Spatial Omics Profiling of Treatment-Naïve Lung Adenocarcinoma with Brain Metastasis as the Initial Presentation
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Samples
2.2. Digital Spatial Protein Profiling and ROI Selection
2.3. Statistics
3. Results
3.1. Study Samples and Protein Profiling
3.2. Differential Protein Expression Between Primary LUAD and Brain Metastases
3.3. Histologic Distribution-Specific Protein Expression in ROI Subgroups
3.4. Intra-Group Comparison of Protein Distribution Within ROI Subtypes
3.5. Protein Expression According to Tumor–Stroma Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BM | brain metastasis |
| CNS | central nervous system |
| CSCs | cancer stem cells |
| DSP | digital spatial profiling |
| FFPE | formalin-fixed paraffin-embedded |
| ICIs | immune checkpoint inhibitors |
| IHC | immunohistochemical staining |
| LUAD | lung adenocarcinoma |
| NSCLC | non-small cell lung cancer |
| PVS | perivascular stroma |
| ROI | region of interest |
| rpS6 | ribosomal protein S6 |
| SC | stromal cell |
| TC | tumor cell |
| TME | tumor microenvironment |
| TSI | tumor–stromal interface |
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Sher, T.; Dy, G.K.; Adjei, A.A. Small cell lung cancer. Mayo Clin. Proc. 2008, 83, 355–367. [Google Scholar] [CrossRef]
- Denisenko, T.V.; Budkevich, I.N.; Zhivotovsky, B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, Q. Analysis of prognostic factors and establishment of prediction model of lung adenocarcinoma based on SEER database. Transl. Cancer Res. 2023, 12, 3346. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Castrucci, W.A.; Knisely, J.P. An update on the treatment of CNS metastases in small cell lung cancer. Cancer J. 2008, 14, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hubbs, J.L.; Boyd, J.A.; Hollis, D.; Chino, J.P.; Saynak, M.; Kelsey, C.R. Factors associated with the development of brain metastases: Analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer 2010, 116, 5038–5046. [Google Scholar] [CrossRef]
- Gril, B.; Evans, L.; Palmieri, D.; Steeg, P.S. Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. Eur. J. Cancer 2010, 46, 1204–1210. [Google Scholar] [CrossRef]
- Pedrosa, R.M.; Mustafa, D.A.; Aerts, J.G.; Kros, J.M. Potential molecular signatures predictive of lung cancer brain metastasis. Front. Oncol. 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhou, X.; Liang, X.; Huang, R.; Chu, Z.; Jiang, J.; Zhan, Q. Brain metastases as the first symptom of lung cancer: A clinical study from an Asian medical center. J. Cancer Res. Clin. Oncol. 2013, 139, 403–408. [Google Scholar] [CrossRef]
- Decalf, J.; Albert, M.L.; Ziai, J. New tools for pathology: A user’s review of a highly multiplexed method for in situ analysis of protein and RNA expression in tissue. J. Pathol. 2019, 247, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.H.E.; Kyle, A.H.; Reinsberg, S.A.; Moosvi, F.; Patrick, H.M.; Cran, J.; Saatchi, K.; Häfeli, U.; Minchinton, A.I. Heterogeneous distribution of trastuzumab in HER2-positive xenografts and metastases: Role of the tumor microenvironment. Clin. Exp. Metastasis 2018, 35, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.W.; Huang, W.C.; Sung, S.Y.; Wu, D.; Odero-Marah, V.; Nomura, T.; Shigemura, K.; Miyagi, T.; Seo, S.; Shi, C.; et al. Stromal-epithelial interaction in prostate cancer progression. Clin. Genitourin. Cancer 2006, 5, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Gallanis, G.T.; Sharif, G.M.; Schmidt, M.O.; Friedland, B.N.; Battina, R.; Rahhal, R.; Davis, J.E., Jr.; Khan, I.S.; Wellstein, A.; Riegel, A.T. Stromal senescence following treatment with the CDK4/6 inhibitor palbociclib alters the lung metastatic niche and increases metastasis of drug-resistant mammary cancer cells. Cancers 2023, 15, 1908. [Google Scholar] [CrossRef]
- Sevenich, L.; Bowman, R.L.; Mason, S.D.; Quail, D.F.; Rapaport, F.; Elie, B.T.; Brogi, E.; Brastianos, P.K.; Hahn, W.C.; Holsinger, L.J.; et al. Analysis of tumour-and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 2014, 16, 876–888. [Google Scholar] [CrossRef]
- Fazilaty, H.; Behnam, B. The perivascular niche governs an autoregulatory network to support breast cancer metastasis. Cell Biol. Int. 2014, 38, 691–694. [Google Scholar] [CrossRef]
- Murgai, M.; Ju, W.; Eason, M.; Kline, J.; Beury, D.W.; Kaczanowska, S.; Miettinen, M.M.; Kruhlak, M.; Lei, H.; Shern, J.F.; et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med. 2017, 23, 1176–1190. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Y.; Liu, Z.; Song, Y.; Ge, L.; Du, T.; Liu, Y.; Liu, L.; Liu, C.; Ma, L. L1CAM deployed perivascular tumor niche promotes vessel wall invasion of tumor thrombus and metastasis of renal cell carcinoma. Cell Death Discov. 2023, 9, 112. [Google Scholar] [CrossRef]
- Perry, S.W.; Schueckler, J.M.; Burke, K.; Arcuri, G.L.; Brown, E.B. Stromal matrix metalloprotease-13 knockout alters Collagen I structure at the tumor-host interface and increases lung metastasis of C57BL/6 syngeneic E0771 mammary tumor cells. BMC Cancer 2013, 13, 411. [Google Scholar] [CrossRef]
- Tagirasa, R.; Yoo, E. Role of serine proteases at the tumor-stroma interface. Front. Immunol. 2022, 13, 832418. [Google Scholar] [CrossRef]
- Allan, A.L.; Vantyghem, S.A.; Tuck, A.B.; Chambers, A.F. Tumor dormancy and cancer stem cells: Implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2007, 26, 87–98. [Google Scholar] [CrossRef]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- Oskarsson, T.; Acharyya, S.; Zhang, X.H.; Vanharanta, S.; Tavazoie, S.F.; Morris, P.G.; Downey, R.J.; Manova-Todorova, K.; Brogi, E.; Massagué, J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 2011, 17, 867–874. [Google Scholar] [CrossRef]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Oskarsson, T. Microenvironment in metastasis: Roadblocks and supportive niches. Am. J. Physiol.-Cell Physiol. 2015, 309, C627–C638. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.J.; Nayyar, N.; Bihun, I.; Dagogo-Jack, I.; Gill, C.M.; Aquilanti, E.; Bertalan, M.; Kaplan, A.; D’Andrea, M.R.; Chukwueke, U.; et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat. Genet. 2020, 52, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.P.; Engel, K.B.; Greytak, S.R.; Moore, H.M. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: How well do you know your FFPE specimen? Arch. Pathol. Lab. Med. 2014, 138, 1520–1530. [Google Scholar] [CrossRef]
- Toki, M.I.; Merritt, C.R.; Wong, P.F.; Smithy, J.W.; Kluger, H.M.; Syrigos, K.N.; Ong, G.T.; Warren, S.E.; Beechem, J.M.; Rimm, D.L. High-plex predictive marker discovery for melanoma immunotherapy–treated patients using digital spatial profiling. Clin. Cancer Res. 2019, 25, 5503–5512. [Google Scholar] [CrossRef]
- Ziai, J.; Caplazi, P.; Decalf, J.; Liang, Y.; Almeida, P.d.; Zollinger, D.; Schoiack, A.V.; Beechem, J.; Grogan, J.; Albert, M. Highly multiplexed analysis of immune cell subsets in non-small cell lung cancer: Validation of protein and RNA analysis by the Nanostring Digital Spatial Profiling (DSP) platform. Cancer Res. 2018, 78, 2089. [Google Scholar] [CrossRef]
- Liu, J.S.; Cai, Y.X.; He, Y.Z.; Xu, J.; Tian, S.F.; Li, Z.Q. Spatial and temporal heterogeneity of tumor immune microenvironment between primary tumor and brain metastases in NSCLC. BMC Cancer 2024, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Mei, W.; Robles, I.; Hagerling, C.; Allen, B.M.; Okholm, T.L.H.; Nanjaraj, A.; Verbeek, T.; Kalavacherla, S.; van Gogh, M.; et al. Cellular architecture of human brain metastases. Cell 2022, 185, 729–745. [Google Scholar] [CrossRef]
- Charles, N.A.; Holland, E.C. The perivascular niche microenvironment in brain tumor progression. Cell Cycle 2010, 9, 3084–3093. [Google Scholar] [CrossRef]
- Wu, J.; Liang, C.; Chen, M.; Su, W. Association between tumor-stroma ratio and prognosis in solid tumor patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 68954. [Google Scholar] [CrossRef]
- Beechem, J.M. High-plex spatially resolved RNA and protein detection using digital spatial profiling: A technology designed for immuno-oncology biomarker discovery and translational research. In Biomarkers for Immunotherapy of Cancer: Methods and Protocols; Humana: New York, NY, USA, 2020; pp. 563–583. [Google Scholar]
- El Rassy, E.; Botticella, A.; Kattan, J.; Le Péchoux, C.; Besse, B.; Hendriks, L. Non-small cell lung cancer brain metastases and the immune system: From brain metastases development to treatment. Cancer Treat. Rev. 2018, 68, 69–79. [Google Scholar] [CrossRef]
- Yawn, B.P.; Wollan, P.C.; Schroeder, C.; Gazzuola, L.; Mehta, M. Temporal and gender-related trends in brain metastases from lung and breast cancer. Minn. Med. 2003, 86, 32–37. [Google Scholar]
- Su, H.; Lin, Z.; Peng, W.; Hu, Z. Identification of potential biomarkers of lung adenocarcinoma brain metastases via microarray analysis of cDNA expression profiles. Oncol. Lett. 2019, 17, 2228–2236. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, K.J.; Cho, B.C.; Liu, B.; Paz-Ares, L.; Cheng, S.; Kitazono, S.; Thiagarajan, M.; Goldman, J.W.; Sabari, J.K.; et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N. Engl. J. Med. 2023, 389, 2039–2051. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Govindan, R.; Szczesna, A.; Ahn, M.J.; Schneider, C.P.; Gonzalez Mella, P.F.; Barlesi, F.; Han, B.; Ganea, D.E.; Von Pawel, J.; Vladimirov, V.; et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non–small-cell lung cancer. J. Clin. Oncol. 2017, 35, 3449–3457. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Schwab, S.; Burghuber, O.C.; Krenbek, D.; Prosch, H. Symptomatic pseudo-progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer 2017, 113, 4–6. [Google Scholar] [CrossRef]
- Parvez, K.; Parvez, A.; Zadeh, G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int. J. Mol. Sci. 2014, 15, 11832–11846. [Google Scholar] [CrossRef]
- Doherty, M.K.; Jao, K.; Shepherd, F.A.; Hazrati, L.N.; Leighl, N.B. Central nervous system pseudoprogression in a patient treated with PD-1 checkpoint inhibitor. J. Thorac. Oncol. 2015, 10, e100–e101. [Google Scholar] [CrossRef]
- Hung, J.J.; Jeng, W.J.; Wu, Y.C.; Chou, T.Y.; Hsu, W.H. Factors predicting organ-specific distant metastasis in patients with completely resected lung adenocarcinoma. Oncotarget 2016, 7, 58261. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Lin, Z.W.; Wu, L.X.; Xie, Y.; Ou, X.; Tian, P.K.; Liu, X.P.; Min, J.; Wang, J.; Chen, R.F.; Chen, Y.J.; et al. The expression levels of transcription factors T-bet, GATA-3, RORγt and FOXP3 in peripheral blood lymphocyte (PBL) of patients with liver cancer and their significance. Int. J. Med Sci. 2015, 12, 7–16. [Google Scholar] [CrossRef]
- Chen, B.; Tan, Z.; Gao, J.; Wu, W.; Liu, L.; Jin, W.; Cao, Y.; Zhao, S.; Zhang, W.; Qiu, Z.; et al. Hyperphosphorylation of ribosomal protein S6 predicts unfavorable clinical survival in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2015, 34, 126. [Google Scholar] [CrossRef]
- Ruvinsky, I.; Meyuhas, O. Ribosomal protein S6 phosphorylation: From protein synthesis to cell size. Trends Biochem. Sci. 2006, 31, 342–348. [Google Scholar] [CrossRef]
- Ruvinsky, I.; Sharon, N.; Lerer, T.; Cohen, H.; Stolovich-Rain, M.; Nir, T.; Dor, Y.; Zisman, P.; Meyuhas, O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005, 19, 2199–2211. [Google Scholar] [CrossRef]
- Karbowniczek, M.; Yu, J.; Henske, E.P. Renal angiomyolipomas from patients with sporadic lymphangiomyomatosis contain both neoplastic and non-neoplastic vascular structures. Am. J. Pathol. 2003, 162, 491–500. [Google Scholar] [CrossRef]
- Robb, V.A.; Astrinidis, A.; Henske, E.P. Frequent [corrected] hyperphosphorylation of ribosomal protein S6 [corrected] in lymphangioleiomyomatosis-associated angiomyolipomas. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc 2006, 19, 839–846. [Google Scholar] [CrossRef]
- Albert, S.; Serova, M.; Dreyer, C.; Sablin, M.P.; Faivre, S.; Raymond, E. New inhibitors of the mammalian target of rapamycin signaling pathway for cancer. Expert Opin. Investig. Drugs 2010, 19, 919–930. [Google Scholar] [CrossRef]
- Benjamin, D.; Colombi, M.; Moroni, C.; Hall, M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011, 10, 868–880. [Google Scholar] [CrossRef]
- Plas, D.R.; Thomas, G. Tubers and tumors: Rapamycin therapy for benign and malignant tumors. Curr. Opin. Cell Biol. 2009, 21, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, Y.H.; Chau, G.C.; Pyo, S.; Um, S.H. Prognostic significance and function of phosphorylated ribosomal protein S6 in esophageal squamous cell carcinoma. Mod. Pathol. 2013, 26, 327–335. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.M.; Pelloski, C.E.; Ledoux, A.; Sun, M.; Raso, G.; Komaki, R.; Wistuba, I.I.; Bekele, B.N.; Aldape, K. Elevated phospho-S6 expression is associated with metastasis in adenocarcinoma of the lung. Clin. Cancer Res. 2008, 14, 7832–7837. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, J.; Huang, L.; Long, F. Efficacy of PD-1/L1 inhibitors in brain metastases of non-small-cell lung cancer: Pooled analysis from seven randomized controlled trials. Future Oncol. 2022, 18, 403–412. [Google Scholar] [CrossRef]
- Chen, W.; Yang, L.; Pang, D.; Liuru, T.; Liang, Z.; MA, L.; Zhang, F.; Xu, W.; Zhang, J.; Luo, M.; et al. Association of IDO immune suppression with brain metastasis in non-small cell lung cancer. J. Clin. Oncol. 2021, 39, e21215. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Yu, Q.; Wu, H.; Liu, B.; Xiong, H.; Hu, G.; Zhao, J.; Yuan, X.; Liao, Z. Associations between single-nucleotide polymorphisms in the PI3K–PTEN–AKT–mTOR pathway and increased risk of brain metastasis in patients with non–small cell lung cancer. Clin. Cancer Res. 2013, 19, 6252–6260. [Google Scholar] [CrossRef]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Ciuffreda, L.; Ferretti, G.; Vari, S.; Ferraresi, V.; Cognetti, F.; Milella, M. PTEN function at the interface between cancer and tumor microenvironment: Implications for response to immunotherapy. Int. J. Mol. Sci. 2020, 21, 5337. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, L.; Li, S.L.; Gu, J.; Cui, X.; Zhou, Y. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer 2021, 21, 429. [Google Scholar] [CrossRef]
- Collins, N.B.; Al Abosy, R.; Miller, B.C.; Bi, K.; Zhao, Q.; Quigley, M.; Ishizuka, J.J.; Yates, K.B.; Pope, H.W.; Manguso, R.T.; et al. PI3K activation allows immune evasion by promoting an inhibitory myeloid tumor microenvironment. J. Immunother. Cancer 2022, 10, e003402. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kang, Y.; Wang, M.; Wu, B.; Su, B.; Yin, H.; Tang, Y.; Li, Q.; Wei, W.; Mei, Q.; et al. Targeting polarized phenotype of microglia via IL6/JAK2/STAT3 signaling to reduce NSCLC brain metastasis. Signal Transduct. Target. Ther. 2022, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, L.; Xiao, D.; Huang, Z.; Wang, W.; Zhai, K.; Fang, X.; Kim, J.; Liu, J.; Liang, W.; et al. CD44+ lung cancer stem cell-derived pericyte-like cells cause brain metastases through GPR124-enhanced trans-endothelial migration. Cancer Cell 2023, 41, 1621–1636. [Google Scholar] [CrossRef]
- Orian-Rousseau, V. CD44 acts as a signaling platform controlling tumor progression and metastasis. Front. Immunol. 2015, 6, 154. [Google Scholar] [CrossRef]
- Morita, S.; Hourai, A.; Miyata, S. Changes in pericytic expression of NG2 and PDGFRB and vascular permeability in the sensory circumventricular organs of adult mouse by osmotic stimulation. Cell Biochem. Funct. 2014, 32, 51–61. [Google Scholar] [CrossRef]
- Tsakonas, G.; Koulouris, A.; Kazmierczak, D.; Botling, J.; Ortiz-Villalon, C.; Nord, H.; Lindskog, M.; Sandelin, M.; Micke, P.; Hydbring, P.; et al. Matched analyses of brain metastases versus primary non-small cell lung cancer reveal a unique microRNA signature. Int. J. Mol. Sci. 2022, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, J.; Wang, L. Machine learning based prediction of brain metastasis of patients with IIIA-N2 lung adenocarcinoma by a three-miRNA signature. Transl. Oncol. 2018, 11, 157–167. [Google Scholar] [CrossRef]
- Martínez-Espinosa, I.; Serrato, J.A.; Ortiz-Quintero, B. MicroRNAs in lung cancer brain metastasis. Int. J. Mol. Sci. 2024, 25, 10325. [Google Scholar] [CrossRef]
- Li, C.; Zheng, H.; Xiong, J.; Huang, Y.; Li, H.; Jin, H.; Ai, S.; Wang, Y.; Su, T.; Sun, G.; et al. miR-596-3p suppresses brain metastasis of non-small cell lung cancer by modulating YAP1 and IL-8. Cell Death Dis. 2022, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.t.; Xu, S.d.; Xu, H.; Zhang, J.f.; Ning, J.f.; Wang, S.f. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med. Oncol. 2012, 29, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yang, X.; Xia, J.; Zhang, C.; Tang, W.; Chu, X.; Fu, R.; Yang, X.; Zhang, X.; Wu, Y.; et al. Proteomic characteristics of lung adenocarcinoma tumors that are small but highly invasive. Med. Adv. 2023, 1, 340–352. [Google Scholar] [CrossRef]
- Gu, A.; Li, J.; Li, M.Y.; Liu, Y. Patient-derived xenograft model in cancer: Establishment and applications. MedComm 2025, 6, e70059. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zeng, T.; Zhang, H.; Li, Y.; Zhu, X.; Liu, H.; Sun, B.; Ji, C.; Li, T.; Huang, L.; et al. Nano-immunotherapy for lung cancer. Nano TransMed 2023, 2, e9130018. [Google Scholar] [CrossRef]
- Yao, H.; Sun, Y.; Wang, C.; Hu, X.; Qin, W.; Sun, W.; Ma, Y.; Wang, Y.; Wang, J.; Li, S.; et al. Single-cell RNA-seq reveals a landscape of developmental heterogeneity and immunosuppressive environment in leptomeningeal metastases. Cell 2020, 183, 1507–1523.e16. [Google Scholar] [CrossRef]
- Kashima, Y.; Shibahara, D.; Suzuki, A.; Muto, K.; Kobayashi, I.S.; Plotnick, D.; Udagawa, H.; Izumi, H.; Shibata, Y.; Tanaka, K.; et al. Single-cell analyses reveal diverse mechanisms of resistance to EGFR tyrosine kinase inhibitors in lung cancer. Cancer Res. 2021, 81, 4835–4848. [Google Scholar] [CrossRef]

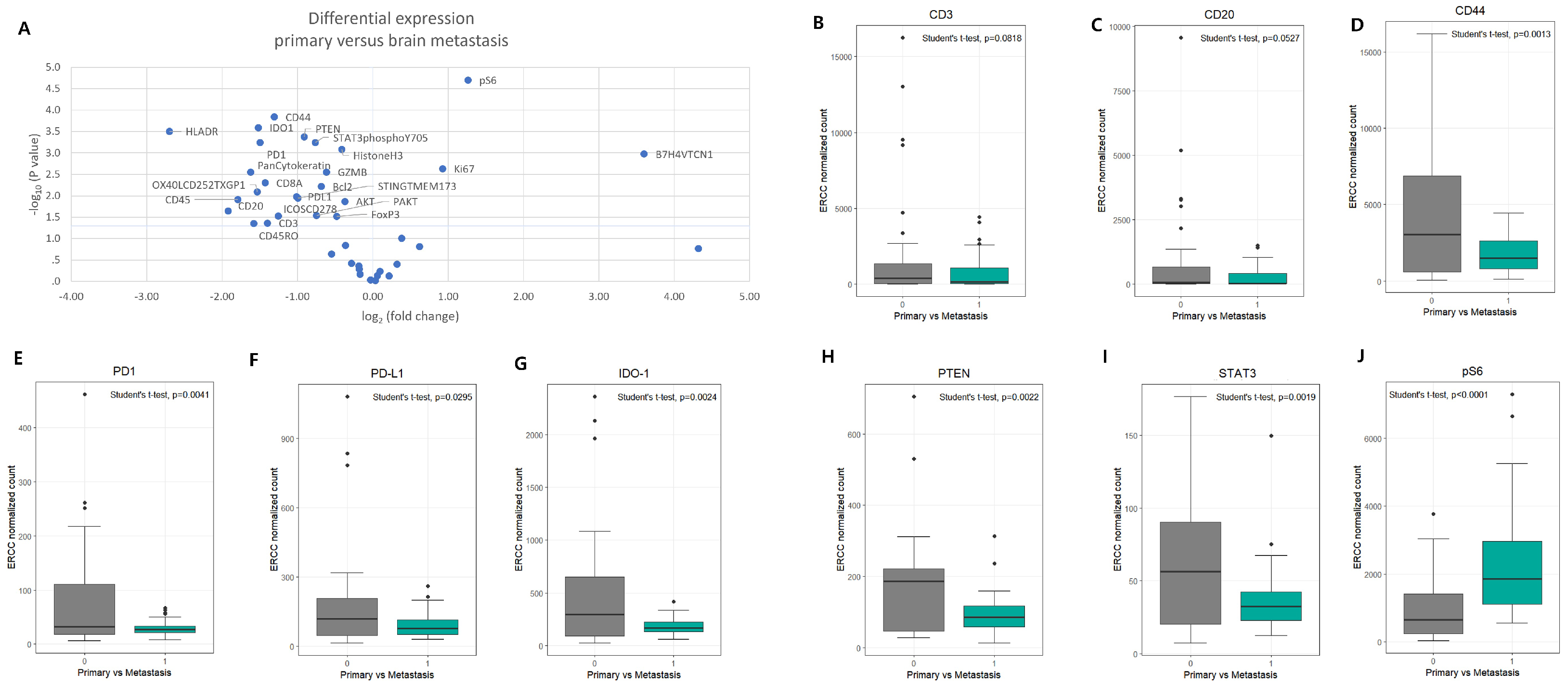
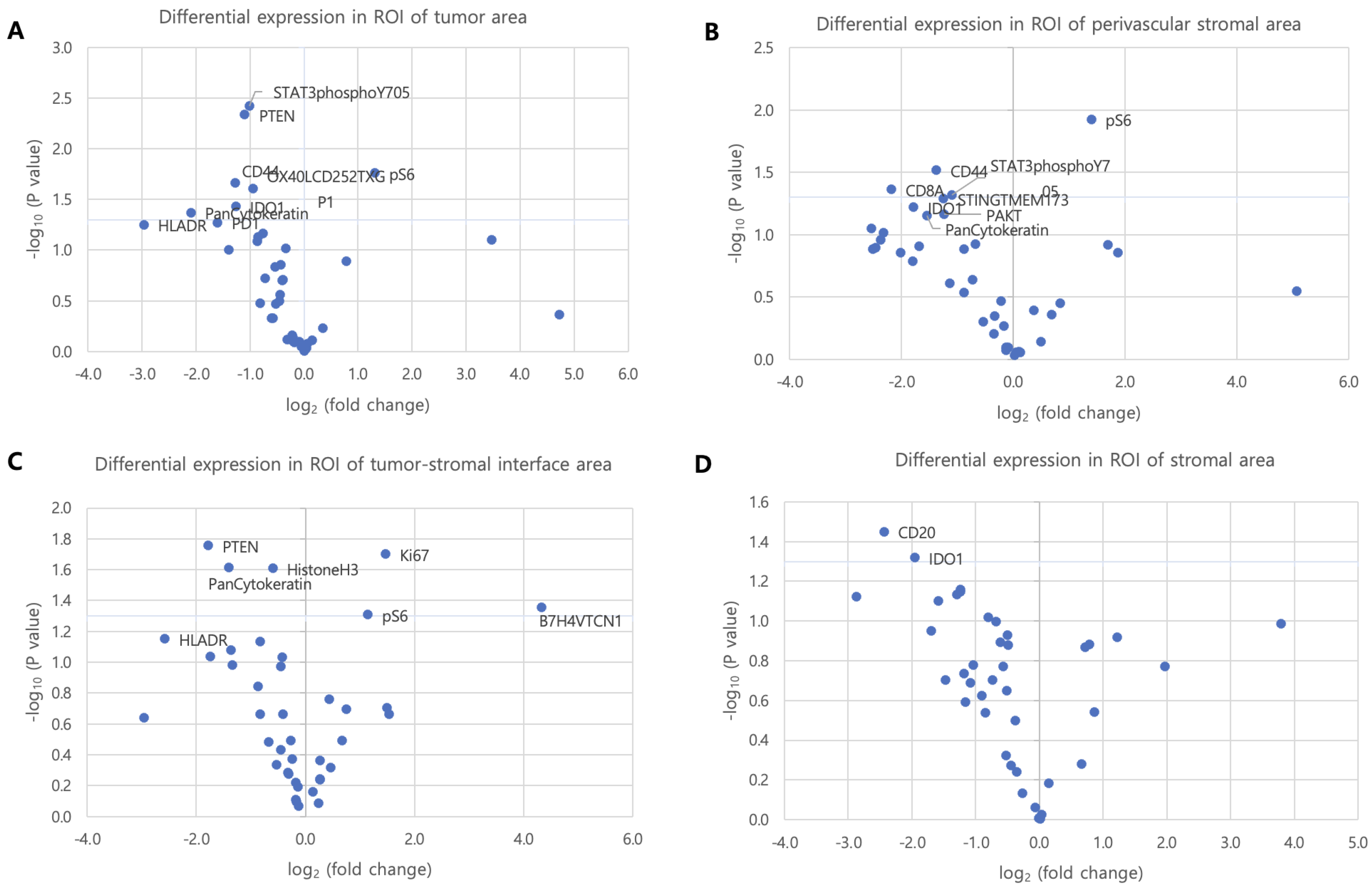
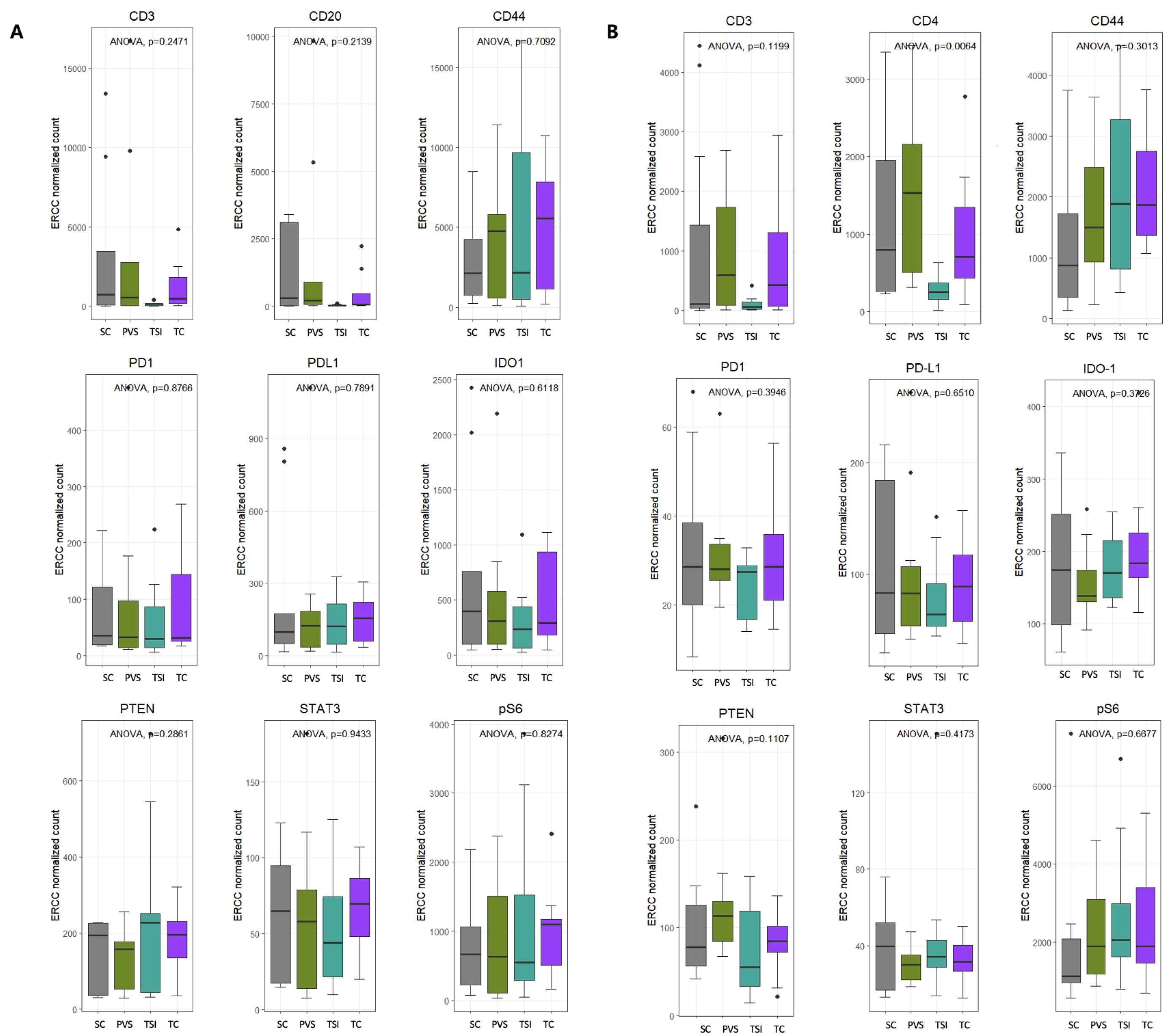

| Marker | TC | TSI | PVS | SC | Relative Expression in BM |
|---|---|---|---|---|---|
| PD-1 | ↓ | ↓ | ↓ | — | Down |
| PD-L1 | ↓ | ↓ | ↓ | — | Down |
| IDO-1 | ↓ | ↓ | ↓ | — | Down |
| STAT3 | — | ↓ | ↓ | — | Down |
| PTEN | ↓ | — | — | — | Down |
| CD44 | ↓ | ↓ | ↓ | ↓ | Down |
| pS6 | ↑ | ↑ | ↑↑ | ↑ | Up (in PVS) |
| CD4 | — | — | ↑ | — | Up (in PVS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, S.; Cho, I.; Lee, J.; Lee, S.Y.; Choi, K.-H.; Kim, T.-J. Spatial Omics Profiling of Treatment-Naïve Lung Adenocarcinoma with Brain Metastasis as the Initial Presentation. Cancers 2025, 17, 2529. https://doi.org/10.3390/cancers17152529
Gwon S, Cho I, Lee J, Lee SY, Choi K-H, Kim T-J. Spatial Omics Profiling of Treatment-Naïve Lung Adenocarcinoma with Brain Metastasis as the Initial Presentation. Cancers. 2025; 17(15):2529. https://doi.org/10.3390/cancers17152529
Chicago/Turabian StyleGwon, Seoyeon, Inju Cho, Jieun Lee, Seung Yun Lee, Kyue-Hee Choi, and Tae-Jung Kim. 2025. "Spatial Omics Profiling of Treatment-Naïve Lung Adenocarcinoma with Brain Metastasis as the Initial Presentation" Cancers 17, no. 15: 2529. https://doi.org/10.3390/cancers17152529
APA StyleGwon, S., Cho, I., Lee, J., Lee, S. Y., Choi, K.-H., & Kim, T.-J. (2025). Spatial Omics Profiling of Treatment-Naïve Lung Adenocarcinoma with Brain Metastasis as the Initial Presentation. Cancers, 17(15), 2529. https://doi.org/10.3390/cancers17152529







