Adverse Events After Carbon-Ion Radiotherapy (CIRT) for Hepatocellular Carcinoma and Risk Factors for Biliary Stricture After CIRT: A Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CIRT
2.3. Clinical Outcome Evaluation
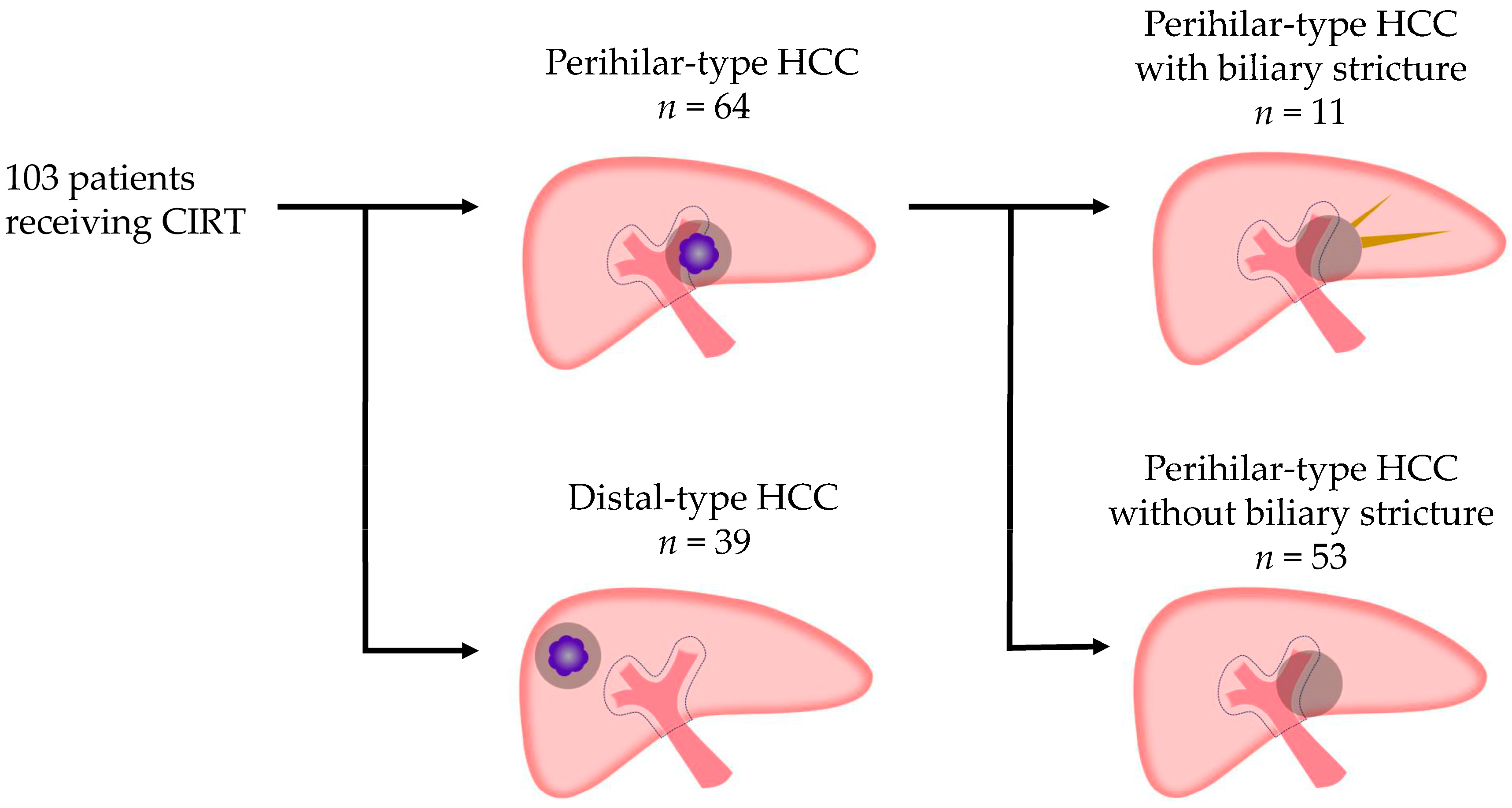
2.4. Definitions of Perihilar-Type HCC and Distal-Type HCC Based on Their Proximity to the Portal Vein Trunk or Primary Branch
2.5. Statistical Analysis
2.6. Ethics Approval
3. Results
3.1. Patient Characteristics in the CIRT Group (Table 1)
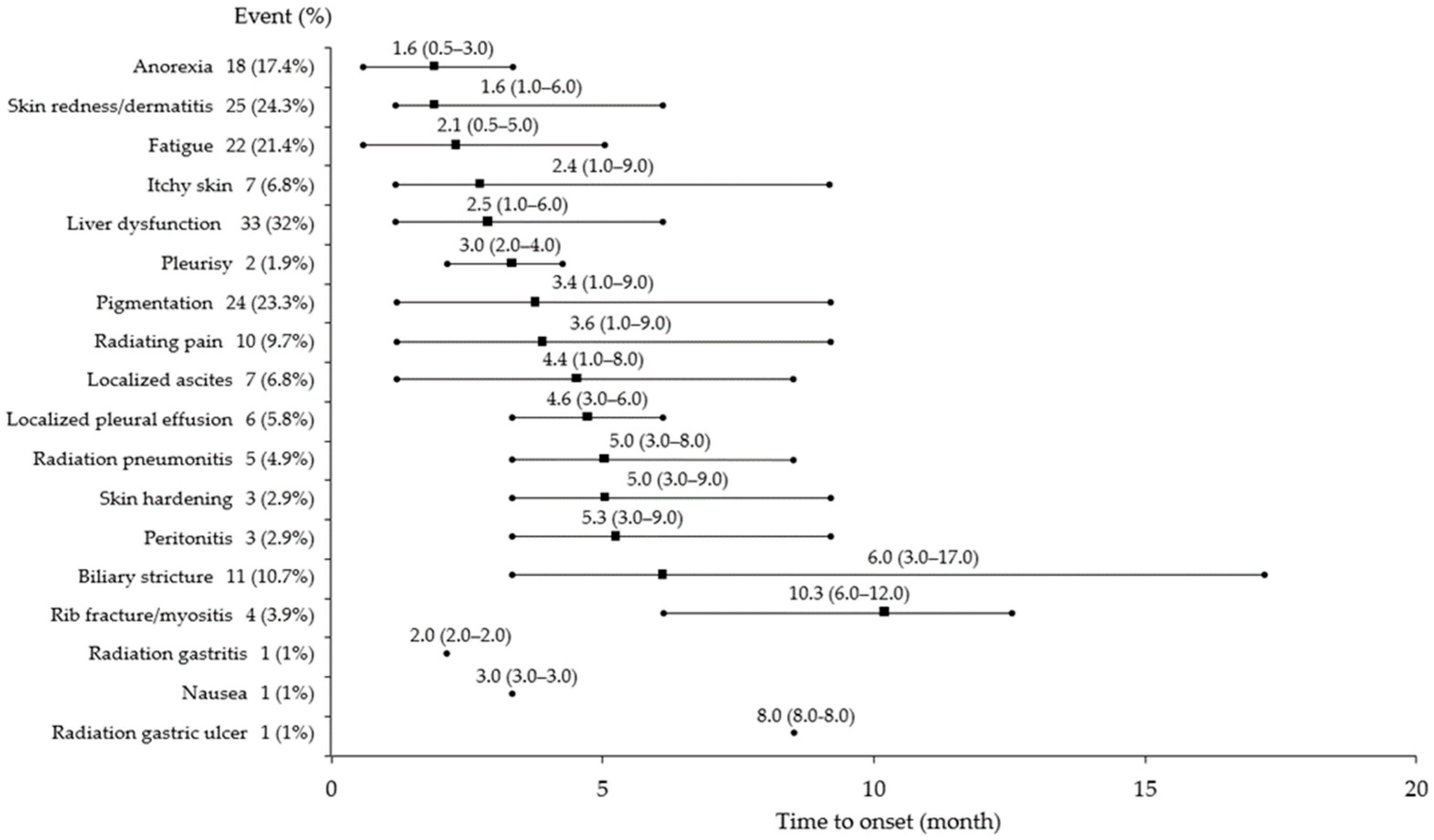
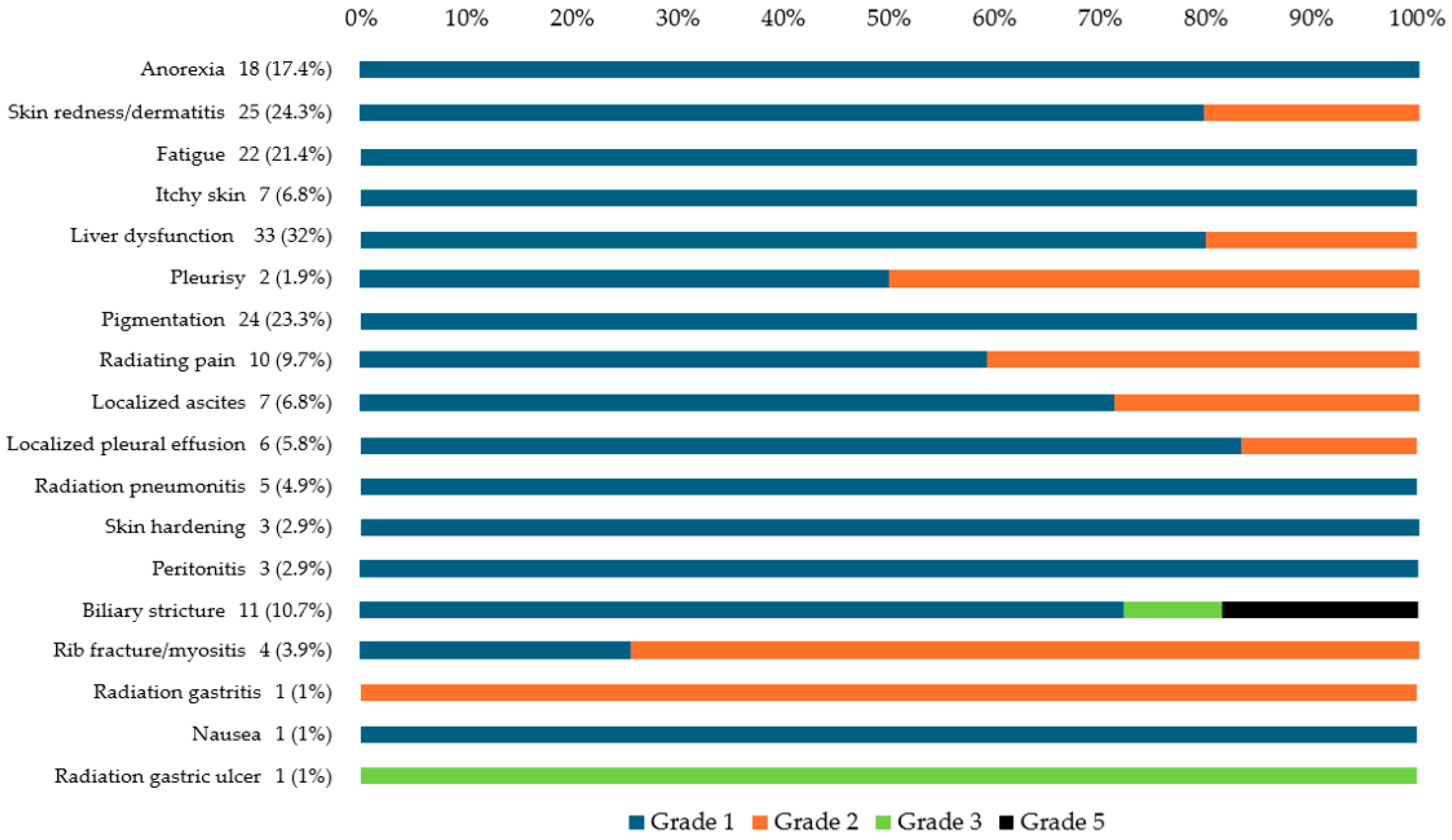
3.2. AEs After CIRT
3.3. Characteristics of Patients with Perihilar-Type HCC and Distal-Type HCC Receiving CIRT (Table 2)
3.4. Comparison of Patients with Perihilar-Type HCC with and Without Biliary Stricture (Table 3)
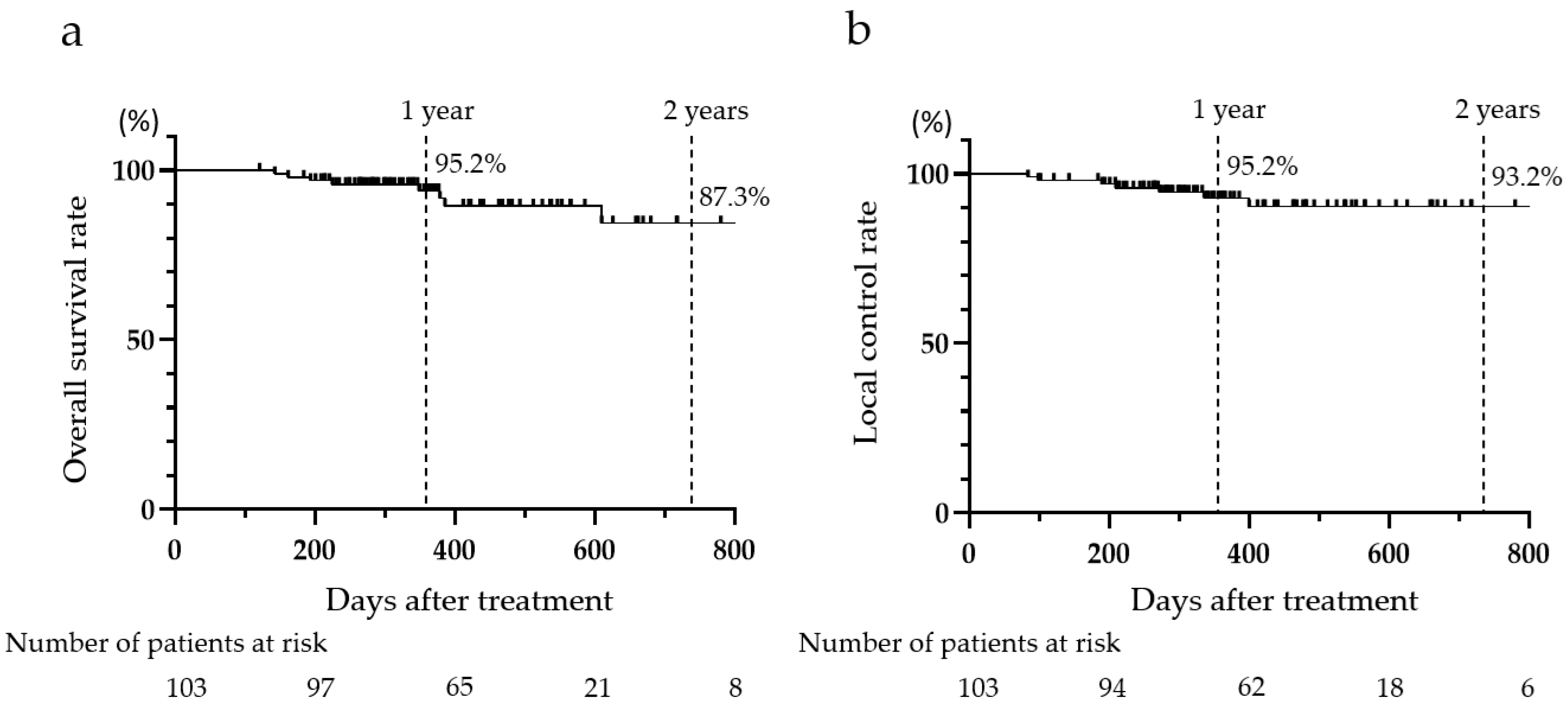
3.5. OS and LC Rates After CIRT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEs | adverse events |
| ABLI score | albumin–bilirubin grade |
| BLCL | Barcelona Clinic for Liver Cancer |
| CIRT | carbon-ion radiotherapy |
| CT | computed tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| EPV | events per variable |
| GTV | gross tumor volume |
| HCC | hepatocellular carcinoma |
| LC | local control |
| mALBI | modified albumin–bilirubin |
| MRI | magnetic resonance imaging |
| MVI | macrovascular invasion |
| OS | overall survival |
| SRT | stereotactic radiation therapy |
| TACE | transarterial chemoembolization |
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Okazaki, S.; Shibuya, K.; Shiba, S.; Okamoto, M.; Miyasaka, Y.; Osu, N.; Kawashima, M.; Kakizaki, S.; Araki, K.; Shirabe, K. Carbon ion radiotherapy for patients with hepatocellular carcinoma in the caudate lobe carbon ion radiotherapy for hepatocellular carcinoma in caudate lobe. Hepatol. Res. 2021, 51, 303–312. [Google Scholar] [CrossRef]
- Tsujii, H.; Mizoe, J.; Kamada, T.; Baba, M.; Tsuji, H.; Kato, H.; Kato, S.; Yamada, S.; Yasuda, S.; Ohno, T.; et al. Clinical results of carbon ion radiotherapy at NIRS. J. Radiat. Res. 2007, 48, A1–A13. [Google Scholar] [CrossRef]
- Chen, G.T.Y.; Castro, J.R.; Quivey, J.M. Heavy charged particle radiotherapy. Ann. Rev. Biophys. Bioeng. 1981, 10, 499–529. [Google Scholar] [CrossRef]
- Elsässer, T.; Krämer, M.; Scholz, M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, J.A. Photon, light ion, and heavy ion cancer radiotherapy: Paths from physics and biology to clinical practice. Ann. Transl. Med. 2015, 3, 336. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T. Particle radiotherapy with carbon ion beams. EPMA J. 2013, 4, 9. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, C.; Seong, J. Carbon ion radiotherapy in the treatment of hepatocellular carcinoma. Clin. Mol. Hepatol. 2023, 29, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- Buglewicz, D.J.; Banks, A.B.; Hirakawa, H.; Fujimori, A.; Kato, T.A. Monoenergetic 290 MeV/n carbon-ion beam biological lethal dose distribution surrounding the Bragg peak. Sci. Rep. 2019, 9, 6157. [Google Scholar] [CrossRef]
- Sokol, O.; Durante, M. Carbon ions for hypoxic tumors: Are we making the most of them? Cancers 2023, 15, 4494. [Google Scholar] [CrossRef]
- Mohamad, O.; Sishc, B.J.; Saha, J.; Pompos, A.; Rahimi, A.; Story, M.D.; Davis, A.J.; Kim, D.N. Carbon ion radiotherapy: A review of clinical experiences and preclinical research, with an emphasis on DNA damage/repair. Cancers 2017, 9, 66. [Google Scholar] [CrossRef]
- Sorin, Y.; Ikeda, K.; Kawamura, Y.; Fujiyama, S.; Kobayashi, M.; Hosaka, T.; Sezaki, H.; Akuta, N.; Saitoh, S.; Suzuki, F. Effectiveness of particle radiotherapy in various stages of hepatocellular carcinoma: A pilot study. Liver Cancer 2018, 7, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Spychalski, P.; Kobiela, J.; Antoszewska, M.; Błażyńska-Spychalska, A.; Jereczek-Fossa, B.A.; Høyer, M. Patient specific outcomes of charged particle therapy for hepatocellular carcinoma: A systematic review and quantitative analysis. Radiother. Oncol. 2019, 132, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cai, X.; Sun, J.; Wang, W.; Zhao, J.; Zhang, Q.; Jiang, G.; Wang, Z. Pencil beam scanning carbon ion radiotherapy for hepatocellular carcinoma. J. Hepatocell. Carcinoma 2023, 10, 2397–2409. [Google Scholar] [CrossRef]
- Yu, J.I.; Park, H.C.; Lim, D.H.; Paik, S.W. Do biliary complications after hypofractionated radiation therapy in hepatocellular carcinoma matter? Cancer Res. Treat. 2016, 48, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, A.H.; Hung, S.P.; Hsieh, C.E.; Tseng, J.H.; Chen, P.J.; Cheng, J.Y.; Chang, J.T.C.; Chan, K.M.; Lin, S.M.; et al. Proton beam therapy in managing unresectable hepatocellular carcinoma with bile duct invasion. Cancers 2022, 14, 1616. [Google Scholar] [CrossRef]
- Eriguchi, T.; Takeda, A.; Sanuki, N.; Oku, Y.; Aoki, Y.; Shigematsu, N.; Kunieda, E. Acceptable toxicity after stereotactic body radiation therapy for liver tumors adjacent to the central biliary system. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1006–1011. [Google Scholar] [CrossRef]
- Miura, Y.; Ashida, R.; Saiga, A.; Sugiura, T.; Ohgi, K.; Yamada, M.; Otsuka, S.; Aramaki, T.; Sato, R.; Uesaka, K. Secondary Budd-Chiari syndrome occurred after adjuvant radio therapy for perihilar cholangiocarcinoma: A case report. World J. Surg. Oncol. 2023, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Yarnold, J.; Vozenin Brotons, M.-C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, C.; Song, B.; Zhang, S.; Chen, C.; Li, C.; Zhang, S. Tissue fibrosis induced by radiotherapy: Current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J. Transl. Med. 2023, 21, 708. [Google Scholar] [CrossRef]
- Sindelar, W.F.; Tepper, J.E.; Kinsella, T.J.; Barnes, M.; DeLuca, A.M.; Terrill, R.; Matthews, D.; Anderson, W.J.; Bollinger, B.K.; Johnstone, P.A. Late effects of intra operative radiation therapy on retroperitoneal tissues, intestine, and bile duct in a large animal model. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 781–788. [Google Scholar] [CrossRef]
- Osmundson, E.C.; Wu, Y.; Luxton, G.; Bazan, J.G.; Koong, A.C.; Chang, D.T. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 986–994. [Google Scholar] [CrossRef]
- Barney, B.M.; Olivier, K.R.; Miller, R.C.; Haddock, M.G. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat. Oncol. 2012, 7, 67. [Google Scholar] [CrossRef]
- Kondo, Y.; Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Goto, E.; Nakagawa, H.; Masuzaki, R.; Asaoka, Y.; et al. Intrahepatic bile duct dilatation after percutaneous radiofrequency ablation for hepatocellular carcinoma: Impact on patient’s prognosis. Liver Int. 2011, 31, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Okuda, M.; Yoshie, Y.; Nakashima, Y.; Ikeno, H.; Orito, N.; Notsumata, K.; Watanabe, H.; Toya, D.; et al. Main bile duct stricture occurring after transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2010, 33, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Katoh, H.; Koyama, Y.; Shiba, S.; Okamoto, M.; Okazaki, S.; Araki, K.; Kakizaki, S.; Shirabe, K.; Ohno, T. Efficacy and safety of 4 fractions of carbon-ion radiation therapy for hepatocellular carcinoma: A prospective study. Liver Cancer 2021, 11, 61–74. [Google Scholar] [CrossRef] [PubMed]
| Carbon-Ion Radiotherapy (n = 103) | |
|---|---|
| Age (year) | Median: 76 (range: 47–97) |
| Sex (male/female) (%) | 79 (76.7)/24 (23.3) |
| Etiology (HBV/HCV/non-B, non-C/alcohol/NASH/AIH/PBC) (%) | 19 (18.4)/25 (24.3)/19 (18.4)/18 (17.5)/18 (17.5)/1 (1)/3 (2.9) |
| mALBI grade (1/2a/2b/3) (%) | 50 (48.5)/24 (23.3)/26 (25.2)/3 (2.9) |
| ALBI score | −2.603 (−3.668 to −1.049) |
| BCLC stage (A/B/C) (%) | 63 (61.2)/21 (20.4)/19 (18.4) |
| First treatment/pretreatment (surgery/RFA/TACE/SRT/systemic therapy) (%) | 48 (46.6)/55 (53.4) |
| Size (mm) | Median: 54.5 (range: 12–142) |
| Total number of intrahepatic tumors (1/2) (%) | 83 (80.6)/20 (19.4) |
| MVI (−/+) (%) | 84 (81.6)/19 (18.4) |
| Blood biochemistry, median (range) | |
| T-bil level (mg/dL) | 0.70 (0.40–2.7) |
| Alb level (g/dL) | 4.0 (2.5–5.1) |
| AST level (U/L) | 31.0 (12–222) |
| ALT level (U/L) | 26.0 (5.0–135) |
| PLT count (103/µL) | 151 (39–382) |
| PT% | 94.0 (40–123) |
| PT-INR | 1.05 (0.87–1.95) |
| AFP level (ng/mL) | 8.3 (2.0–20,000) |
| PIVKA-II level (mAU/mL) | 232.0 (6.7–81,886) |
| Perihilar-Type HCC (n = 64) | Distal-Type HCC (n = 39) | p-Value | |
|---|---|---|---|
| Age (year), median (range) | 75 (47–91) | 77 (49–97) | 0.7652 |
| Sex (male/female) (%) | 47 (73.4)/17 (26.6) | 32 (82.1)/7 (17.9) | 0.3481 |
| Etiology (HBV/HCV/non-B, non-C/alcohol/NASH/AIH/PBC) (%) | 11 (17.2)/14 (21.9)/14 (21.9)/ 10 (15.6)/12 (18.7)/1 (1.6)/2 (3.1) | 8 (20.5)/11 (28.2)/5 (12.8)/8 (20.5)/ 6 (15.4)/0 (0)/1 (2.6) | 0.8859 |
| mALBI grade (1/2a/2b/3) (%) | 30 (46.9)/12 (18.7)/21 (32.8/1 (1.6) | 20 (51.3)/12 (30.8)/5 (12.8)/2 (5.1) | 0.0652 |
| ALBI score | −2.586 (−3.415 to −1.143) | −2.586 (−3.415 to −1.143) | 0.4496 |
| BCLC stage (A/B/C) (%) | 36 (56.2)/10 (15.6)/18 (28.1) | 27 (69.2)/10 (25.6)/2 (5.1) | 0.0095 |
| First treatment/pretreatment (surgery/RFA/TACE/SRT/systemic therapy) (%) | 28 (43.8)/36 (56.2) | 20 (51.3)/19 (48.7) | 0.5425 |
| Size (mm), median (range) | 63.0 (11.5–142) | 46.0 (15–130) | 0.0059 |
| Total number of intrahepatic tumors (1/2) (%) | 50 (78.1)/14 (21.9) | 33 (84.6)/6 (15.4) | 0.4555 |
| MVI (−/+) (%) | 47 (73.4)/17 (26.6) | 37 (94.9)/2 (5.1) | 0.0078 |
| Biliary stricture (−/+) (%) | 53 (82.8)/11 (17.2) | 39 (100)/0 (0) | 0.0061 |
| Blood biochemistry, median (range) | |||
| T-bil level (mg/dL) | 0.80 (0.40–2.7) | 0.75 (0.40–2.5) | 0.6754 |
| Alb level (g/dL) | 4.0 (2.5–4.9) | 3.9 (2.5–5.1) | 0.3298 |
| AST level (U/L) | 36.0 (13–222) | 26.0 (12–83) | 0.0676 |
| ALT level (U/L) | 28.0 (8.0–135) | 24.0 (5–95) | 0.2279 |
| PLT count (103/µL) | 150 (39–346) | 155 (55–382) | 0.6248 |
| PT% | 94.0 (41–123) | 93.0 (40–112) | 0.6165 |
| PT-INR | 1.04 (0.87–1.95) | 1.05 (0.93–1.90) | 0.7841 |
| AFP level (ng/mL) | 14.6 (2.0–20,000) | 4.9 (2.0–20,000) | 0.2621 |
| PIVKA-II level (mAU/mL) | 217.0 (6.7–51,166) | 288.0 (12.0–81,886) | 0.9624 |
| Perihilar-Type HCC with Biliary Stricture (+) (n = 11) | Perihilar-Type HCC Without Biliary Stricture (−) (n = 53) | p-Value | |
|---|---|---|---|
| Age (year), median (range) | 75 (66–90) | 76 (47–91) | 0.9786 |
| Sex (male/female) (%) | 9 (81.8)/2 (18.2) | 38 (71.7)/15 (28.3) | 0.7124 |
| Etiology (HBV/HCV/non-B, non-C/alcohol/NASH/AIH/PBC) (%) | 2 (18.2)/3 (27.3)/4 (36.4)/0 (0)/ 2 (18.2)/0 (0)/0 (0) | 9 (17.0)/11 (20.8)/10 (18.8)/10 (18.8)/10 (18.8)/1 (1.9)/2 (3.8) | 0.6755 |
| mALBI grade (1/2a/2b/3) (%) | 6 (54.5)/1 (9.1)/4 (36.4)/0 (0) | 24 (45.3)/11 (20.8)/17 (32.1)/1 (1.9) | 0.8542 |
| ALBI score | −2.652 (−3.415 to −1.984) | −2.586 (−3.019 to −1.143) | 0.3662 |
| BCLC stage (A/B/C) (%) | 4 (36.4)/0 (0)/7 (63.6) | 32 (60.4)/9 (17.0)/12 (22.6) | 0.0247 |
| First treatment/pretreatment (surgery/RFA/TACE/SRT/systemic therapy) (%) | 4 (36.4)/7 (63.6) | 24 (45.3)/29 (54.7) | 0.7426 |
| Previous local therapy targeting the perihilar region (−/+) (%) | 6 (54.5)/5 (45.5) | 45 (84.9)/8 (15.1) | 0.0371 |
| Size (mm), median (range) | 64.0 (42–110) | 60.0 (12–142) | 0.7551 |
| Total number of intrahepatic tumors (1/2) (%) | 10 (90.9)/1 (9.1) | 40 (75.5)/13 (24.5) | 0.4306 |
| MVI (−/+) (%) | 4 (36.4)/7 (63.6) | 43 (81.1)/10 (18.8) | 0.0052 |
| Location (primary portal vein branch area/portal vein trunk branch area) (%) | 1 (9.1)/10 (90.9) | 33 (62.3)/20 (37.7) | 0.0018 |
| Blood biochemistry, median (range) | |||
| T-bil level (mg/dL) | 0.80 (0.50–2.5) | 0.70 (0.40–2.7) | 0.7140 |
| Alb level (g/dL) | 4.0 (3.3–4.9) | 3.9 (2.5–4.4) | 0.2574 |
| AST level (U/L) | 33.5 (13–67) | 36.0 (14–222) | 0.4833 |
| ALT level (U/L) | 29.5 (10–70) | 28.0 (8–135) | 0.6963 |
| PLT count (103/µL) | 148 (59–291) | 151 (39–346) | 0.6665 |
| PT% | 100 (65–111) | 94.0 (41–123) | 0.5041 |
| PT-INR | 1.00 (0.94–1.35) | 1.04 (0.87–1.95) | 0.5359 |
| AFP level (ng/mL) | 4.2 (2.0–20,000) | 18.2 (2.0–20,000) | 0.8742 |
| PIVKA-II level (mAU/mL) | 270.5 (29–14,678) | 150.0 (6.7–51,166) | 0.4299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maki, K.; Haga, H.; Katsumi, T.; Hoshikawa, K.; Suzuki, F.; Uchiyama, F.; Kaneko, T.; Koto, M.; Ueno, Y. Adverse Events After Carbon-Ion Radiotherapy (CIRT) for Hepatocellular Carcinoma and Risk Factors for Biliary Stricture After CIRT: A Retrospective Study. Cancers 2025, 17, 2542. https://doi.org/10.3390/cancers17152542
Maki K, Haga H, Katsumi T, Hoshikawa K, Suzuki F, Uchiyama F, Kaneko T, Koto M, Ueno Y. Adverse Events After Carbon-Ion Radiotherapy (CIRT) for Hepatocellular Carcinoma and Risk Factors for Biliary Stricture After CIRT: A Retrospective Study. Cancers. 2025; 17(15):2542. https://doi.org/10.3390/cancers17152542
Chicago/Turabian StyleMaki, Keita, Hiroaki Haga, Tomohiro Katsumi, Kyoko Hoshikawa, Fumiya Suzuki, Fumi Uchiyama, Takashi Kaneko, Masashi Koto, and Yoshiyuki Ueno. 2025. "Adverse Events After Carbon-Ion Radiotherapy (CIRT) for Hepatocellular Carcinoma and Risk Factors for Biliary Stricture After CIRT: A Retrospective Study" Cancers 17, no. 15: 2542. https://doi.org/10.3390/cancers17152542
APA StyleMaki, K., Haga, H., Katsumi, T., Hoshikawa, K., Suzuki, F., Uchiyama, F., Kaneko, T., Koto, M., & Ueno, Y. (2025). Adverse Events After Carbon-Ion Radiotherapy (CIRT) for Hepatocellular Carcinoma and Risk Factors for Biliary Stricture After CIRT: A Retrospective Study. Cancers, 17(15), 2542. https://doi.org/10.3390/cancers17152542







