Real-World Toxicity and Effectiveness Study of Abemaciclib in Greek Patients with Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: A Multi-Institutional Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
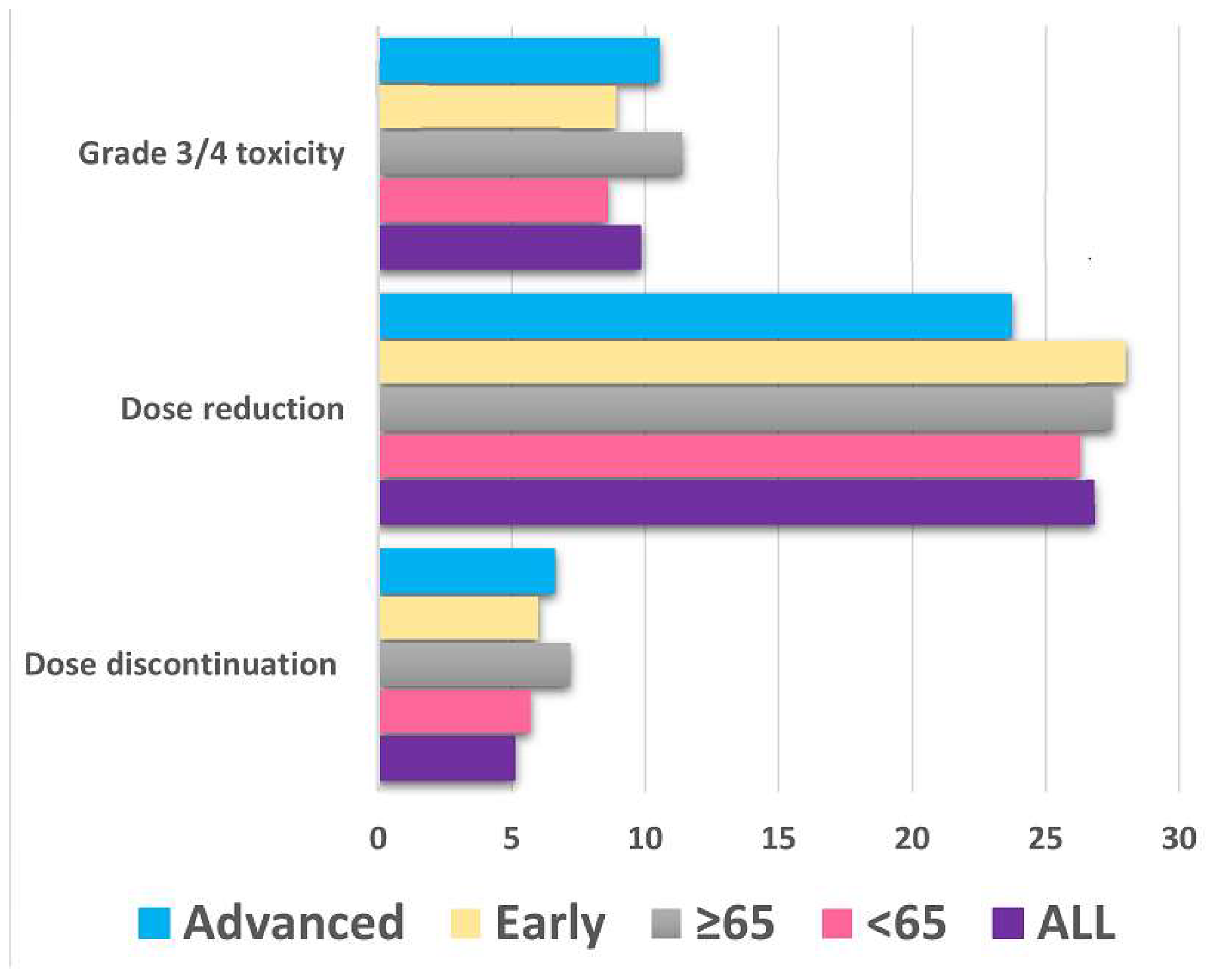
3.2. Toxicity
3.3. Clinical Outcomes
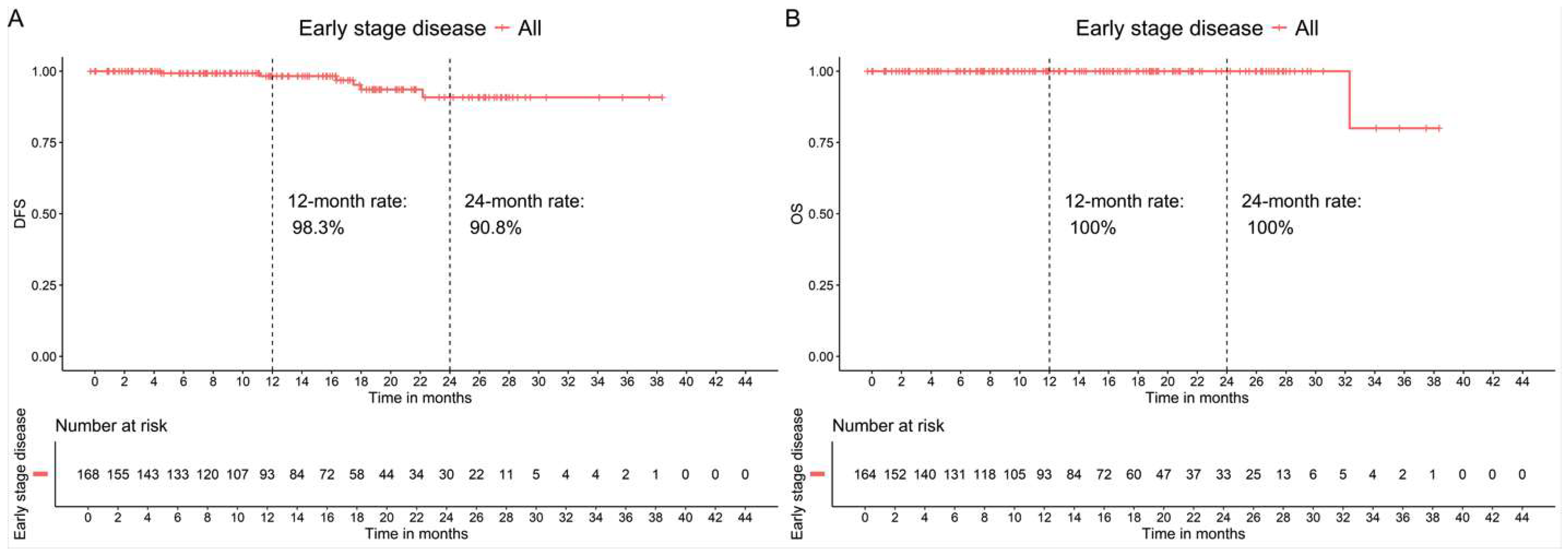
3.3.1. Patients in Early-Stage High-Risk Breast Cancer
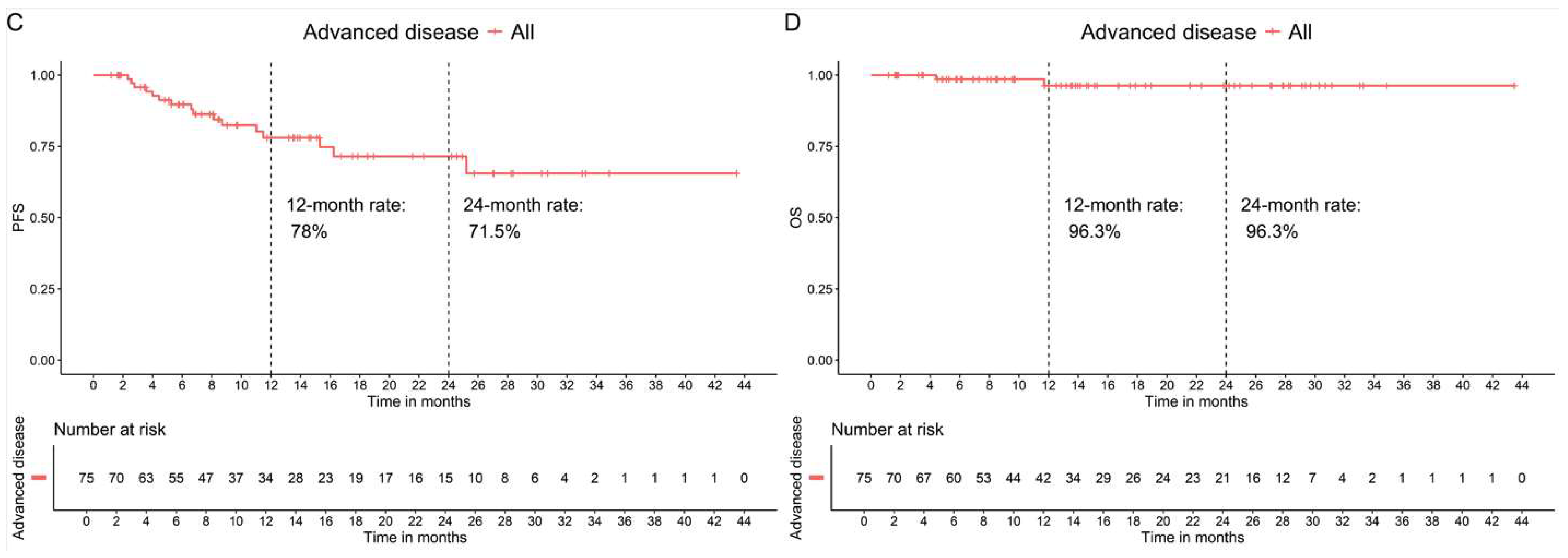
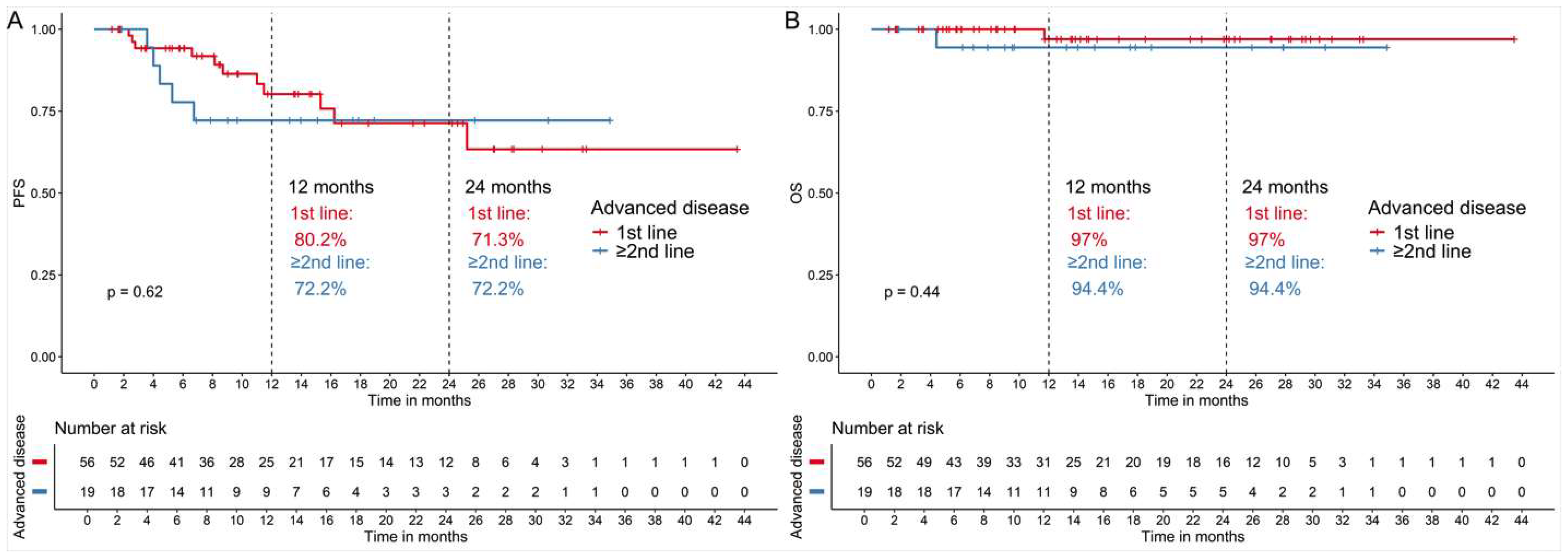
3.3.2. Patients with Advanced Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | adverse event |
| BC | advanced breast cancer |
| CDK | cyclin-dependent kinases |
| DFS | disease-free survival |
| EMA | European Medicines Agency |
| FDA | U.S. Food and Drug Administration |
| HeCOG | Hellenic Cooperative Oncology Group |
| HER2 | human epidermal growth factor receptor 2 |
| HR | hormone receptor |
| OS | overall survival |
| PFS | progression-free survival |
References
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.-A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Lipatov, O.; Nowecki, Z.; McAndrew, N.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; Fasching, P.A.; Crown, J.; et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N. Engl. J. Med. 2024, 390, 1080–1091. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef]
- Rastogi, P.; O’Shaughnessy, J.; Martin, M.; Boyle, F.; Cortes, J.; Rugo, H.S.; Goetz, M.P.; Hamilton, E.P.; Huang, C.S.; Senkus, E.; et al. Adjuvant Abemaciclib Plus Endocrine Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, High-Risk Early Breast Cancer: Results From a Preplanned monarchE Overall Survival Interim Analysis, Including 5-Year Efficacy Outcomes. J. Clin. Oncol. 2024, 42, 987–993. [Google Scholar] [CrossRef]
- Di Maio, M.; Perrone, F.; Conte, P. Real-World Evidence in Oncology: Opportunities and Limitations. Oncologist 2020, 25, e746–e752. [Google Scholar] [CrossRef]
- Saesen, R.; Van Hemelrijck, M.; Bogaerts, J.; Booth, C.M.; Cornelissen, J.J.; Dekker, A.; Eisenhauer, E.A.; Freitas, A.; Gronchi, A.; Hernán, M.A.; et al. Defining the role of real-world data in cancer clinical research: The position of the European Organisation for Research and Treatment of Cancer. Eur. J. Cancer 2023, 186, 52–61. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Onar-Thomas, A.; Wheeler, S.B. Real-World Database Studies in Oncology: A Call for Standards. J. Clin. Oncol. 2024, 42, 977–980. [Google Scholar] [CrossRef]
- Fountzilas, E.; Koliou, G.A.; Vozikis, A.; Rapti, V.; Nikolakopoulos, A.; Boutis, A.; Christopoulou, A.; Kontogiorgos, I.; Karageorgopoulou, S.; Lalla, E.; et al. Real-world clinical outcome and toxicity data and economic aspects in patients with advanced breast cancer treated with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors combined with endocrine therapy: The experience of the Hellenic Cooperative Oncology Group. ESMO Open 2020, 5, e000774. [Google Scholar] [CrossRef]
- Drowne, T.; Armgardt, E.; Svoboda, A. Real-world experience of abemaciclib for adjuvant and metastatic breast cancer. J. Oncol. Pharm. Pract. 2025, 31, 141–146. [Google Scholar] [CrossRef]
- Shimoi, T.; Pathadka, S.; Sekine, N.; Cai, Z.; Tanizawa, Y.; Kawaguchi, T.; Saji, S.; Yamashita, T. Real-world data on patients with early breast cancer who were prescribed abemaciclib adjuvant therapy in Japan. Future Oncol. 2024, 20, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, L.; Pellat, A.; Martins-Branco, D.; Valachis, A.; Derksen, J.W.G.; Suijkerbuijk, K.P.M.; Dafni, U.; Dellaporta, T.; Vogel, A.; Prelaj, A.; et al. ESMO Guidance for Reporting Oncology real-World evidence (GROW). Ann. Oncol 2023, 34, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2024, 22, 331–357. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Malorni, L.; Curigliano, G.; Minisini, A.M.; Cinieri, S.; Tondini, C.A.; D’Hollander, K.; Arpino, G.; Bernardo, A.; Martignetti, A.; Criscitiello, C.; et al. Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann. Oncol. 2018, 29, 1748–1754. [Google Scholar] [CrossRef]
- Hamilton, E.; Cortes, J.; Ozyilkan, O.; Chen, S.C.; Petrakova, K.; Manikhas, A.; Jerusalem, G.; Hegg, R.; Huober, J.; Chapman, S.C.; et al. nextMONARCH: Abemaciclib Monotherapy or Combined With Tamoxifen for Metastatic Breast Cancer. Clin. Breast Cancer 2021, 21, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil Gil, M.J.; Ruiz Borrego, M.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Palbociclib with Fulvestrant or Letrozole in Endocrine-Sensitive Patients with HR-Positive/HER2-Negative Advanced Breast Cancer: A Detailed Safety Analysis of the Randomized PARSIFAL Trial. Oncologist 2023, 28, 23–32. [Google Scholar] [CrossRef]
- Rugo, H.S.; Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Cristofanilli, M.; Torres, M.A.; Curigliano, G.; et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer 2022, 8, 114. [Google Scholar] [CrossRef]
- Wong, V.; de Boer, R.; Baron-Hay, S.; Blum, R.; Boyle, F.; Chua, S.; Clarke, K.; Cuff, K.; Green, M.; Lim, E.; et al. Real-World Outcomes of Ribociclib and Aromatase Inhibitor Use in First Line Hormone Receptor Positive, HER2-Negative Metastatic Breast Cancer. Clinical Breast Cancer 2022, 22, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Layman, R.M.; Lynce, F.; Liu, X.; Li, B.; McRoy, L.; Cohen, A.B.; Estevez, M.; Curigliano, G.; Brufsky, A. Comparative overall survival of CDK4/6 inhibitors plus an aromatase inhibitor in HR+/HER2- metastatic breast cancer in the US real-world setting. ESMO Open 2025, 10, 104103. [Google Scholar] [CrossRef] [PubMed]
- Cuyun Carter, G.; Sheffield, K.M.; Gossai, A.; Huang, Y.J.; Zhu, Y.E.; Bowman, L.; Nash Smyth, E.; Mathur, R.; Cohen, A.B.; Rasmussen, E.; et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2- metastatic breast cancer. Curr. Med. Res. Opin. 2021, 37, 1179–1187. [Google Scholar] [CrossRef]
- Gehrchen, M.L.; Berg, T.; Garly, R.; Jensen, M.B.; Eßer-Naumann, S.; Rønlev, J.D.; Nielsen, H.M.; Knoop, A.; Kümler, I. Real-world effectiveness of CDK 4/6 inhibitors in estrogen-positive metastatic breast cancer. BJC Rep. 2024, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Cejuela, M.; Gil-Torralvo, A.; Castilla, M.; Domínguez-Cejudo, M.; Falcón, A.; Benavent, M.; Molina-Pinelo, S.; Ruiz-Borrego, M.; Salvador Bofill, J. Abemaciclib, Palbociclib, and Ribociclib in Real-World Data: A Direct Comparison of First-Line Treatment for Endocrine-Receptor-Positive Metastatic Breast Cancer. Int. J. Mol. Sci. 2023, 24, 8488. [Google Scholar] [CrossRef]
- Provenzano, L.; Dieci, M.V.; Curigliano, G.; Giuliano, M.; Botticelli, A.; Lambertini, M.; Rizzo, G.; Pedersini, R.; Sirico, M.; La Verde, N.; et al. Real-world effectiveness comparison of first-line palbociclib, ribociclib or abemaciclib plus endocrine therapy in advanced HR-positive/HER2-negative BC patients: Results from the multicenter PALMARES-2 study. Ann. Oncol. 2025, 36, 762–774. [Google Scholar] [CrossRef]
- Premji, S.; Sarah, K.P.; Tanmayi, S.P.; Savannah, S.L.; Nathaniel, E.W.; Prasad, D.K.; Whitney, R.H.; Lisa, L.E.; Jenna, E.H.; Shikha, P.; et al. Abstract P1-11-08: Practice patterns and uptake of adjuvant abemaciclib in high risk localized hormone receptor positive, HER2-negative breast cancer. Clin. Cancer Res. 2025, 31 (Suppl. S12), 1–11. [Google Scholar] [CrossRef]
- Phan, V.H.; Moore, M.M.; McLachlan, A.J.; Piquette-Miller, M.; Xu, H.; Clarke, S.J. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin. Drug Metab. Toxicol. 2009, 5, 243–257. [Google Scholar] [CrossRef]
- Karihtala, P.; Schiza, A.; Fountzilas, E.; Geisler, J.; Meattini, I.; Risi, E.; Biganzoli, L.; Valachis, A. Clinical trials in older patients with cancer—Typical challenges, possible solutions, and a paradigm of study design in breast cancer. Acta Oncol. 2024, 63, 441–447. [Google Scholar] [CrossRef]
- Valachis, A.; Biganzoli, L.; Christopoulou, A.; Fjermeros, K.; Fountzila, E.; Geisler, J.; Gomez-Bravo, R.; Karihtala, P.; Kosmidis, P.; Koutras, A.; et al. Implementing geriatric assessment for dose optimization of CDK4/6 inhibitors in older breast cancer patients. Future Oncol. 2024, 20, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Accordino, M.K.; Bedard, P.L.; Cervantes, A.; Gambardella, V.; Hamilton, E.; Italiano, A.; Kalinsky, K.; Krop, I.E.; Oliveira, M.; et al. Phase I/Ib Trial of Inavolisib Plus Palbociclib and Endocrine Therapy for PIK3CA-Mutated, Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced or Metastatic Breast Cancer. J. Clin. Oncol. 2024, 42, 3947–3956. [Google Scholar] [CrossRef]
- Loibl, S.; Metzger, O.; Mandrekar, S.J.; Mundhenke, C.; Seiler, S.; Valagussa, P.; DeMichele, A.; Lim, E.; Tripathy, D.; Winer, E.P.; et al. PATINA: A randomized, open label, phase III trial to evaluate the efficacy and safety of palbociclib + Anti-HER2 therapy + endocrine therapy (ET) vs. anti-HER2 therapy + ET after induction treatment for hormone receptor positive (HR+)/HER2-positive metastatic breast cancer (MBC). Ann. Oncol. 2018, 29, viii121. [Google Scholar] [CrossRef]
- Navarro, G.; Gómez-Autet, M.; Morales, P.; Rebassa, J.B.; Llinas Del Torrent, C.; Jagerovic, N.; Pardo, L.; Franco, R. Homodimerization of CB(2) cannabinoid receptor triggered by a bivalent ligand enhances cellular signaling. Pharmacol. Res. 2024, 208, 107363. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Berrón, S.; Garau, I.; Gramaje, M.; Castelo, B.; Cortes Salgado, A.; Sanchez-Rovira, P.; Bueno, A.; García, A.; Gener Gromova, P.; et al. 271P Eribulin (E) plus endocrine therapy (ET) in patients (pts) with HR[+]/HER2[-] metastatic breast cancer (mBC) after progression on previous ET: The REVERT study. Ann. Oncol. 2022, 33, S661–S662. [Google Scholar] [CrossRef]
| Early-Stage (n = 169) | Advanced (n = 76) | Total (n = 245) | |
|---|---|---|---|
| Age at diagnosis Median [IQR] | 56.0 [16.0] | 59.5 [21.3] | 57.0 [18.0] |
| The patient comes from urban centers, n = 244 | 115 (68.5%) | 42 (55.3%) | 157 (64.3%) |
| Previous history of other cancers, n = 244 | 8 (4.8%) | 3 (3.9%) | 11 (4.5%) |
| Family history of breast or ovarian cancer, n = 237 | 38 (23.3%) | 15 (20.3%) | 53 (22.4%) |
| Family history of other cancers, n = 234 | 54 (34.0%) | 23 (31.1%) | 77 (32.9%) |
| Location, n = 243 | |||
| Bilateral | 8 (4.7%) | 4 (5.4%) | 12 (4.9%) |
| Left breast | 83 (49.1%) | 38 (51.4%) | 121 (49.8%) |
| Right breast | 78 (46.2%) | 32 (43.2%) | 110 (45.3%) |
| Tumor size, n = 194 | |||
| T1 0–2 cm | 44 (29.3%) | 18 (40.9%) | 62 (32.0%) |
| T2 2.1–5 cm | 72 (48.0%) | 15 (34.1%) | 87 (44.8%) |
| T3 ≥ 5 cm | 34 (22.7%) | 11 (25.0%) | 45 (23.2%) |
| Histology grade, n = 239 | |||
| 1 | 8 (4.7%) | 3 (4.3%) | 11 (4.6%) |
| 2 | 105 (62.1%) | 43 (61.4%) | 148 (61.9%) |
| 3 | 56 (33.1%) | 24 (34.3%) | 80 (33.5%) |
| LVI/PNI, n = 208 | 78 (50.3%) | 26 (49.1%) | 104 (50.0%) |
| Nodal status, n = 209 | |||
| 0 | 9 (5.4%) | 15 (35.7%) | 24 (11.5%) |
| 1–3 | 43 (25.7%) | 14 (33.3%) | 57 (27.3%) |
| ≥4 | 115 (68.9%) | 13 (31.0%) | 128 (61.2%) |
| Estrogen Receptor status, n = 245 | |||
| ER-positive | 169 (100%) | 76 (100%) | 245 (100%) |
| ER positivity value (%) | 95.0 [15.0] | 95.0 [20.0] | 95.0 [15.0] |
| Progesterone Receptor status, n = 245 | |||
| Positive | 144 (85.2%) | 61 (80.3%) | 203 (83.5%) |
| PR positivity value (%) | 80.0 [55.0] | 70.0 [62.0] | 80.0 [55.0] |
| Ki67 (%) | 20.0 [15.0] | 20.0 [16.5] | 20.0 [15.0] |
| Genetic testing, result, n = 86 | |||
| Mutation | 16 (23.9%) | 4 (23.5%) | 20 (23.8%) |
| Negative | 47 (70.1%) | 13 (76.5%) | 60 (71.4%) |
| VUS | 4 (6.0%) | 0 (0.0%) | 4 (4.8%) |
| If mutation, specify # | |||
| BRCA1 | 5 (31.3%) | 0 (0.0%) | 5 (25.0%) |
| BRCA2 | 3 (18.8%) | 1 (25.0%) | 4 (20.0%) |
| CHEK2 | 1 (6.3%) | 1 (25.0%) | 2 (10.0%) |
| PALB2 | 3 (18.8%) | 0 (0.0%) | 3 (15.0%) |
| ATM | 4 (25.0%) | 0 (0.0%) | 4 (20.0%) |
| OTHER | 3 (18.8%) | 2 (50.0%) | 5 (25.0%) |
| Menopausal status (n = 236) | |||
| Premenopausal/Perimenopausal | 50 (30.9%) | 19 (25.7%) | 69 (29.2.0%) |
| Postmenopausal | 112 (69.1%) | 55 (74.3%) | 167 (70.8%) |
| Chemotherapy adjuvant/neoadjuvant | 163 (96.4%) | ΝA | |
| Endocrine therapy (n = 245) | 169 (100%) | 76 (100%) | |
| Tamoxifen | 18 (10.8%) | 5 (6.6%) | |
| Aromatase inhibitor | 150 (88.8%) | 52 (68.4%) | |
| Fulvestrant | 1 (0.6%) | 20 (26.3%) | |
| De novo metastatic disease | 40 (52.6%) | ||
| Site of metastasis at diagnosis | |||
| Locoregional (axillary and supraclavicular nodes, skin, breast) | 18 (23.7%) | ||
| Bones | 28 (36.8%) | ||
| Lung | 12 (15.8%) | ||
| Liver | 3 (3.9%) | ||
| Other * | 14 |
| Adverse Events | n (%) | Grade | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Diarrhea | 125 (51.0%) | 64 (26.1%) | 46 (18.8%) | 13 (5.3%) | 1 (0.4%) |
| Fatigue | 43 (17.6%) | 31 (12.7%) | 10 (4.1%) | 1 (0.4%) | |
| Arthralgia | 17 (6.9%) | 13 (5.3%) | 4 (1.6%) | ||
| Leukopenia | 15 (6.1%) | 8 (3.3%) | 6 (2.4%) | 1 (0.4%) | |
| Nausea | 13 (5.3%) | 10 (4.1%) | 3 (1.2%) | ||
| Anemia | 13 (5.3%) | 7 (2.9%) | 5 (2.0%) | 1 (0.4%) | |
| ALT/AST increased | 12 (4.9%) | 9 (3.7%) | 1 (0.4%) | 2 (0.8%) | |
| Neutropenia | 11 (4.5%) | 6 (2.4%) | 3 (1.2%) | 2 (0.8%) | |
| Abdominal Pain | 10 (4.1%) | 8 (3.3%) | 1 (0.4%) | ||
| Stomatitis/Dry Mouth | 9 (3.7%) | 6 (2.4%) | 2 (0.8%) | ||
| Anorexia | 9 (3.7%) | 6 (2.4%) | 3 (1.2%) | ||
| Headache | 6 (2.4%) | 6 (2.4%) | |||
| Dyspepsia | 6 (2.4%) | 2 (0.8%) | 4 (1.6%) | ||
| Hot Flashes/night sweats | 5 (2.0%) | 5 (2.0%) | |||
| Vomiting | 5 (2.0%) | 5 (2.0%) | |||
| Skin Rash | 4 (1.6%) | 2 (0.8%) | 1 (0.4%) | ||
| Dry skin | 4 (1.6%) | 2 (0.8%) | 1 (0.4%) | ||
| Constipation | 3 (1.2%) | 2 (0.8%) | 1 (0.4%) | ||
| Infection | 3 (1.2%) | 2 (0.8%) | 1 (0.4%) | ||
| Changes in food taste | 3 (1.2%) | 1 (0.4%) | 2 (0.8%) | ||
| Depression | 3 (1.2%) | 1 (0.4%) | 2 (0.8%) | ||
| Pruritus | 2 (0.8%) | 2 (0.8%) | |||
| Vaginal dryness | 2 (0.8%) | 2 (0.8%) | |||
| Dizziness | 2 (0.8%) | 2 (0.8%) | |||
| Insomnia | 2 (0.8%) | 2 (0.8%) | |||
| Thrombocytopenia | 1 (0.4%) | 1 (0.4%) | |||
| Other * | 11 (4.5%) | 7 (2.9%) | 2 (0.8%) | 2 (0.8%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fountzilas, E.; Aravantinou-Fatorou, E.; Dadouli, K.; Economopoulou, P.; Tryfonopoulos, D.; Vernadou, A.; Vorrias, E.; Vagionas, A.; Nikolaidi, A.; Karageorgopoulou, S.; et al. Real-World Toxicity and Effectiveness Study of Abemaciclib in Greek Patients with Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: A Multi-Institutional Study. Cancers 2025, 17, 2543. https://doi.org/10.3390/cancers17152543
Fountzilas E, Aravantinou-Fatorou E, Dadouli K, Economopoulou P, Tryfonopoulos D, Vernadou A, Vorrias E, Vagionas A, Nikolaidi A, Karageorgopoulou S, et al. Real-World Toxicity and Effectiveness Study of Abemaciclib in Greek Patients with Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: A Multi-Institutional Study. Cancers. 2025; 17(15):2543. https://doi.org/10.3390/cancers17152543
Chicago/Turabian StyleFountzilas, Elena, Eleni Aravantinou-Fatorou, Katerina Dadouli, Panagiota Economopoulou, Dimitrios Tryfonopoulos, Anastasia Vernadou, Eleftherios Vorrias, Anastasios Vagionas, Adamantia Nikolaidi, Sofia Karageorgopoulou, and et al. 2025. "Real-World Toxicity and Effectiveness Study of Abemaciclib in Greek Patients with Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: A Multi-Institutional Study" Cancers 17, no. 15: 2543. https://doi.org/10.3390/cancers17152543
APA StyleFountzilas, E., Aravantinou-Fatorou, E., Dadouli, K., Economopoulou, P., Tryfonopoulos, D., Vernadou, A., Vorrias, E., Vagionas, A., Nikolaidi, A., Karageorgopoulou, S., Koumarianou, A., Boukovinas, I., Mauri, D., Kokkali, S., Christopoulou, A., Tsoukalas, N., Assi, A., Spathas, N., Kosmidis, P., ... Psyrri, A. (2025). Real-World Toxicity and Effectiveness Study of Abemaciclib in Greek Patients with Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: A Multi-Institutional Study. Cancers, 17(15), 2543. https://doi.org/10.3390/cancers17152543







