Medication Adherence in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedure
2.2. Participants
2.3. Sociodemographic and Clinical Data
2.4. Outcome Measures
2.4.1. Objective Measures of Adherence
2.4.2. Patient-Reported Measures of Adherence
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
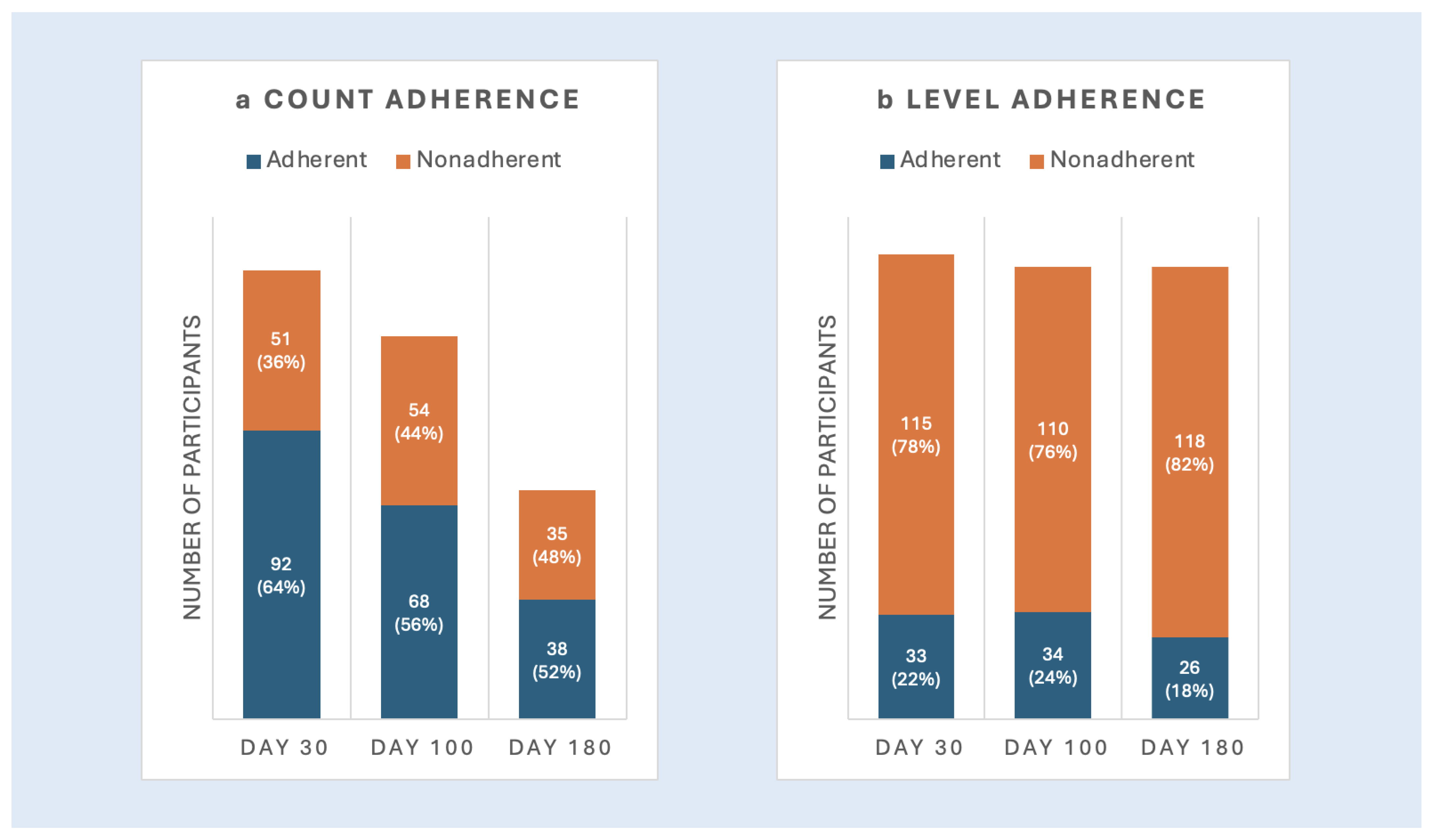
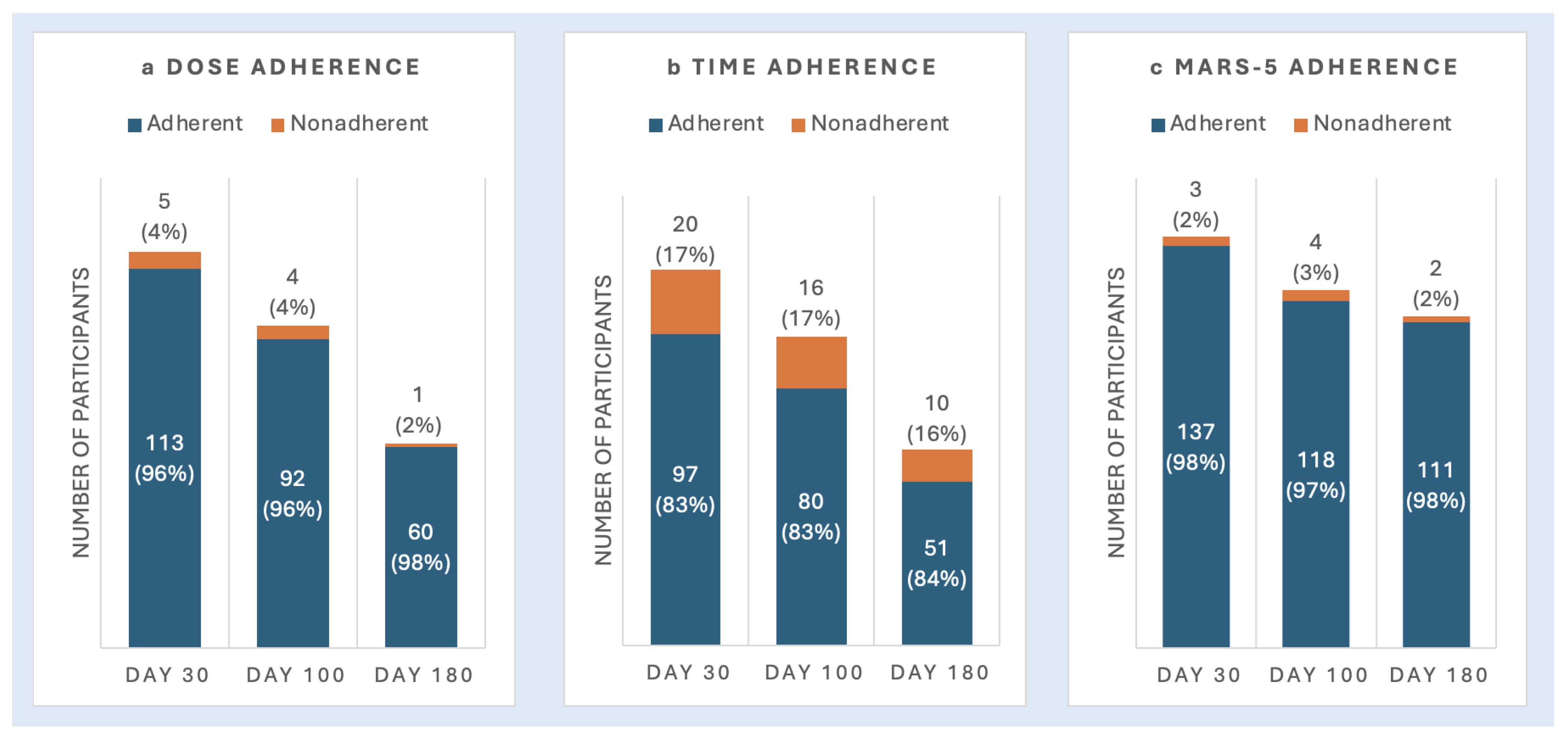
3.1.1. Objective Measures of Adherence
3.1.2. Patient-Reported Measures of Adherence
3.1.3. Level of Agreement Across Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, N.; Zhao, Q.; Ni, B.; Elder, P.; Puto, M.; Benson, D.M.; Rosko, A.; Chaudhry, M.; Devarakonda, S.; Bumma, N.; et al. Effect of Early Post-Transplantation Tacrolimus Concentration on the Risk of Acute Graft-Versus-Host Disease in Allogenic Stem Cell Transplantation. Cancers 2021, 13, 613. [Google Scholar] [CrossRef]
- Pai, A.L.H.; Rausch, J.; Drake, S.; Morrison, C.F.; Lee, J.L.; Nelson, A.; Tackett, A.; Berger, S.; Szulczewski, L.; Mara, C.; et al. Poor Adherence is Associated with More Infections after Pediatric Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transpl. 2018, 24, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.F.; Martsolf, D.M.; Wehrkamp, N.; Tehan, R.; Pai, A.L.H. Medication Adherence in Hematopoietic Stem Cell Transplantation: A Review of the Literature. Biol. Blood Marrow Transpl. 2017, 23, 562–568. [Google Scholar] [CrossRef]
- Gresch, B.; Kirsch, M.; Fierz, K.; Halter, J.P.; Nair, G.; Denhaerynck, K.; De Geest, S. Medication nonadherence to immunosuppressants after adult allogeneic haematopoietic stem cell transplantation: A multicentre cross-sectional study. Bone Marrow Transpl. 2017, 52, 304–306. [Google Scholar] [CrossRef]
- Lehrer, J.; Brissot, E.; Ruggeri, A.; Dulery, R.; Vekhoff, A.; Battipaglia, G.; Giannotti, F.; Fernandez, C.; Mohty, M.; Antignac, M. Medication adherence among allogeneic hematopoietic stem cell transplant recipients: A pilot single-center study. Bone Marrow Transpl. 2018, 53, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Noens, L.; van Lierde, M.A.; De Bock, R.; Verhoef, G.; Zachée, P.; Berneman, Z.; Martiat, P.; Mineur, P.; Van Eygen, K.; MacDonald, K.; et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: The ADAGIO study. Blood 2009, 113, 5401–5411. [Google Scholar] [CrossRef]
- Jönsson, S.; Olsson, B.; Söderberg, J.; Wadenvik, H. Good adherence to imatinib therapy among patients with chronic myeloid leukemia--a single-center observational study. Ann. Hematol. 2012, 91, 679–685. [Google Scholar] [CrossRef]
- Puts, M.T.E.; Tu, H.A.; Tourangeau, A.; Howell, D.; Fitch, M.; Springall, E.; Alibhai, S.M.H. Factors influencing adherence to cancer treatment in older adults with cancer: A systematic review. Ann. Oncol. 2014, 25, 564–577. [Google Scholar] [CrossRef]
- Marco, D.N.; Salas, M.Q.; Gutiérrez-García, G.; Monge, I.; Riu, G.; Carcelero, E.; Roma, J.R.; Llobet, N.; Arcarons, J.; Suárez-Lledó, M.; et al. Impact of Early Intrapatient Variability of Tacrolimus Concentrations on the Risk of Graft-Versus-Host Disease after Allogeneic Stem Cell Transplantation Using High-Dose Post-Transplant Cyclophosphamide. Pharmaceuticals 2022, 15, 1529. [Google Scholar] [CrossRef]
- Beattie, S.; Lebel, S.; Tay, J. The influence of social support on hematopoietic stem cell transplantation survival: A systematic review of literature. PLoS ONE 2013, 8, e61586. [Google Scholar] [CrossRef]
- Amonoo, H.L.; Deary, E.C.; Wang, A.; Newcomb, R.A.; Daskalakis, E.; Weber, D.; Holmbeck, K.E.; Choe, J.J.; Nabily, A.; Cutler, C.; et al. Medication Adherence in Patients with Hematologic Malignancies Who Are Hematopoietic Stem Cell Transplantation Survivors: A Qualitative Study. Transpl. Cell Ther. 2023, 29, 620.e1–620.e11. [Google Scholar] [CrossRef] [PubMed]
- Visintini, C.; Mansutti, I.; Palese, A. Medication Adherence among Allogeneic Haematopoietic Stem Cell Transplant Recipients: A Systematic Review. Cancers 2023, 15, 2452. [Google Scholar] [CrossRef]
- Joost, R.; Dörje, F.; Schwitulla, J.; Eckardt, K.U.; Hugo, C. Intensified pharmaceutical care is improving immunosuppressive medication adherence in kidney transplant recipients during the first post-transplant year: A quasi-experimental study. Nephrol. Dial. Transpl. 2014, 29, 1597–1607. [Google Scholar] [CrossRef]
- Mathes, T.; Großpietsch, K.; Neugebauer, E.A.M.; Pieper, D. Interventions to increase adherence in patients taking immunosuppressive drugs after kidney transplantation: A systematic review of controlled trials. Syst. Rev. 2017, 6, 236. [Google Scholar] [CrossRef]
- Su, G.C.; Greanya, E.D.; Partovi, N.; Yoshida, E.M.; Shapiro, R.J.; Levy, R.D. Assessing medication adherence in solid-organ transplant recipients. Exp. Clin. Transpl. 2013, 11, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.Y.; Fresco, P. Medication Adherence Measures: An Overview. Biomed. Res. Int. 2015, 2015, 217047. [Google Scholar] [CrossRef] [PubMed]
- Vik, S.A.; Maxwell, C.J.; Hogan, D.B. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann. Pharmacother. 2004, 38, 303–312. [Google Scholar] [CrossRef]
- Lim, M.T.; Ab Rahman, N.; Teh, X.R.; Chan, C.L.; Thevendran, S.; Ahmad Hamdi, N.; Lim, K.K.; Sivasampu, S. Optimal cut-off points for adherence measure among patients with type 2 diabetes in primary care clinics: A retrospective analysis. Ther. Adv. Chronic Dis. 2021, 12, 2040622321990264. [Google Scholar] [CrossRef]
- Gellad, W.F.; Thorpe, C.T.; Steiner, J.F.; Voils, C.I. The myths of medication adherence. Pharmacoepidemiol. Drug Saf. 2017, 26, 1437–1441. [Google Scholar] [CrossRef]
- Karve, S.; Cleves, M.A.; Helm, M.; Hudson, T.J.; West, D.S.; Martin, B.C. Good and poor adherence: Optimal cut-point for adherence measures using administrative claims data. Curr. Med. Res. Opin. 2009, 25, 2303–2310. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Urata, S.; Yasuda, K.; Sekiya, H.; Hirabara, Y.; Okumura, M.; Ikeda, R. Retrospective analysis of the correlation between tacrolimus concentrations measured in whole blood and variations of blood cell counts in patients undergoing allogeneic haematopoietic stem cell transplantation. Eur. J. Hosp. Pharm. 2020, 27, e7–e11. [Google Scholar] [CrossRef]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.P.; Siminoff, L.A. Adherence to immunosuppression: A prospective diary study. Transpl. Proc. 2007, 39, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, J.W.; Gregoski, M.J.; Weiland, A.K.; Rock, R.A.; Brunner-Jackson, B.M.; Patel, S.K.; Thomas, B.S.; Taber, D.J.; Chavin, K.D.; Baliga, P.K.; et al. Mobile Health Medication Adherence and Blood Pressure Control in Renal Transplant Recipients: A Proof-of-Concept Randomized Controlled Trial. JMIR Res. Protoc. 2013, 2, e32. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.Y.; Horne, R.; Hankins, M.; Chisari, C. The Medication Adherence Report Scale: A measurement tool for eliciting patients’ reports of nonadherence. Br. J. Clin. Pharmacol. 2020, 86, 1281–1288. [Google Scholar] [CrossRef]
- Konstantinou, P.; Kasinopoulos, O.; Karashiali, C.; Georgiou, G.; Panayides, A.; Papageorgiou, A.; Wozniak, G.; Kassianos, A.P.; Karekla, M. A Scoping Review of Methods Used to Assess Medication Adherence in Patients with Chronic Conditions. Ann. Behav. Med. 2022, 56, 1201–1217. [Google Scholar] [CrossRef]
- Stone, J.K.; Shafer, L.A.; Graff, L.A.; Lix, L.; Witges, K.; Targownik, L.E.; Haviva, C.; Sexton, K.; Bernstein, C.N. Utility of the MARS-5 in Assessing Medication Adherence in IBD. Inflamm. Bowel Dis. 2021, 27, 317–324. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Ice, L.L.; Bartoo, G.T.; McCullough, K.B.; Wolf, R.C.; Dierkhising, R.A.; Mara, K.C.; Jowsey-Gregoire, S.G.; Damlaj, M.; Litzow, M.R.; Merten, J.A. A Prospective Survey of Outpatient Medication Adherence in Adult Allogeneic Hematopoietic Stem Cell Transplantation Patients. Biol. Blood Marrow Transpl. 2020, 26, 1627–1634. [Google Scholar] [CrossRef]
- Kelly, K.; Grau-Sepulveda, M.V.; Goldstein, B.A.; Spratt, S.E.; Wolfley, A.; Hatfield, V.; Murphy, M.; Jones, E.; Granger, B.B. The agreement of patient-reported versus observed medication adherence in type 2 diabetes mellitus (T2DM). BMJ Open Diabetes Res. Care 2016, 4, e000182. [Google Scholar] [CrossRef]
- Héritier, J.; Medinger, M.; Heim, D.; Baldomero, H.; Arranto, C.; Halter, J.P.; Passweg, J.R.; Kleber, M. Optimized cyclosporine starting dose may reduce risk of acute GvHD after allogeneic hematopoietic cell transplantation: A single-center cohort study. Bone Marrow Transpl. 2022, 57, 613–619. [Google Scholar] [CrossRef]
- Maruyama, Y.; Maejima, Y.; Hirabayashi, K.; Morokawa, H.; Okura, E.; Saito, S.; Nakazawa, Y. Factors Affecting Day-to-Day Variations in Tacrolimus Concentration among Children and Young Adults Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Transpl. Cell Ther. 2023, 29, 270.e1–270.e8. [Google Scholar] [CrossRef]
- Fructuoso-González, L.; Najera-Perez, M.D.; Manresa-Ramón, N.; Torrano-Belmonte, P.; Caracena-López, S.; Pacheco-López, P. Isavuconazole-tacrolimus drug-drug interactions in HSCT patients. J. Antimicrob. Chemother. 2023, 78, 2559–2562. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.M.; Lewis, J.S., 2nd; Le, H.; Bubalo, J.S. Comparative effects of fluconazole, posaconazole, and isavuconazole upon tacrolimus and cyclosporine serum concentrations. J. Oncol. Pharm. Pr. 2022, 28, 1357–1362. [Google Scholar] [CrossRef]
- Huynh, D.A.H.; Freeman, T.; Marshall, V.; Markstrom, D.; Frame, D. Evaluation of Tacrolimus Levels and Risk of Acute Graft-Versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transpl. 2019, 25, S282. [Google Scholar] [CrossRef]
- Spetz, K.; Olbers, T.; Östbring, M.; Moon, Z.; Horne, R.; Andersson, E. Using the 5-Item Medication Adherence Report Scale (MARS-5) to Screen for Non-adherence to Vitamin and Mineral Supplementation After Bariatric Surgery. Obes. Surg. 2024, 34, 576–582. [Google Scholar] [CrossRef]
- Friis, K.; Lasgaard, M.; Rowlands, G.; Osborne, R.H.; Maindal, H.T. Health Literacy Mediates the Relationship Between Educational Attainment and Health Behavior: A Danish Population-Based Study. J. Health Commun. 2016, 21 (Suppl. S2), 54–60. [Google Scholar] [CrossRef]
- Miller, T.A. Health literacy and adherence to medical treatment in chronic and acute illness: A meta-analysis. Patient Educ. Couns. 2016, 99, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Jin, H.; Mao, J.; Zhu, W.; Zhou, Y.; Ge, X.; Yu, D. Impact of health literacy and social support on medication adherence in patients with hypertension: A cross-sectional community-based study. BMC Cardiovasc. Disord. 2023, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Blecker, S.; Li, X.; Kronish, I.M.; Chunara, R.; Zheng, Y.; Lawrence, S.; Dodson, J.A.; Kozloff, S.; Adhikari, S. Neighborhood-Level Socioeconomic Status and Prescription Fill Patterns Among Patients With Heart Failure. JAMA Netw. Open 2023, 6, e2347519. [Google Scholar] [CrossRef]
- Ashok Kumar, P.; Ghimire, K.; Haroun, E.; Kassab, J.; Saba, L.; Gentile, T.; Dutta, D.; Lim, S.H. Utilization and outcome disparities in allogeneic hematopoietic stem cell transplant in the United States. Eur. J. Haematol. 2024, 112, 328–338. [Google Scholar] [CrossRef]
- McQuaid, E.L.; Landier, W. Cultural Issues in Medication Adherence: Disparities and Directions. J. Gen. Intern. Med. 2018, 33, 200–206. [Google Scholar] [CrossRef]
- Stirratt, M.J.; Dunbar-Jacob, J.; Crane, H.M.; Simoni, J.M.; Czajkowski, S.; Hilliard, M.E.; Aikens, J.E.; Hunter, C.M.; Velligan, D.I.; Huntley, K.; et al. Self-report measures of medication adherence behavior: Recommendations on optimal use. Transl. Behav. Med. 2015, 5, 470–482. [Google Scholar] [CrossRef]
- Ralph, J.E.; Sezgin, E.; Stanek, C.J.; Landier, W.; Pai, A.L.H.; Gerhardt, C.A.; Skeens, M.A. Improving medication adherence monitoring and clinical outcomes through mHealth: A randomized controlled trial protocol in pediatric stem cell transplant. PLoS ONE 2023, 18, e0289987. [Google Scholar] [CrossRef] [PubMed]
- Shellmer, D.A.; Zelikovsky, N. The challenges of using medication event monitoring technology with pediatric transplant patients. Pediatr. Transpl. 2007, 11, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Coste, G.; Lemaitre, F. The Role of Intra-Patient Variability of Tacrolimus Drug Concentrations in Solid Organ Transplantation: A Focus on Liver, Heart, Lung and Pancreas. Pharmaceutics 2022, 14, 379. [Google Scholar] [CrossRef] [PubMed]
| Total | |
|---|---|
| N = 150 | |
| Age, m (SD) | 57.5 (13.5) |
| Sex, n (%) | |
| Female | 62 (41.3%) |
| Male | 88 (58.7%) |
| Race, n (%) | |
| White | 138 (92.0%) |
| Asian | 3 (2.0%) |
| Black | 3 (2.0%) |
| Middle Eastern | 1 (0.7%) |
| Native American | 1 (0.7%) |
| Not listed | 4 (2.7%) |
| Hispanic, n (%) | |
| No | 10 (6.7%) |
| Yes | 128 (85.3%) |
| Missing | 12 (8.0%) |
| Relationship Status, n (%) | |
| Single | 16 (10.7%) |
| Relationship/Not living together | 8 (5.3%) |
| Married/Living Together | 110 (73.3%) |
| Separated/Divorced | 5 (3.3%) |
| Widowed/Loss of Partner | 4 (2.7%) |
| Missing | 7 (4.7%) |
| Religion, n (%) | |
| Agnostic | 12 (8.0%) |
| Atheist | 6 (4.0%) |
| Buddhist | 1 (0.7%) |
| Catholic Christian | 63 (42.0%) |
| Other Christian | 37 (24.7%) |
| Jewish | 9 (6.0%) |
| Muslim | 1 (0.7%) |
| None | 18 (12.0%) |
| Other | 3 (2.0%) |
| Education, n (%) | |
| <High School Diploma | 2 (1.3%) |
| High School Diploma (GED) | 19 (12.7%) |
| Some College | 45 (30.0%) |
| College | 36 (24.0%) |
| Some Postgraduate/Professional Education | 7 (4.7%) |
| Post-Graduate/Professional | 33 (22.0%) |
| Missing | 8 (5.3%) |
| Employment, n (%) | |
| Employed | 34 (22.7%) |
| Home-Maker | 3 (2.0%) |
| Disability | 61 (40.7%) |
| Retired | 42 (28.0%) |
| Other | 3 (2.0%) |
| Missing | 7 (4.7%) |
| Cancer Type Combined, n (%) | |
| Other | 36 (24.0%) |
| Leukemia | 83 (55.3%) |
| Myelodysplastic Syndrome | 31 (20.7%) |
| Transplant Type, n (%) | |
| Myeloablative Allogeneic | 56 (37.3%) |
| Reduced Intensity Allogeneic | 94 (62.7%) |
| Transplant Setting, n (%) | |
| Inpatient | 138 (92.0%) |
| Outpatient | 12 (8.0%) |
| Total Body Radiation, n (%) | |
| No | 125 (83.3%) |
| Yes | 25 (16.7%) |
| Donor Source, n (%) | |
| Matched | 133 (88.7%) |
| Mismatched | 17 (11.3%) |
| Graft-versus-host Disease, n (%) | |
| No | 140 (93.3%) |
| Yes | 10 (6.7%) |
| Graft-versus-host Disease Prophylaxis, n (%) | |
| Cyclophosphamide-Based | 33 (22.0%) |
| Tacrolimus-Based | 117 (78.0%) |
| Combined ecog, n (%) | |
| 2–3 | 59 (39.3%) |
| 0–1 | 89 (59.3%) |
| Missing | 2 (1.3%) |
| Transplant Length of Stay, m (SD) | 20.0 (8.7) |
| Adherence Measures | Kappa | |||||
|---|---|---|---|---|---|---|
| Pill Count | Dose Adherence | DA Y | DA N | Total | 0.025 | |
| PC Y | 71 | 2 | 73 | |||
| PC N | 40 | 2 | 42 | |||
| Total | 111 | 4 | 115 | |||
| Pill Count | Time Adherence | TA Y | TA N | Total | −0.097 | |
| PC Y | 58 | 15 | 73 | |||
| PC N | 37 | 5 | 42 | |||
| Total | 95 | 20 | 115 | |||
| Pill Count | MARS-5 | M Y | M N | Total | −0.044 | |
| PC Y | 83 | 3 | 86 | |||
| PC N | 49 | 0 | 49 | |||
| Total | 132 | 3 | 135 | |||
| Pill Count | Drug Level | LA Y | LA N | Total | 0.035 | |
| PC Y | 8 | 83 | 91 | |||
| PC N | 2 | 48 | 50 | |||
| Total | 10 | 131 | 141 | |||
| Dose Adherence | Time Adherence | TA Y | TA N | Total | 0.116 | |
| DA Y | 95 | 18 | 113 | |||
| DA N | 2 | 2 | 4 | |||
| Total | 97 | 20 | 117 | |||
| Dose Adherence | MARS-5 | M Y | M N | Total | −0.035 | |
| DA Y | 104 | 3 | 107 | |||
| DA N | 5 | 0 | 5 | |||
| Total | 109 | 3 | 112 | |||
| Dose Adherence | Drug Levels | LA Y | LA N | Total | 0.006 | |
| DA Y | 8 | 105 | 113 | |||
| DA N | 0 | 5 | 5 | |||
| Total | 8 | 110 | 118 | |||
| Time Adherence | MARS-5 | M Y | M N | Total | 0.042 | |
| TA Y | 89 | 2 | 91 | |||
| TA N | 19 | 1 | 20 | |||
| Total | 108 | 3 | 111 | |||
| Time Adherence | Drug Level | LA Y | LA N | Total | 0.008 | |
| TA Y | 7 | 90 | 97 | |||
| TA N | 1 | 19 | 20 | |||
| Total | 8 | 109 | 117 | |||
| MARS-5 | Drug Level | LA Y | LA N | Total | 0.004 | |
| M Y | 10 | 125 | 135 | |||
| M N | 0 | 3 | 3 | |||
| Total | 10 | 128 | 138 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amonoo, H.L.; Wolfe, E.D.; Keane, E.P.; Larizza, I.S.; Boardman, A.C.; Healy, B.C.; Traeger, L.N.; Cutler, C.; Lee, S.J.; Greer, J.A.; et al. Medication Adherence in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Cancers 2025, 17, 2546. https://doi.org/10.3390/cancers17152546
Amonoo HL, Wolfe ED, Keane EP, Larizza IS, Boardman AC, Healy BC, Traeger LN, Cutler C, Lee SJ, Greer JA, et al. Medication Adherence in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Cancers. 2025; 17(15):2546. https://doi.org/10.3390/cancers17152546
Chicago/Turabian StyleAmonoo, Hermioni L., Emma D. Wolfe, Emma P. Keane, Isabella S. Larizza, Annabella C. Boardman, Brian C. Healy, Lara N. Traeger, Corey Cutler, Stephanie J. Lee, Joseph A. Greer, and et al. 2025. "Medication Adherence in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation" Cancers 17, no. 15: 2546. https://doi.org/10.3390/cancers17152546
APA StyleAmonoo, H. L., Wolfe, E. D., Keane, E. P., Larizza, I. S., Boardman, A. C., Healy, B. C., Traeger, L. N., Cutler, C., Lee, S. J., Greer, J. A., & El-Jawahri, A. (2025). Medication Adherence in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Cancers, 17(15), 2546. https://doi.org/10.3390/cancers17152546






