako, B.; Sonanini, D.; Krishnamachary, B.; et al. Acidosis-mediated increase in IFN-γ-induced PD-L1 expression on cancer cells as an immune escape mechanism in solid tumors. Mol. Cancer 2023, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Wu, J.; Huang, J.; Zhu, W.; Sun, H.; Wang, X.; Lin, D.; Li, X.; Shi, D.; Liu, Z.; et al. FLI1 promotes IFN-γ-induced kynurenine production to impair anti-tumor immunity. Nat. Commun. 2024, 15, 4590. [Google Scholar] [CrossRef]
- Schelker, R.C.; Fioravanti, J.A.-O.; Mastrogiovanni, F.; Baldwin, J.G.; Rana, N.; Li, P.; Chen, P.; Vadász, T.; Spolski, R.; Heuser-Loy, C.A.-O.; et al. LIM-domain-only 4 (LMO4) enhances CD8(+) T-cell stemness and tumor rejection by boosting IL-21-STAT3 signaling. Signal Transduct. Target. Ther. 2024, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Yang, B.; Chen, J.; Wang, S.; Zhang, W.; Guo, Y.A.-O.; Han, Y.; Li, H.; Dang, Y.; Yuan, Y.; et al. Gallic acid induces T-helper-1-like T(reg) cells and strengthens immune checkpoint blockade efficacy. J. Immunother. Cancer 2022, 10, e004037. [Google Scholar] [CrossRef] [PubMed]
- Sumaria, N.; Fiala, G.A.-O.; Inácio, D.; Curado-Avelar, M.; Cachucho, A.; Pinheiro, R.; Wiesheu, R.A.-O.; Kimura, S.A.-O.X.; Courtois, L.; Blankenhaus, B.; et al. Perinatal thymic-derived CD8αβ-expressing γδ T cells are innate IFN-γ producers that expand in IL-7R-STAT5B-driven neoplasms. Nat. Immunol. 2024, 25, 1207–1217. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. STAT4 and STAT6, their role in cellular and humoral immunity and in diverse human diseases. Int. Rev. Immunol. 2024, 43, 394–418. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Garza-Davila, V.F.; Valdespino-Valdes, J.; Barrera, F.J.; Ocampo-Candiani, J.; Garza-Rodríguez, V. Clinical impact of immunotherapy in Merkel cell carcinoma patients: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2022, 87, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404.e9. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Haoyue, W.; Kexiang, S.; Shan, T.W.; Jiamin, G.; Luyun, Y.; Junkai, W.; Wanli, D. Icariin promoted ferroptosis by activating mitochondrial dysfunction to inhibit colorectal cancer and synergistically enhanced the efficacy of PD-1 inhibitors. Phytomedicine 2025, 136, 156224. [Google Scholar] [CrossRef]
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, D.R.; Rimm, D.L. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol. Res. 2015, 3, 326–332. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Slingluff, C.L.; Zarour, H.M.; Tawbi, H.A.; Kirkwood, J.M.; Postow, M.A.; Friedlander, P.; Devoe, C.E.; Gaughan, E.M.; Mauldin, I.S.; Olson, W.C.; et al. A phase 1 study of NY-ESO-1 vaccine + anti-CTLA4 antibody Ipilimumab (IPI) in patients with unresectable or metastatic melanoma. Oncoimmunology 2021, 10, 1898105. [Google Scholar] [CrossRef]
- Wang, Y.; Radfar, S.; Khong, H.T. Activated CD4+ T cells enhance radiation effect through the cooperation of interferon-gamma and TNF-alpha. BMC Cancer 2010, 10, 60. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, Q.; Xu, Y.; Wang, L. Improving the efficacy of combined radiotherapy and immunotherapy: Focusing on the effects of radiosensitivity. Radiat. Oncol. 2023, 18, 89. [Google Scholar] [CrossRef]
- Kong, F.; Sun, T.; Kong, X.; Xie, D.; Li, Z.; Xie, K. Krüppel-like Factor 4 Suppresses Serine/Threonine Kinase 33 Activation and Metastasis of Gastric Cancer through Reversing Epithelial-Mesenchymal Transition. Clin. Cancer Res. 2018, 24, 2440–2451. [Google Scholar] [CrossRef]
- Harms, P.W.; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef]
- Kervarrec, T.; Gaboriaud, P.; Berthon, P.; Zaragoza, J.; Schrama, D.; Houben, R.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Aubin, F.; Bens, G.; et al. Merkel cell carcinomas infiltrated with CD33(+) myeloid cells and CD8(+) T cells are associated with improved outcome. J. Am. Acad. Dermatol. 2018, 78, 973–982.e8. [Google Scholar] [CrossRef] [PubMed]
- Mitteldorf, C.; Berisha, A.; Tronnier, M.; Pfaltz, M.C.; Kempf, W. PD-1 and PD-L1 in neoplastic cells and the tumor microenvironment of Merkel cell carcinoma. J. Cutan. Pathol. 2017, 44, 740–746. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Nunoi, H.; Nakamura, H.; Nishimura, T.; Matsukura, M. Recent topics and advanced therapies in chronic granulomatous disease. Hum. Cell 2023, 36, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Crispe, I.N.; Pierce, R.H. Killer T cells find meaningful encounters through iMATEs. Nat. Immunol. 2013, 14, 533–534. [Google Scholar] [CrossRef]
- Schadendorf, D.; Lebbé, C.; Zur Hausen, A.; Avril, M.F.; Hariharan, S.; Bharmal, M.; Becker, J.C. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur. J. Cancer 2017, 71, 53–69. [Google Scholar] [CrossRef]
- Razaghi, A.; Durand-Dubief, M.; Brusselaers, N.; Björnstedt, M. Combining PD-1/PD-L1 blockade with type I interferon in cancer therapy. Front. Immunol. 2023, 14, 1249330. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Zhou, J.; Li, L.; Song, C.; Chen, E. Optimal Treatment Based on Interferon No Longer Makes Clinical Cure of Chronic Hepatitis B Far Away: An Evidence-Based Review on Emerging Clinical Data. Clin. Pharmacol. Ther. 2024, 116, 295–303. [Google Scholar] [CrossRef]
- Jochems, C.; Fantini, M.; Fernando, R.I.; Kwilas, A.R.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Kim, Y.S.; Brechbiel, M.W.; Gulley, J.L.; et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget 2016, 7, 37762–37772. [Google Scholar] [CrossRef]
- Chen, X.; Pan, X.; Zhang, W.; Guo, H.; Cheng, S.; He, Q.; Yang, B.; Ding, L. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm. Sin. B 2020, 10, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, L.; Yang, X.; Gao, Q.; Qu, Y.; Wu, L. Design, synthesis, and biological evaluation of novel napabucasin-melatonin hybrids as potent STAT3 inhibitors. Bioorg. Chem. 2023, 136, 106541. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.I.; Barrett, J.; Wong, S.; Chang, M.J.; Muranski, P.J.; Brownell, I. Robust Production of Merkel Cell Polyomavirus Oncogene Specific T Cells From Healthy Donors for Adoptive Transfer. Front. Immunol. 2020, 11, 592721. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, F.C.A.; Kreuz, M.; de Lara, I.C.A.; Lôbo, A.O.M.; Burbano, R.M.R. Efficacy and safety of PD-1/PD-L1 inhibitors in patients with Merkel Cell Carcinoma: A systematic review and Meta-analysis. BMC Cancer 2024, 24, 1357. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Guan, J.; Zhou, Q.; Liu, W.; Becker, J.C.; Deng, D. IFN γ and the IFN γ Signaling Pathways in Merkel Cell Carcinoma. Cancers 2025, 17, 2547. https://doi.org/10.3390/cancers17152547
Song L, Guan J, Zhou Q, Liu W, Becker JC, Deng D. IFN γ and the IFN γ Signaling Pathways in Merkel Cell Carcinoma. Cancers. 2025; 17(15):2547. https://doi.org/10.3390/cancers17152547
Chicago/Turabian StyleSong, Lina, Jinye Guan, Qunmei Zhou, Wenshang Liu, Jürgen C. Becker, and Dan Deng. 2025. "IFN γ and the IFN γ Signaling Pathways in Merkel Cell Carcinoma" Cancers 17, no. 15: 2547. https://doi.org/10.3390/cancers17152547
APA StyleSong, L., Guan, J., Zhou, Q., Liu, W., Becker, J. C., & Deng, D. (2025). IFN γ and the IFN γ Signaling Pathways in Merkel Cell Carcinoma. Cancers, 17(15), 2547. https://doi.org/10.3390/cancers17152547







