Efficacy of Adding Immune Checkpoint Inhibitors to Chemotherapy Plus Bevacizumab in Metastatic Colorectal Cancer: A Meta-Analysis of Randomized Controlled Trials
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Bias Risk Assessment
2.3. Data Extraction Process
2.4. Statistical Analysis
3. Results
3.1. Literature Identification and Inclusion
3.2. Risk of Bias Assessment
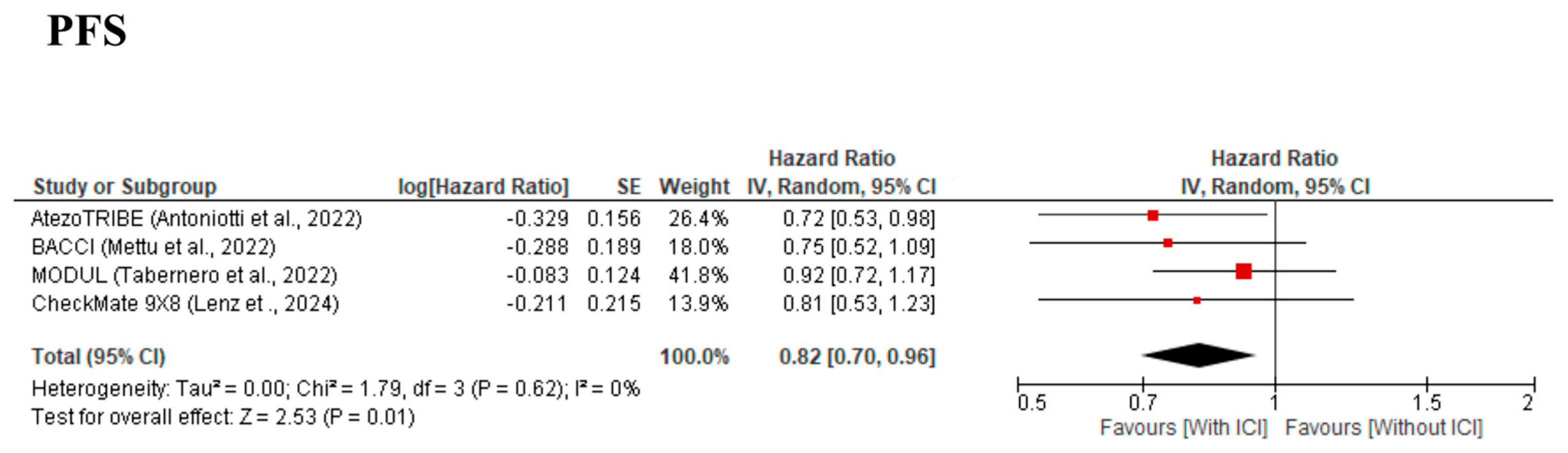
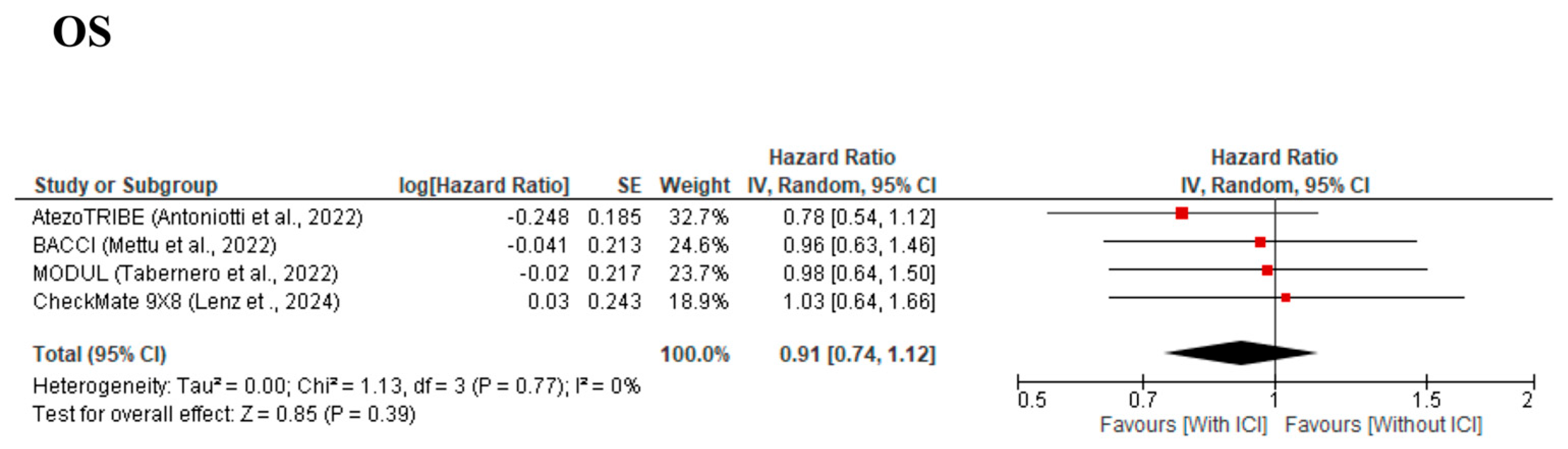
3.3. Primary Outcomes
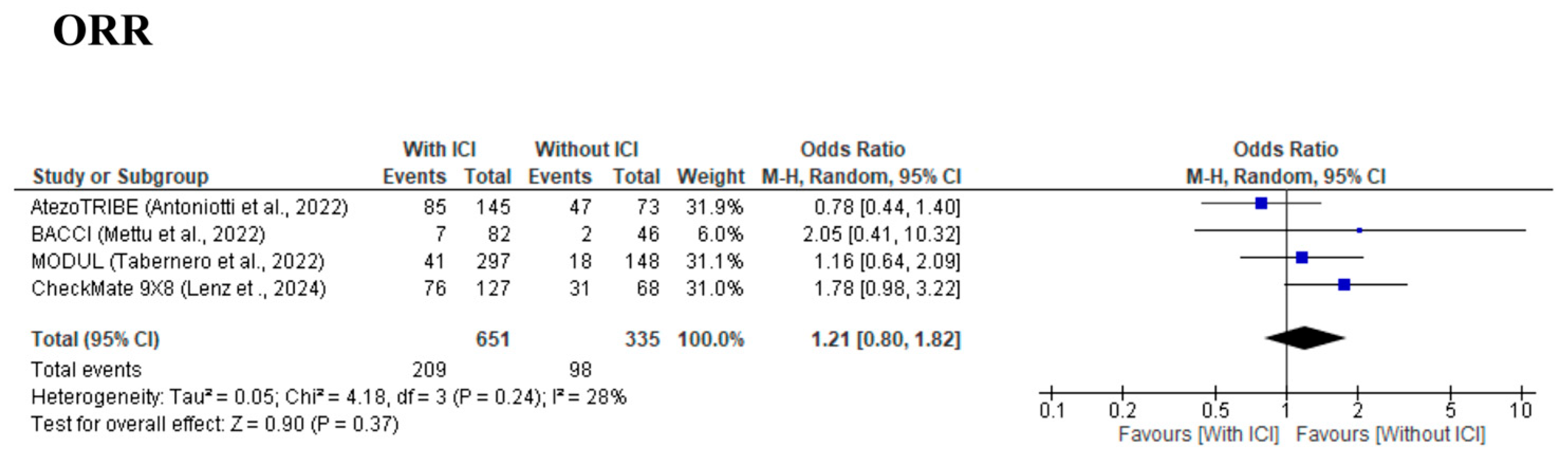
3.4. Secondary Outcomes
3.5. Subgroup Analysis
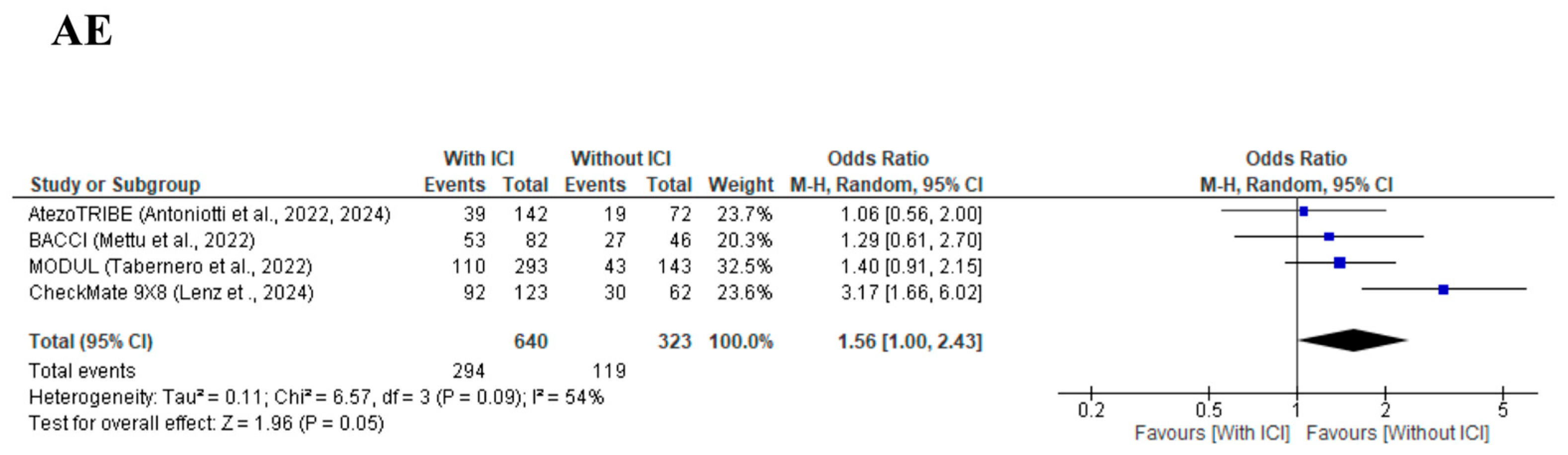
3.6. Safety Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trullas, A.; Delgado, J.; Genazzani, A.; Mueller-Berghaus, J.; Migali, C.; Muller-Egert, S.; Zander, H.; Enzmann, H.; Pignatti, F. The EMA assessment of pembrolizumab as monotherapy for the first-line treatment of adult patients with metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer. ESMO Open 2021, 6, 100145. [Google Scholar] [CrossRef]
- Andre, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Hegde, P.S.; Wallin, J.J.; Mancao, C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018, 52, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wallin, J.J.; Bendell, J.C.; Funke, R.; Sznol, M.; Korski, K.; Jones, S.; Hernandez, G.; Mier, J.; He, X.; Hodi, F.S.; et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016, 7, 12624. [Google Scholar] [CrossRef] [PubMed]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Salvatore, L.; Lonardi, S.; Tamberi, S.; Marmorino, F.; Moretto, R.; Prisciandaro, M.; Tamburini, E.; et al. Upfront Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Bevacizumab With or Without Atezolizumab for Patients with Metastatic Colorectal Cancer: Updated and Overall Survival Results of the ATEZOTRIBE Study. J. Clin. Oncol. 2024, 42, 2637–2644. [Google Scholar] [CrossRef]
- Lenz, H.J.; Parikh, A.; Spigel, D.R.; Cohn, A.L.; Yoshino, T.; Kochenderfer, M.; Elez, E.; Shao, S.H.; Deming, D.; Holdridge, R.; et al. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: Phase 2 results from the CheckMate 9X8 randomized clinical trial. J. Immunother. Cancer 2024, 12, e008409. [Google Scholar] [CrossRef]
- Mettu, N.B.; Ou, F.S.; Zemla, T.J.; Halfdanarson, T.R.; Lenz, H.J.; Breakstone, R.A.; Boland, P.M.; Crysler, O.V.; Wu, C.; Nixon, A.B.; et al. Assessment of Capecitabine and Bevacizumab with or Without Atezolizumab for the Treatment of Refractory Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2149040. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Arnold, D.; de Gramont, A.; Ducreux, M.; O’Dwyer, P.; Tahiri, A.; Gilberg, F.; Irahara, N.; Schmoll, H.J.; et al. MODUL cohort 2: An adaptable, randomized, signal-seeking trial of fluoropyrimidine plus bevacizumab with or without atezolizumab maintenance therapy for BRAF(wt) metastatic colorectal cancer. ESMO Open 2022, 7, 100559. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Matsuyama, K. Nippon Medical School’s Ethical Review Processes for Studies Involving Human Subjects. J. Nippon Med. Sch. 2024, 91, 136–139. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Chen, E.X.; Jonker, D.J.; Loree, J.M.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients with Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020, 6, 831–838. [Google Scholar] [CrossRef]
- Guler, I.; Askan, G.; Klostergaard, J.; Sahin, I.H. Precision medicine for metastatic colorectal cancer: An evolving era. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer. 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; Luo, H.; Meng, X.; Chen, M.; Zhu, D. Wnt signaling pathway in cancer immunotherapy. Cancer Lett. 2022, 525, 84–96. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yanez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients with Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Powles, T.; Plimack, E.R.; Soulieres, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, S.; Jiang, T.; Zhu, B.; Li, X.; Zhao, C.; Jia, Y.; Shi, J.; Zhang, L.; Liu, X.; et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol. Res. 2019, 7, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, J.X.; Zhang, Y.X.; Li, S.Q. Effectiveness of immune checkpoint inhibitors in combination with tyrosine kinase inhibitors in patients with advanced or metastatic colorectal carcinoma with either mismatch repair proficient or metastatic microsatellite stable disease: A systematic review and meta-analysis. Oncol. Lett. 2024, 27, 153. [Google Scholar] [PubMed]
- Zhang, W.; Vallboehmer, D.; Mizutomo, A. Differential gene expression levels of vascular endothelial growth factor (VEGF) and its receptors in renal cell cancer and colorectal cancer patients. Cancer Res. 2006, 66 (Suppl. S8), 1060. [Google Scholar]
- Macarulla, T.; Ren, Z.; Chon, H.J.; Park, J.O.; Kim, J.W.; Pressiani, T.; Li, D.; Zhukova, L.; Zhu, A.X.; Chen, M.H.; et al. Atezolizumab Plus Chemotherapy with or Without Bevacizumab in Advanced Biliary Tract Cancer: Clinical and Biomarker Data From the Randomized Phase II IMbrave151 Trial. J. Clin. Oncol. 2025, 43, 545–557. [Google Scholar] [CrossRef]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gumus, M.; Vicente, D.; Mazieres, J.; Rodriguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ando, F.; Kashiwada, T.; Kuroda, S.; Fujii, T.; Takano, R.; Miyabe, Y.; Kunugi, S.; Sakatani, T.; Miyanaga, A.; Asatsuma-Okumura, T.; et al. Combination of plasma MMPs and PD-1-binding soluble PD-L1 predicts recurrence in gastric cancer and the efficacy of immune checkpoint inhibitors in non-small cell lung cancer. Front. Pharmacol. 2024, 15, 1384731. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, T.; Takano, R.; Ando, F.; Kuroda, S.; Miyabe, Y.; Owada, R.; Miyanaga, A.; Asatsuma-Okumura, T.; Hashiguchi, M.; Kanazawa, Y.; et al. Lysosomal degradation of PD-L1 is associated with immune-related adverse events during anti-PD-L1 immunotherapy in NSCLC patients. Front. Pharmacol. 2024, 15, 1384733. [Google Scholar] [CrossRef] [PubMed]
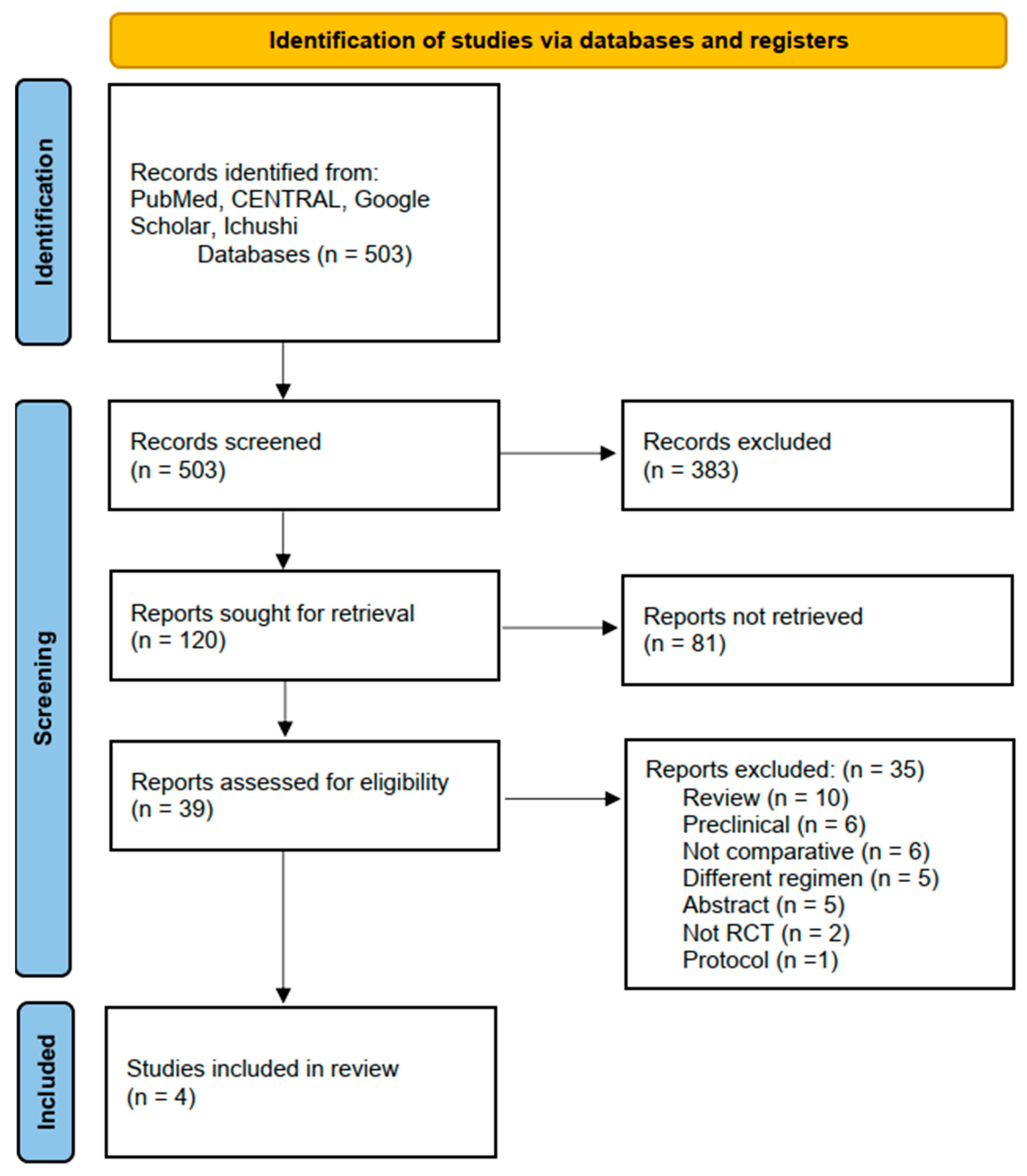
| Author | Year | Country | Study Design | Study Period | Institutions | Total Cases (With/Without ICI) | dMMR or MSI-H (With/Without ICI) | Treatment Line | ICI | Backbone Chemotherapy | Primary Endpoint | Median Follow Up (With/Without ICI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AtezoTRIBE (Antoniotti et al.) [9,10] | 2022, 2024 | Italy | RCT (Phase 2, Open-label) | 2018–2020 | Multi | 218 (145/73) | (7%/6%) | First-line | Atezolizumab | FOLFOXIRI + Bevacizumab | PFS | 19.9 M (2022) 45.2 M (2024) |
| BACCI (Mettu et al.) [12] | 2022 | United States | RCT (Phase 2, double-blind) | 2017–2018 | Multi | 128 (82/46) | 7.3% (7.7%/6.7%) | Second-line or later | Atezolizumab | Capecitabine + Bevacizumab | PFS | 20.9 M (for PFS) |
| MODUL (Tabernero et al.) [13] | 2022 | Europe, Asia, Africa, America | RCT (Phase 2, Open-label) | 2015–2016 | Multi | 445 (297/148) | (2.0%/1.6%) | First-line | Atezolizumab | Fluoropyrimidine + Bevacizumab | PFS | 10.5 M (10.6 M/10.4 M) |
| CheckMate 9X8 (Lenz et al.) [11] | 2024 | United States | RCT (Phase 2/3, Open-label) | 2018–2019 | Multi | 195 (127/68) | (5%/10%) | First-line | Nivolumab | mFOLFOX6 + Bevacizumab | PFS | 21.5 M (minimum) (23.7 M/23.2 M) |
| Author | Randomization | Allocation Concealment | Blinding | Missing Data | Selective Reporting | Overall Risk |
|---|---|---|---|---|---|---|
| AtezoTRIBE (Antoniotti et al.) [9,10] | Low | Low | Some concerns | Low | Low | Some concerns |
| BACCI (Mettu et al.) [12] | Low | Low | Low | Low | Low | Low |
| MODUL (Tabernero et al.) [13] | Low | Low | Some concerns | Low | Low | Some concerns |
| CheckMate 9X8 (Lenz et al.) [11] | Low | Low | Some concerns | Low | Low | Some concerns |
| Outcome | Overall Quality | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Comments |
|---|---|---|---|---|---|---|---|
| PFS | Low | ⚫⚫⚫⚪ (Some concerns) | ⚫⚫⚫⚫ (No concerns) | ⚫⚫⚫⚫ (No concerns) | ⚫⚫⚪⚪ (Serious) | ⚫⚫⚫⚫ (No concerns) | Wider confidence interval and lack of blinding in some trials reduce certainty. |
| OS | Low | ⚫⚫⚫⚪ (Some concerns) | ⚫⚫⚫⚫ (No concerns) | ⚫⚫⚫⚫ (No concerns) | ⚫⚫⚪⚪ (Serious) | ⚫⚫⚪⚪ (Serious) | No significant difference in mortality observed; wide Confidence interval reduces certainty. |
| ORR | Low | ⚫⚫⚫⚪ (Some concerns) | ⚫⚫⚫⚫ (No concerns) | ⚫⚫⚫⚫ (No concerns) | ⚫⚫⚪⚪ (Serious) | ⚫⚫⚫⚫ (No concerns) | Improvement in response rate observed, but inconsistency across trials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, F.; Matsuda, A.; Miyamoto, Y.; Sunakawa, Y.; Asatsuma-Okumura, T.; Iwai, Y.; Yoshida, H. Efficacy of Adding Immune Checkpoint Inhibitors to Chemotherapy Plus Bevacizumab in Metastatic Colorectal Cancer: A Meta-Analysis of Randomized Controlled Trials. Cancers 2025, 17, 2538. https://doi.org/10.3390/cancers17152538
Ando F, Matsuda A, Miyamoto Y, Sunakawa Y, Asatsuma-Okumura T, Iwai Y, Yoshida H. Efficacy of Adding Immune Checkpoint Inhibitors to Chemotherapy Plus Bevacizumab in Metastatic Colorectal Cancer: A Meta-Analysis of Randomized Controlled Trials. Cancers. 2025; 17(15):2538. https://doi.org/10.3390/cancers17152538
Chicago/Turabian StyleAndo, Fumihiko, Akihisa Matsuda, Yuji Miyamoto, Yu Sunakawa, Tomoko Asatsuma-Okumura, Yoshiko Iwai, and Hiroshi Yoshida. 2025. "Efficacy of Adding Immune Checkpoint Inhibitors to Chemotherapy Plus Bevacizumab in Metastatic Colorectal Cancer: A Meta-Analysis of Randomized Controlled Trials" Cancers 17, no. 15: 2538. https://doi.org/10.3390/cancers17152538
APA StyleAndo, F., Matsuda, A., Miyamoto, Y., Sunakawa, Y., Asatsuma-Okumura, T., Iwai, Y., & Yoshida, H. (2025). Efficacy of Adding Immune Checkpoint Inhibitors to Chemotherapy Plus Bevacizumab in Metastatic Colorectal Cancer: A Meta-Analysis of Randomized Controlled Trials. Cancers, 17(15), 2538. https://doi.org/10.3390/cancers17152538







