Balancing Innovation and Safety: Prediction, Prevention, and Management of Pneumonitis in Lung Cancer Patients Receiving Novel Anti-Cancer Agents
Simple Summary
Abstract
1. Introduction
2. Novel Anti-Cancer Agents
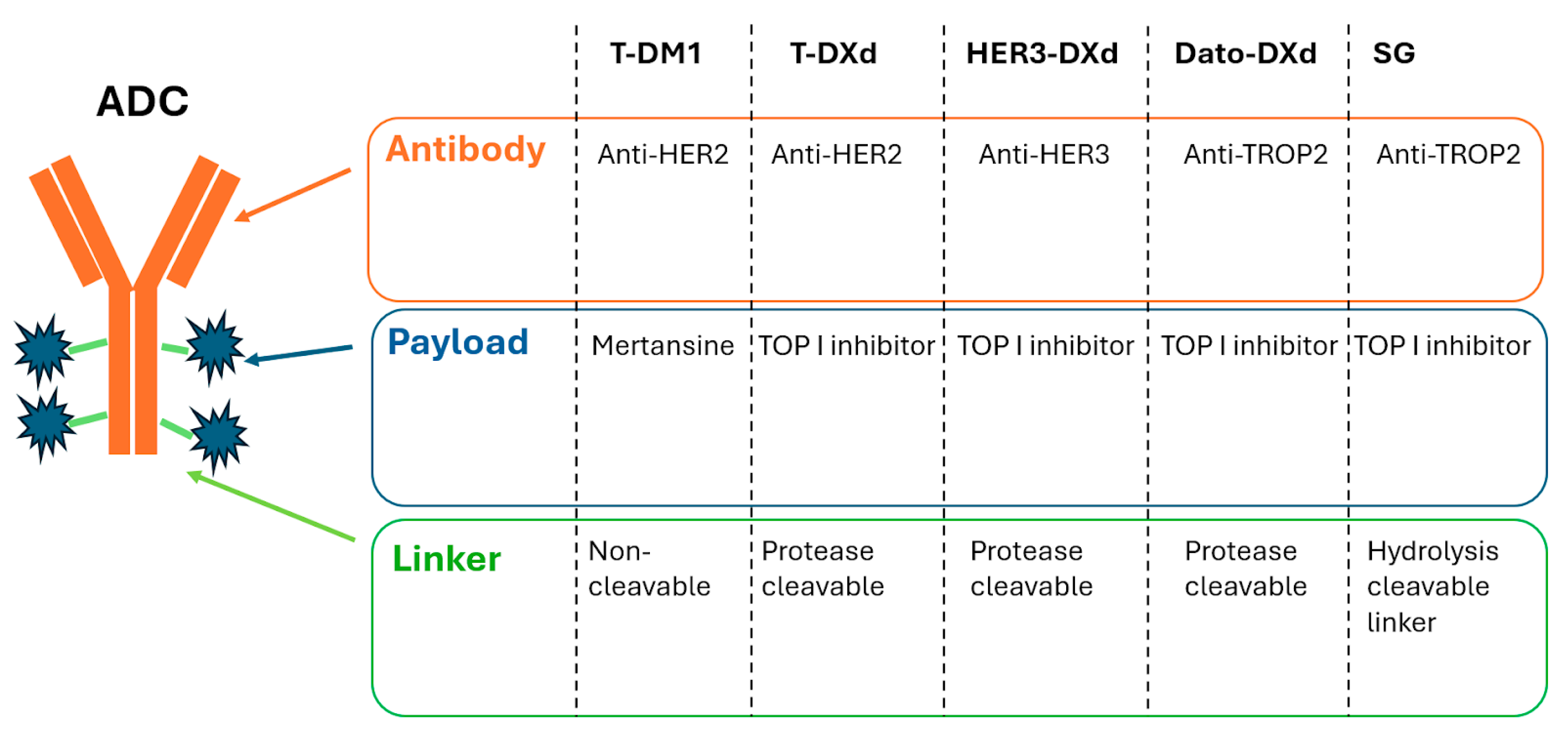
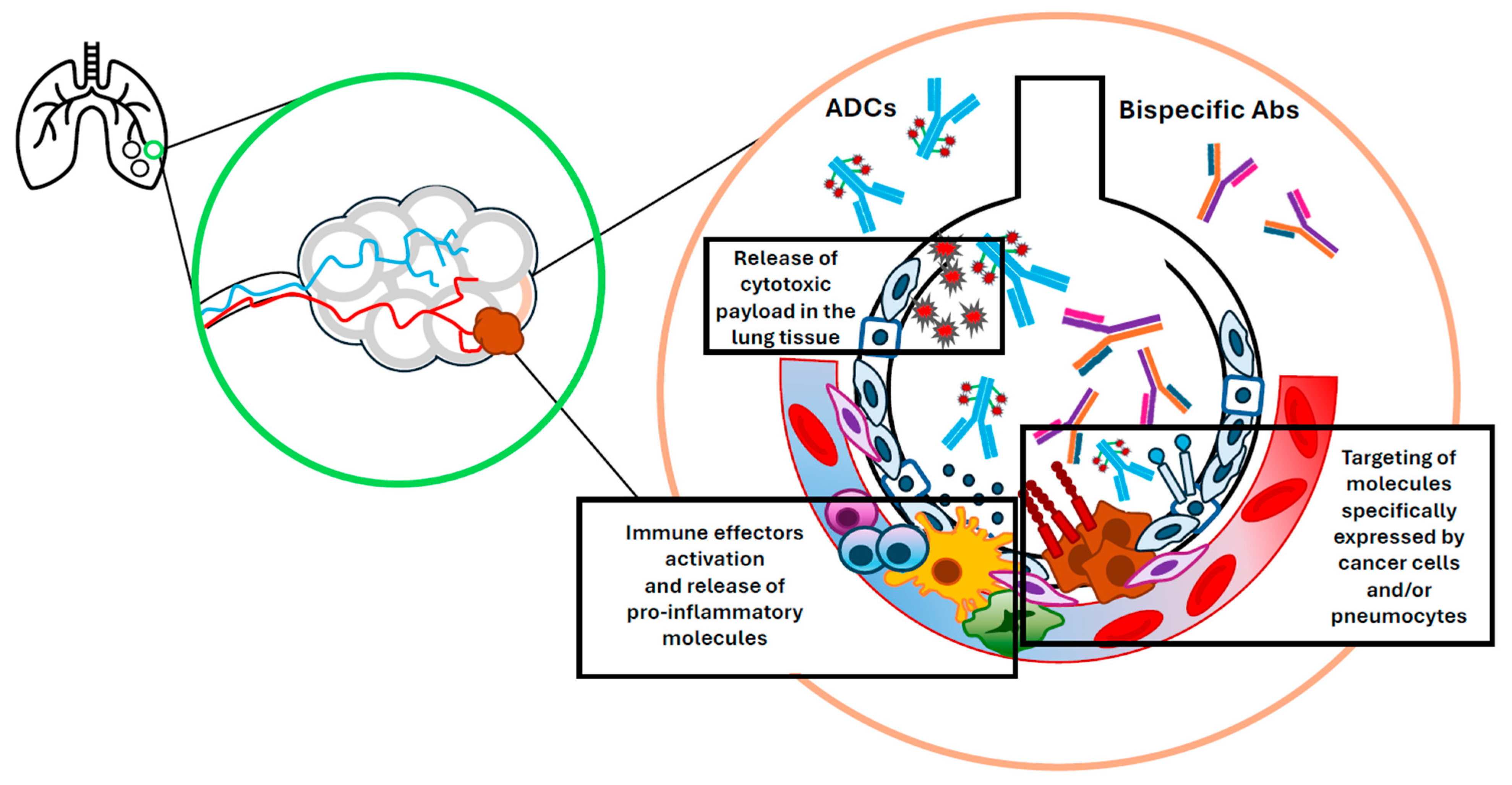
2.1. Antibody–Drug Conjugates
2.1.1. Anti-HER2 ADCs
2.1.2. Anti-HER3 ADCs
2.1.3. Anti-TROP2 ADCs
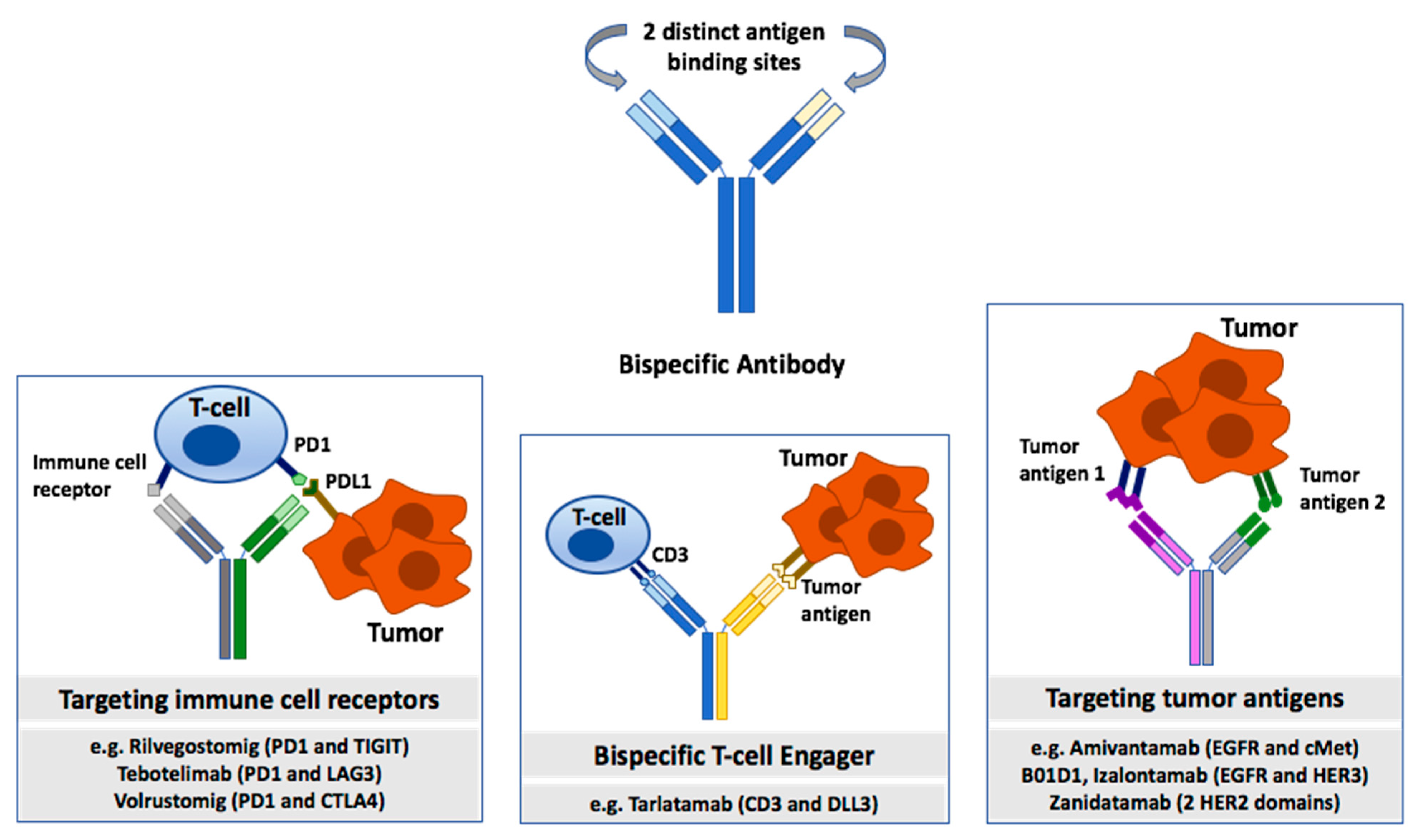
2.2. Bispecific Antibodies
3. Risk Factors and Predictive Biomarkers of Treatment-Related Pneumonitis
4. Management of Treatment-Related Pneumonitis and Treatment Rechallenge Following Drug-Induced Pneumonitis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Antibody–drug conjugate |
| BsAb | Bispecific antibody |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DAR | Drug–antibody ratio |
| Dato-DXd | Datopotamab Deruxtecan |
| DLL3 | Delta-like Ligand 3 |
| EGFR | Epidermal growth factor receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| HER3 | Human epidermal growth factor receptor 3 |
| HER3-DXd | Patritumab-deruxtecan |
| ICI | Immune checkpoint inhibitor |
| ILD | Interstitial lung disease |
| irAE | Immune-related adverse event |
| ITT | Intention to treat |
| LAG3 | Lymphocyte-activation gene 3 |
| NSCLC | Non-small cell lung cancer |
| ORR | Objective response rate |
| OS | Overall survival |
| PD1 | Programmed cell death protein 1 |
| PDL1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| SCLC | Small cell lung cancer |
| SG | Sacituzumab Govitecan |
| TCR | T-cell receptor |
| TROP2 | Trophoblast Cell Surface Antigen 2 |
| T-DM1 | Trastuzumab Emtansine |
| T-DXd | Trastuzumab Deruxtecan |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| TKI | Tyrosine Kinase Inhibitor |
References
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. (Engl.) 2023, 136, 1583–1590. [Google Scholar] [CrossRef]
- Brenner, D.R.; Gillis, J.; Demers, A.A.; Ellison, L.F.; Billette, J.-M.; Zhang, S.X.; Liu, J.L.; Woods, R.R.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2024. Can. Med. Assoc. J. 2024, 196, E615–E623. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-X.; Zang, D.; Liu, C.-G.; Han, X.; Chen, J. Immune checkpoint inhibitor-related pneumonitis: Research advances in prediction and management. Front. Immunol. 2024, 15, 1266850. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, C.; Huang, Y.; Huang, R.; Huang, S.-M.; Larkins, E.; Stapleford, L.; Rivera, D.R.; Kluetz, P.G.; Wang, S.; et al. Evaluating Pneumonitis Incidence in Patients with Non–small Cell Lung Cancer Treated with Immunotherapy and/or Chemotherapy Using Real-world and Clinical Trial Data. Cancer Res. Commun. 2023, 3, 258–266. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B.; He, S. Advances and challenges in the treatment of lung cancer. Biomed. Pharmacother. 2023, 169, 115891. [Google Scholar] [CrossRef]
- Tiu, B.C.; Zubiri, L.; Iheke, J.; Pahalyants, V.; Theodosakis, N.; Ugwu-Dike, P.; Seo, J.; Tang, K.; E Sise, M.; Sullivan, R.; et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: A multi-institutional cohort study. J. Immunother. Cancer 2022, 10, e004670. [Google Scholar] [CrossRef]
- Arroyo-Hernández, M.; Maldonado, F.; Lozano-Ruiz, F.; Muñoz-Montaño, W.; Nuñez-Baez, M.; Arrieta, O. Radiation-induced lung injury: Current evidence. BMC Pulm. Med. 2021, 21, 9. [Google Scholar] [CrossRef]
- Pozzessere, C.; Lazor, R.; Jumeau, R.; Peters, S.; Prior, J.O.; Beigelman-Aubry, C. Imaging Features of Pulmonary Immune-related Adverse Events. J. Thorac. Oncol. 2021, 16, 1449–1460. [Google Scholar] [CrossRef]
- Aiad, M.; Fresco, K.; Prenatt, Z.; Tahir, A.; Ramos-Feliciano, K.; Stoltzfus, J.; Harmouch, F.; Wilson, M. Comparison of Pneumonitis Rates and Severity in Patients With Lung Cancer Treated by Immunotherapy, Radiotherapy, and Immunoradiotherapy. Cureus 2022, 14, e25665. [Google Scholar] [CrossRef]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.V.; Mazzone, P.; Stevenson, J.; Pennell, N.A.; Velcheti, V. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer. Chest 2017, 152, 271–281. [Google Scholar] [CrossRef]
- Yirmibesoglu, E.; Higginson, D.S.; Fayda, M.; Rivera, M.P.; Halle, J.; Rosenman, J.; Xie, L.; Marks, L.B. Challenges scoring radiation pneumonitis in patients irradiated for lung cancer. Lung Cancer 2012, 76, 350–353. [Google Scholar] [CrossRef]
- Altan, M.; Soto, F.; Zhong, L.L.; O Akhmedzhanov, F.; Wilson, N.R.; Zarifa, A.; A Albittar, A.; Yang, V.; Lewis, J.; Rinsurongkawong, W.; et al. Incidence and Risk Factors for Pneumonitis Associated with Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer: A Single Center Experience. Oncologist 2023, 28, e1065–e1074. [Google Scholar] [CrossRef]
- Suh, C.H.; Park, H.S.; Kim, K.W.; Pyo, J.; Hatabu, H.; Nishino, M. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients. Lung Cancer 2018, 123, 60–69. [Google Scholar] [CrossRef]
- Abuhelwa, Z.; Alloghbi, A.; Alqahtani, A.; Nagasaka, M. Trastuzumab Deruxtecan-Induced Interstitial Lung Disease/Pneumonitis in ERBB2-Positive Advanced Solid Malignancies: A Systematic Review. Drugs 2022, 82, 979–987. [Google Scholar] [CrossRef]
- Zhu, Z.; Shen, G.; Li, J.; Qiu, T.; Fang, Q.; Zheng, Y.; Xin, Y.; Liu, Z.; Zhao, F.; Ren, D.; et al. Incidence of antibody–drug conjugates-related pneumonitis in patients with solid tumors: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2023, 184, 103960. [Google Scholar] [CrossRef]
- Jiménez-Labaig, P.; Rullan, A.; Hernando-Calvo, A.; Llop, S.; Bhide, S.; O’lEary, B.; Braña, I.; Harrington, K.J. A systematic review of antibody-drug conjugates and bispecific antibodies in head and neck squamous cell carcinoma and nasopharyngeal carcinoma: Charting the course of future therapies. Cancer Treat. Rev. 2024, 128, 102772. [Google Scholar] [CrossRef]
- Qie, W.; Zhao, Q.; Yang, L.; Zou, B.; Duan, Y.; Yao, Y.; Wang, L. Incidence of pneumonitis following the use of different anaplastic lymphoma kinase tyrosine kinase inhibitor regimens: An updated systematic review and meta-analysis. Cancer Med. 2023, 12, 13873–13884. [Google Scholar] [CrossRef]
- Kuang, Y.; Pierce, C.M.; Chang, H.C.; Sosinsky, A.Z.; Deitz, A.C.; Keller, S.M.; Samkari, A.; Uyei, J. Chemoradiation-induced pneumonitis in patients with unresectable stage III non-small cell lung cancer: A systematic literature review and meta-analysis. Lung Cancer 2022, 174, 174–185. [Google Scholar] [CrossRef]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Rodríguez, L.P.-A.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef]
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.W.; Planchard, D.; Ahn, M.J.; Smit, E.F.; De Langen, A.J.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients With HER2 -Mutant Metastatic Non–Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. J. Clin. Oncol. 2023, 41, 4852–4863. [Google Scholar] [CrossRef]
- Yu, H.A.; Goto, Y.; Hayashi, H.; Felip, E.; Yang, J.C.-H.; Reck, M.; Yoh, K.; Lee, S.-H.; Paz-Ares, L.; Besse, B.; et al. HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor–Mutated Non–Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J. Clin. Oncol. 2023, 41, 5363–5375. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Yu, H.A.; Lim, S.M.; Okamoto, I.; Perol, M.; Novello, S.; Dooms, C.A.; Sun, J.-M.; Kao, S.C.-H.; Jänne, P.A.; et al. Patritumab deruxtecan (HER3-DXd) in resistant EGFR -mutated (EGFR m) advanced non-small cell lung cancer (NSCLC) after a third-generation EGFR TKI: The phase 3 HERTHENA-Lung02 study. J. Clin. Oncol. 2025, 43, 8506. [Google Scholar] [CrossRef]
- Ahn, M.J.; Tanaka, K.; Paz-Ares, L.; Cornelissen, R.; Girard, N.; Pons-Tostivint, E.; Vicente Baz, D.; Sugawara, S.; Cobo, M.; Pérol, M.; et al. Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study. J. Clin. Oncol. 2025, 43, 260–272. [Google Scholar] [CrossRef]
- Shimizu, T.; Sands, J.; Yoh, K.; Spira, A.; Garon, E.B.; Kitazono, S.; Johnson, M.L.; Meric-Bernstam, F.; Tolcher, A.W.; Yamamoto, N.; et al. First-in-Human, Phase I Dose-Escalation and Dose-Expansion Study of Trophoblast Cell-Surface Antigen 2–Directed Antibody-Drug Conjugate Datopotamab Deruxtecan in Non–Small-Cell Lung Cancer: TROPION-PanTumor01. J. Clin. Oncol. 2023, 41, 4678–4687. [Google Scholar] [CrossRef]
- Santin, A.D.; Corr, B.R.; Spira, A.; Willmott, L.; Butrynski, J.; Tse, K.Y.; Patel, J.; Mekan, S.; Wu, T.; Lin, K.-W.; et al. Efficacy and Safety of Sacituzumab Govitecan in Patients With Advanced Solid Tumors (TROPiCS-03): Analysis in Patients With Advanced Endometrial Cancer. J. Clin. Oncol. 2024, 42, 3421–3429. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Champiat, S.; Lai, W.V.; Izumi, H.; Govindan, R.; Boyer, M.; Hummel, H.-D.; Borghaei, H.; Johnson, M.L.; Steeghs, N.; et al. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J. Clin. Oncol. 2023, 41, 2893–2903. [Google Scholar] [CrossRef]
- Sands, J.M.; Champiat, S.; Hummel, H.; Paulson, K.G.; Borghaei, H.; Alvarez, J.B.; Carbone, D.P.; Carlisle, J.W.; Choudhury, N.J.; Clarke, J.M.; et al. Practical management of adverse events in patients receiving tarlatamab, a delta-like ligand 3–targeted bispecific T-cell engager immunotherapy, for previously treated small cell lung cancer. Cancer 2025, 131, e35738. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.-Y.; Hanna, D.L.; Kang, Y.-K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion–Mutated Non–Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, Y.; Zhao, Y.; Zhao, S.; Xue, J.; Yang, Y.; Fang, W.; Guo, Y.; Han, Y.; Yang, K.; et al. BL-B01D1, a first-in-class EGFR–HER3 bispecific antibody–drug conjugate, in patients with locally advanced or metastatic solid tumours: A first-in-human, open-label, multicentre, phase 1 study. Lancet Oncol. 2024, 25, 901–911. [Google Scholar] [CrossRef]
- Xue, J.; Ma, Y.; Zhao, Y.; Wang, Y.; Hong, W.; Huang, Y.; Yang, Y.; Fang, W.; Hong, S.; Zhang, Y.; et al. Izalontamab (SI-B001), a novel EGFRxHER3 bispecific antibody in patients with Locally Advanced or Metastatic Epithelial Tumor: Results from first-in-human phase I/Ib study. Clin. Cancer Res. 2025, OF1–OF8. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Shastry, M.; Gupta, A.; Chandarlapaty, S.; Young, M.; Powles, T.; Hamilton, E. Rise of Antibody-Drug Conjugates: The Present and Future. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390094. [Google Scholar] [CrossRef]
- Powell, C.; Modi, S.; Iwata, H.; Takahashi, S.; Smit, E.; Siena, S.; Chang, D.-Y.; Macpherson, E.; Qin, A.; Singh, J.; et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 2022, 7, 100554. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef]
- Lin, W.; Xu, J.; Liao, Y.; Lin, X.; Yang, J.; Zhuang, W. Assessing safety concerns of interstitial lung disease associated with antibody-drug conjugates: A real-world pharmacovigilance evaluation of the FDA adverse event reporting system. Int. J. Clin. Pharm. 2024, 46, 614–622. [Google Scholar] [CrossRef]
- Waliany, S.; Neal, J.W.; Engel-Nitz, N.; Lam, C.; Lin, F.; Park, L.; Le, L.; Nagasaka, M. HER2-Mutant Advanced and/or Metastatic Non–Small-Cell Lung Cancer: A US Electronic Health Records Database Analysis of Clinical Characteristics, Treatment Practice Patterns, and Outcomes. Clin. Lung Cancer 2024, 25, 319–328.e1. [Google Scholar] [CrossRef]
- Ren, S.; Wang, J.; Ying, J.; Mitsudomi, T.; Lee, D.; Wang, Z.; Chu, Q.; Mack, P.; Cheng, Y.; Duan, J.; et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open 2022, 7, 100395. [Google Scholar] [CrossRef]
- Li, R.; Hua, M.; Li, J.; Chen, W.; Xu, L.; Meng, H.; Zhang, Z.; Liu, Q.; Cui, Y.; Xiang, Q. The safety of trastuzumab deruxtecan (DS-8201) with a focus on interstitial lung disease and/or pneumonitis: A systematic review and single-arm meta-analysis. Cancer 2024, 130, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Iwama, E.; Zenke, Y.; Sugawara, S.; Daga, H.; Morise, M.; Yanagitani, N.; Sakamoto, T.; Murakami, H.; Kishimoto, J.; Matsumoto, S.; et al. Trastuzumab emtansine for patients with non–small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur. J. Cancer 2022, 162, 99–106. [Google Scholar] [CrossRef]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.D.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non–Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef]
- Hsu, R.; Benjamin, D.J. A narrative review of antibody–drug conjugates in EGFR-mutated non-small cell lung cancer. Front. Oncol. 2023, 13, 1252652. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, K.; Pirazzoli, V.; Arcila, M.E.; Nebhan, C.A.; Song, X.; de Stanchina, E.; Ohashi, K.; Janjigian, Y.Y.; Spitzler, P.J.; Melnick, M.A.; et al. HER2 Amplification: A Potential Mechanism of Acquired Resistance to EGFR Inhibition in EGFR -Mutant Lung Cancers That Lack the Second-Site EGFR T790M Mutation. Cancer Discov. 2012, 2, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Baik, C.; Su, W.C.; Johnson, M.L.; Hayashi, H.; Nishio, M.; Kim, D.W.; Koczywas, M.; Gold, K.A.; Steuer, C.E.; et al. Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor–Resistant, EGFR -Mutated Non–Small Cell Lung Cancer. Cancer Discov. 2022, 12, 74–89. [Google Scholar] [CrossRef]
- Belluomini, L.; Avancini, A.; Sposito, M.; Milella, M.; Rossi, A.; Pilotto, S. Antibody-drug conjugates (ADCs) targeting TROP-2 in lung cancer. Expert Opin. Biol. Ther. 2023, 23, 1077–1087. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Yuan, Y.; Chen, W.; Sun, W.; Wang, Y.; Huang, H.; Liang, B.; Ming, T.; Wen, J.; et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol. Ther. 2022, 239, 108296. [Google Scholar] [CrossRef]
- Sands, J.; Lisberg, A.; Bardia, A.; Shimizu, T.; Ahn, M.-J.; Paz-Ares, L.G.; Meric-Bernstam, F.; Kitazono, S.; Krop, I.E.; Girard, N.; et al. Analysis of drug-related interstitial lung disease (ILD) inpatients (pts) treated with datopotamab deruxtecan (Dato-DXd). J. Clin. Oncol. 2024, 42, 8623. [Google Scholar] [CrossRef]
- Bardia, A.; Krop, I.E.; Kogawa, T.; Juric, D.; Tolcher, A.W.; Hamilton, E.P.; Mukohara, T.; Lisberg, A.; Shimizu, T.; Spira, A.I.; et al. Datopotamab Deruxtecan in Advanced or Metastatic HR+/HER2– and Triple-Negative Breast Cancer: Results from the Phase I TROPION-PanTumor01 Study. J. Clin. Oncol. 2024, 42, 2281–2294. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Juan-Vidal, O.; Mountzios, G.S.; Felip, E.; Reinmuth, N.; de Marinis, F.; Girard, N.; Patel, V.M.; Takahama, T.; Owen, S.P.; et al. Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study. J. Clin. Oncol. 2024, 42, 2860–2872. [Google Scholar] [CrossRef]
- Patel, J.D.; Cho, B.C.; Cobo, M.; Cabanillas, R.R.; Vicente, D.; Pradera, J.F.; Garon, E.B.; Mok, T.S.K.; Cappuzzo, F.; Neal, J.W.; et al. Sacituzumab govitecan (SG) + pembrolizumab (pembro) in first-line (1L) metastatic non-small cell lung cancer (mNSCLC) with PD-L1 ≥ 50%: Cohort A of EVOKE-02. J. Clin. Oncol. 2024, 42, 8592. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef]
- Tong, Y.; Fan, X.; Liu, H.; Liang, T. Advances in Trop-2 targeted antibody-drug conjugates for breast cancer: Mechanisms, clinical applications, and future directions. Front. Immunol. 2024, 15, 1495675. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Gökbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Brüggemann, M.; Horst, H.-A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Cho, B.C.; Felip, E.; Korantzis, I.; Ohashi, K.; Majem, M.; Juan-Vidal, O.; Handzhiev, S.; Izumi, H.; Lee, J.-S.; et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 2063–2075. [Google Scholar] [CrossRef]
- Mountzios, G.; Sun, L.; Cho, B.C.; Demirci, U.; Baka, S.; Gümüş, M.; Lugini, A.; Zhu, B.; Yu, Y.; Korantzis, I.; et al. Tarlatamab in Small-Cell Lung Cancer after Platinum-Based Chemotherapy. N. Engl. J. Med. 2025, 393, 349–361. [Google Scholar] [CrossRef]
- Khosla, A.A.; Jatwani, K.; Singh, R.; Reddy, A.; Jaiyesimi, I.; Desai, A. Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review. Pharmaceuticals 2023, 16, 1461. [Google Scholar] [CrossRef]
- Moores, S.L.; Chiu, M.L.; Bushey, B.S.; Chevalier, K.; Luistro, L.; Dorn, K.; Brezski, R.J.; Haytko, P.; Kelly, T.; Wu, S.-J.; et al. A Novel Bispecific Antibody Targeting EGFR and cMet Is Effective against EGFR Inhibitor–Resistant Lung Tumors. Cancer Res. 2016, 76, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.; Larkins, E.; Chatterjee, S.; Mishra-Kalyani, P.S.; Aungst, S.; Wearne, E.; Subramaniam, S.; Li, Y.; Liu, J.; Sun, J.; et al. FDA Approval Summary: Amivantamab for the Treatment of Patients with Non–Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations. Clin. Cancer Res. 2023, 29, 3262–3266. [Google Scholar] [CrossRef]
- Cho, B.C.; Lu, S.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.-S.; Lee, S.-H.; Ostapenko, Y.; Danchaivijitr, P.; Liu, B.; et al. Amivantamab plus Lazertinib in Previously Untreated EGFR -Mutated Advanced NSCLC. N. Engl. J. Med. 2024, 391, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.-H.; Melosky, B.; Shih, J.-Y.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; Felip, E.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef]
- Brandão, M.; Hiltermann, J.; Wauters, E.; Solomon, B.; Alvarez, E.C.; Felip, E.; Gort, E.; Izumi, H.; Kim, D.-W.; Paz-Ares, L.; et al. 1446P Preliminary efficacy and safety of rilvegostomig (AZD2936), a bispecific antibody targeting PD-1 and TIGIT, in checkpoint inhibitor (CPI)-pretreated advanced/metastatic non-small-cell lung cancer (NSCLC): ARTEMIDE-01. Ann. Oncol. 2023, 34, S822–S823. [Google Scholar] [CrossRef]
- Johnson, M.L.; Arriola, E.; Kato, T.; Girard, N.; Gadgeel, S.M.; Wang, J.; Li, X.; Lowery, C.; Krug, L.M.; Ahn, M.-J. eVOLVE-Lung02: A phase 3 study of first-line (1L) volrustomig plus chemotherapy (CT) versus pembrolizumab plus CT in metastatic non-small-cell lung cancer (mNSCLC) with PD-L1 tumor cell (TC) expression <50%. J. Clin. Oncol. 2024, 42, TPS8652. [Google Scholar]
- Luke, J.J.; Patel, M.R.; Blumenschein, G.R.; Hamilton, E.; Chmielowski, B.; Ulahannan, S.V.; Connolly, R.M.; Santa-Maria, C.A.; Wang, J.; Bahadur, S.W.; et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: A phase 1 trial. Nat. Med. 2023, 29, 2814–2824. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Reuss, J.E.; Suresh, K.; Naidoo, J. Checkpoint Inhibitor Pneumonitis: Mechanisms, Characteristics, Management Strategies, and Beyond. Curr. Oncol. Rep. 2020, 22, 56. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, X.; Wang, G. Risk Factors for Immune Checkpoint Inhibitor-Related Pneumonitis in Cancer Patients: A Systemic Review and Meta-Analysis. Respiration 2022, 101, 1035–1050. [Google Scholar] [CrossRef]
- Kong, Y.; Hong, L.; Xu, X.; Chen, Y.; Xu, J. The relative risk of immune checkpoint inhibitor pneumonitis in advanced non-small- cell lung cancer: Meta-analyses of controlled clinical trials. PLoS ONE 2024, 19, e0301931. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, L.; Qin, W.; Yi, M.; Liu, B.; Yuan, X. Validation study of the association between genetic variant of IL4 and severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Radiother. Oncol. 2019, 141, 86–94. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, L.; Qin, W.; Yi, M.X.; Liu, B.; Yuan, X. Impact of genetic variant of HIPK2 on the risk of severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Radiat. Oncol. 2020, 15, 9. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, M.; Bi, N.; Fang, M.; Sun, T.; Ji, W.; Tan, W.; Zhao, L.; Yu, D.; Lin, D.; et al. ATM Polymorphisms Are Associated With Risk of Radiation-Induced Pneumonitis. Int. J. Radiat. Oncol. 2010, 77, 1360–1368. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, B.; Li, J.; Wu, H.; Yang, J.; Zhou, X.; Yi, M.; Li, Q.; Yu, S.; Yuan, X. Genetic variants in PI 3K/ AKT pathway are associated with severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Cancer Med. 2016, 5, 24–32. [Google Scholar] [CrossRef]
- Yang, J.; Xu, T.; Gomez, D.R.; Yuan, X.; Nguyen, Q.-N.; Jeter, M.; Song, Y.; Hahn, S.; Liao, Z. Polymorphisms in BMP2/BMP4, with estimates of mean lung dose, predict radiation pneumonitis among patients receiving definitive radiotherapy for non-small cell lung cancer. Oncotarget 2017, 8, 43080–43090. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Zhou, J.; Hsu, S.; Ding, N.; Li, J.; Zhang, Y.; Xu, X.; Tang, X.; Wei, T.; Zhu, Z.; et al. Risk factors for immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer. Transl. Lung Cancer Res. 2022, 11, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ploch, M.; Zhao, S.; Wei, L.; Englert, J.A.; Cohen, S.P.; Inks, M.A.; Meara, A.S.; Fussner, L.A.; Owen, D.H.; Ho, K. Cytokine profile of bronchoalveolar lavage in patients with and without checkpoint inhibitor pneumonitis. Cancer Immunol. Immunother. 2025, 74, 46. [Google Scholar] [CrossRef]
- Nuñez, N.G.; Berner, F.; Friebel, E.; Unger, S.; Wyss, N.; Gomez, J.M.; Purde, M.-T.; Niederer, R.; Porsch, M.; Lichtensteiger, C.; et al. Immune signatures predict development of autoimmune toxicity in patients with cancer treated with immune checkpoint inhibitors. Med 2023, 4, 113–129.e7. [Google Scholar] [CrossRef] [PubMed]
- Berner, F.; Flatz, L. Autoimmunity in immune checkpoint inhibitor-induced immune-related adverse events: A focus on autoimmune skin toxicity and pneumonitis. Immunol. Rev. 2023, 318, 37–50. [Google Scholar] [CrossRef]
- Sung, C.; An, J.; Lee, S.; Park, J.; Lee, K.S.; Kim, I.-H.; Han, J.-Y.; Park, Y.H.; Kim, J.H.; Kang, E.J.; et al. Integrative analysis of risk factors for immune-related adverse events of checkpoint blockade therapy in cancer. Nat. Cancer 2023, 4, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Genta, S.; Lajkosz, K.; Yee, N.R.; Spiliopoulou, P.; Heirali, A.; Hansen, A.R.; Siu, L.L.; Saibil, S.; Stayner, L.-A.; Yanekina, M.; et al. Autoimmune PaneLs as PrEdictors of Toxicity in Patients TReated with Immune Checkpoint InhibiTors (ALERT). J. Exp. Clin. Cancer Res. 2023, 42, 276. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.A.; Gao, J.; Miura, Y.; Blando, J.; Tidwell, R.S.S.; Zhao, H.; Subudhi, S.K.; Tawbi, H.; Keung, E.; Wargo, J.; et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc. Natl. Acad. Sci. USA 2019, 116, 22246–22251. [Google Scholar] [CrossRef]
- Murray, J.C.; Sivapalan, L.; Hummelink, K.; Balan, A.; White, J.R.; Niknafs, N.; Rhymee, L.; Pereira, G.; Rao, N.; Weksler, B.; et al. Elucidating the Heterogeneity of Immunotherapy Response and Immune-Related Toxicities by Longitudinal ctDNA and Immune Cell Compartment Tracking in Lung Cancer. Clin. Cancer Res. 2024, 30, 389–403. [Google Scholar] [CrossRef]
- Ponvilawan, B.; Khan, A.W.; Subramanian, J.; Bansal, D. Non-Invasive Predictive Biomarkers for Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors. Cancers 2024, 16, 1225. [Google Scholar] [CrossRef]
- Kawahara, D.; Imano, N.; Nishioka, R.; Ogawa, K.; Kimura, T.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; Hattori, N.; Nagata, Y. Prediction of radiation pneumonitis after definitive radiotherapy for locally advanced non-small cell lung cancer using multi-region radiomics analysis. Sci. Rep. 2021, 11, 16232. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, S.; Jia, X.; Zhang, X.; Li, X.; Pan, L.; Li, Z.; Niu, G.; Liang, T.; Guo, H. Radiomics Biomarkers to Predict Checkpoint Inhibitor Pneumonitis in Non-small Cell Lung Cancer. Acad. Radiol. 2025, 32, 1685–1695. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yi, G.; Li, X.; Yi, B.; Bao, X.; Zhang, Y.; Zhang, X.; Yang, Z.; Guo, Z. Predicting radiation pneumonitis in lung cancer using machine learning and multimodal features: A systematic review and meta-analysis of diagnostic accuracy. BMC Cancer 2024, 24, 1355. [Google Scholar] [CrossRef]
- Nicolò, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining antibody-drug conjugates with immunotherapy in solid tumors: Current landscape and future perspectives. Cancer Treat. Rev. 2022, 106, 102395. [Google Scholar] [CrossRef]
- Chen, A.W.; Brady, L.K.; Cohen, E.A.; Mankowski, W.C.; Roshkovan, L.; Qutaish, M.; Katz, S.; Castro, P.G.; Pencheva, N.; Jure-Kunkel, M.; et al. Abstract 2587: Evaluating the potential of radiomics features to predict outcome to an antibody-drug conjugate therapy in non-small cell lung cancer patients. Cancer Res. 2024, 84, 2587. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, B.; Li, T.; Gao, Q.; Zhao, L. Immune-related pneumonitis requiring low-dose prednisone maintenance in one patient with durable complete response. J. Oncol. Pharm. Pract. 2023, 29, 986–991. [Google Scholar] [CrossRef]
- Conte, P.; Ascierto, P.; Patelli, G.; Danesi, R.; Vanzulli, A.; Sandomenico, F.; Tarsia, P.; Cattelan, A.; Comes, A.; De Laurentiis, M.; et al. Drug-induced interstitial lung disease during cancer therapies: Expert opinion on diagnosis and treatment. ESMO Open 2022, 7, 100404. [Google Scholar] [CrossRef]
- Karayama, M.; Inui, N.; Inoue, Y.; Yasui, H.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Fujisawa, T.; Enomoto, N.; Asada, K.; et al. Six-week oral prednisolone therapy for immune-related pneumonitis: A single-arm phase II study. J. Immunother. Cancer 2023, 11, e007056. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; E Gerber, D.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef]
- Moodabagil, M.; Easterling, R.; Peng, J.; Abu-Sbeih, H.; Meara, A.; Donnelly, E.; Owen, D.H.; Ho, K. Investigating risk factors and treatment options for severe, partially steroid responsive, and steroid-refractory checkpoint inhibitor pneumonitis. Oncologist 2024, 29, e1575–e1585. [Google Scholar] [CrossRef]
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT Diagnosis and Clinical Management of Drug-Related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors. Chest 2021, 159, 1107–1125. [Google Scholar] [CrossRef]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef]
- Campochiaro, C.; Farina, N.; Tomelleri, A.; Ferrara, R.; Lazzari, C.; De Luca, G.; Bulotta, A.; Signorelli, D.; Palmisano, A.; Vignale, D.; et al. Tocilizumab for the treatment of immune-related adverse events: A systematic literature review and a multicentre case series. Eur. J. Intern. Med. 2021, 93, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fa’ak, F.; Buni, M.; Falohun, A.; Lu, H.; Song, J.; Johnson, D.H.; Zobniw, C.M.; Trinh, V.A.; Awiwi, M.O.; Tahon, N.H.; et al. Selective immune suppression using interleukin-6 receptor inhibitors for management of immune-related adverse events. J. Immunother. Cancer 2023, 11, e006814corr1. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef]
- Xu, S.; Shukuya, T.; Tamura, J.; Shimamura, S.; Kurokawa, K.; Miura, K.; Miyawaki, T.; Hayakawa, D.; Asao, T.; Yamamoto, K.; et al. Heterogeneous Outcomes of Immune Checkpoint Inhibitor Rechallenge in Patients with NSCLC: A Systematic Review and Meta-Analysis. JTO Clin. Res. Rep. 2022, 3, 100309. [Google Scholar] [CrossRef]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; Da-Silva, A.; Plane, A.-F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pdf.hres.ca/dpd_pm/00078295.PDF (accessed on 20 July 2025).
- Available online: https://dhpp.hpfb-dgpsa.ca/dhpp/resource/100355 (accessed on 20 July 2025).
- Delanian, S.; Porcher, R.; Balla-Mekias, S.; Lefaix, J.-L. Randomized, Placebo-Controlled Trial of Combined Pentoxifylline and Tocopherol for Regression of Superficial Radiation-Induced Fibrosis. J. Clin. Oncol. 2003, 21, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Porcher, R.; Rudant, J.; Lefaix, J.-L. Kinetics of Response to Long-Term Treatment Combining Pentoxifylline and Tocopherol in Patients With Superficial Radiation-Induced Fibrosis. J. Clin. Oncol. 2005, 23, 8570–8579. [Google Scholar] [CrossRef]
- Haddad, P.; Kalaghchi, B.; Amouzegar-Hashemi, F. Pentoxifylline and vitamin E combination for superficial radiation-induced fibrosis: A phase II clinical trial. Radiother. Oncol. 2005, 77, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Willett, A.; Silverman, C.L.; Woo, S.Y.; Gaskins, J.; Dunlap, N.E. Pentoxifylline and Vitamin E in Preventing Pneumonitis Using Stereotactic Ablative Radiotherapy in Previously Irradiated Patients. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e74. [Google Scholar] [CrossRef]
- Goffin, J.; Kreisman, H.; Sandor, V. Bleomycin-Induced Lung Toxicity and Pentoxifylline. J. Clin. Oncol. 2001, 19, 597–598. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ito, S.; Hashimoto, N.; Hasegawa, Y. Pirfenidone as salvage treatment for refractory bleomycin-induced lung injury: A case report of seminoma. BMC Cancer 2017, 17, 526. [Google Scholar] [CrossRef]
- Chhajed, P.; Vaidya, P.; Sandeepa, H.; Singh, T.; Kumar, S.S.; Bhargava, R.; Ramakrishnan, G. Combined prednisolone and pirfenidone in bleomycin-induced lung disease. J. Cancer Res. Ther. 2016, 12, 1198. [Google Scholar] [CrossRef]
- Miao, K.; Xu, Y.; Xu, W.; Zhang, Y.; Xu, Y.; Tian, X.; Zhang, L. Treatment of steroid-resistant checkpoint inhibitor pneumonitis with pirfenidone: A case report. Thorac. Cancer 2021, 12, 2214–2216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Huang, H.B.; Ye, X.M.; Zeng, B.; Huang, F.B.; Chen, L. A retrospective study of combination therapy with glucocorticoids and pirfenidone for PD-1 inhibitor-related immune pneumonitis. Medicine 2024, 103, e37808. [Google Scholar] [CrossRef]
- Desai, A.; Subbiah, V.; Roy-Chowdhuri, S.; Sheshadri, A.; Deshmukh, S.; Peters, S. Association of Antibody–Drug Conjugate (ADC) Target Expression and Interstitial Lung Disease (ILD) in Non-Small-Cell Lung Cancer (NSCLC): Association or Causation or Neither? Cancers 2024, 16, 3753. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Wang, J.; Duan, J.; Sun, Y.; Xing, L.; Han, L.; Wang, Q.; Wu, L.; Chen, J.; Lu, P.; Guo, W.; et al. ARTEMIS-001: Data from a phase 1a/b study of HS-20093 in patients with relapsed small cell lung cancer (SCLC). J. Clin. Oncol. 2024, 42, 8093. [Google Scholar] [CrossRef]
- Rudin, C.; Ahn, M.-J.; Johnson, M.; Hann, C.; Girard, N.; Nishio, M.; Cheng, Y.; Hayashi, H.; Kim, Y.J.; Navarro, A.; et al. OA04.03 Ifinatamab Deruxtecan (I-DXd) in Extensive-Stage Small Cell Lung Cancer (ES-SCLC): Interim Analysis of Ideate-lung01. J. Thorac. Oncol. 2024, 19, S15–S16. [Google Scholar] [CrossRef]
- Patnaik, A.; Call, J.A.; Spreafico, A.; Nabell, L.; Yan, M.; Forero-Torres, A.; Gillison, M.L. Phase 1 study of SGN-PDL1V, a novel, investigational vedotin antibody–drug conjugate directed to PD-L1, in patients with advanced solid tumors (SGNPDL1V-001, trial in progress). J. Clin. Oncol. 2022, 40, TPS3154. [Google Scholar] [CrossRef]
- Schoenfeld, A.; Arbour, K.; Rizvi, H.; Iqbal, A.; Gadgeel, S.; Girshman, J.; Kris, M.; Riely, G.; Yu, H.; Hellmann, M.D. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann. Oncol. 2019, 30, 839–844. [Google Scholar] [CrossRef] [PubMed]
| Treatment Type | Study Type | No. of Patients | Patient Population | Incidence of Pneumonitis | ||
|---|---|---|---|---|---|---|
| All Grade | Grade 3 or Higher | Grade 5 | ||||
| PD-1 and PD-L1 inhibitors | Systematic review and meta-analysis PMID 28499515 [12] | Subgroup 1: 3232 Subgroup 2: 1806 | Subgroup 1: PD-1 inhibitors Subgroup 2: PD-L1 inhibitors | Subgroup 1: 3.6% (95% CI: 2.4–4.9%) Subgroup 2: 1.3% (95% CI: 0.8–1.9%) | Subgroup 1: 1.1% (95% CI: 0.6–1.7%) Subgroup 2: 0.4% (95% CI: 0–0.8%) | Not reported |
| EGFR-TKIs | Meta-analysis PMID 30089596 [15] | 15,713 | Subgroup 1: NSCLC patients without prior exposure to EGFR-TKIs Subgroup 2: NSCLC patients with prior exposure to EGFR-TKIs | Subgroup 1: 1.12% (95% CI: 0.79–1.58%) Subgroup 2: 1.13% (95% CI:0.40–3.15%) | Subgroup 1: 0.61% (95% CI: 0.40–0.93%) Subgroup 2: 0.49% (95% CI: 0.21–1.11%) | Subgroup 1: 0.20% (95% CI: 0.11–0.38%) Subgroup 2: 0.16% (95% CI: 0.04–0.65%) |
| ALK-TKIs | Systematic review and meta-analysis PMID 37017467 [19] | 4752 | NSCLC patients | 2.92% (95% CI: 1.79–4.27%) | 1.42% (95% CI: 0.84–2.12%) | 0.09% (95% CI: 0.00–0.28%) |
| Chemo-radiation | Systemic review and meta-analysis PMID 35717343 [20] | 1788 | Unresectable stage 3 NSCLC | Not reported | RCTs: 3.62% (95% CI: 1.65–6.21) Observational studies: 5.98% (95% CI: 2.26–12.91) | RCTs: 0.37% (95% CI: 0–2.78) Observational studies: 1.73% (95% CI: 0.53–4.33) |
| Drug or Therapy | Study Name and Type | No. of Patients | Drug Dose | Incidence of Pneumonitis | Median Time to Onset of Pneumonitis | Median Duration of Pneumonitis | ||
|---|---|---|---|---|---|---|---|---|
| All Grade | Grade 3 or Higher | Grade 5 | ||||||
| Trastuzumab deruxtecan (ADC) | DESTINY-Lung01 [21] (phase 2 study) PMID 38547891 | Cohort 1: 49 Cohort 1A: 41 | Cohort 1: 6.4 mg/kg dose once every 3 weeks Cohort 1A: 5.4 mg/kg dose once every 3 weeks | Cohort 1: 20% Cohort 1A: 5% | Cohort 1: 6% Cohort 1A: 2% | Cohort 1: 6% Cohort 1A: 2% | Not reported | Not reported |
| Trastuzumab deruxtecan (ADC) | DESTINY-Lung02 [22] (phase 2 study) PMID 37694347 | Arm 1: 102 Arm 2: 50 | Arm 1: 5.4 mg/kg dose once every 3 weeks Arm 2: 6.4 mg/kg dose once every 3 weeks | Arm 1: 12.9% (95% CI: 7.0–2.10) Arm 2: 28.0% (95% CI: 16.2–42.5%) | Arm 1: 2% Arm 2: 2% | Arm 1: 1% Arm 2: 2% | Arm 1: 88.0 (range: 20–421) days Arm 2: 83.5 (range 36–386) days | Not reported |
| Patritumab-deruxtecan (ADC) | HERTHENA-Lung01 [23] (phase 2 study) PMID 37689979 | 225 | 5.6 mg/kg once every 3 weeks | 5.3% | 1.3% | 0.4% | 53 (range: 9–230) days | Not reported |
| Patritumab-deruxtecan (ADC) | HERTHENA-Lung02 [24] (phase 3 study) | ~293 | 5.6 mg/kg once every 3 weeks | 5% | 1% | >1% | Not reported | Not reported |
| Datopotamab deruxtecan (ADC) | TROPION-LUNG01 [25] (phase 3 study) PMID 39250535 | 299 | 6 mg/kg once every 3 weeks | 8.8% | 2.7% | None | 52 days | Not reported |
| Datopotamab deruxtecan (ADC) | TROPION-PanTumour01 [26] (phase 1 study) PMID 37327461 | 6 mg/kg dose: 50 8 mg/kg dose: 80 | 0.27–10 mg/kg once every 3 weeks during escalation 4, 6, or 8 mg/kg once every 3 weeks during expansion | 6 mg/kg dose: 6% 8 mg/kg dose: 13.8% | 6 mg/kg dose: 2% 8 mg/kg dose: 5% | 6 mg/kg dose: none 8 mg/kg dose: 3.75% | Not reported | Not reported |
| Sacituzumab govitecan (ADC) | TROPiCS-03 [27] (phase 2 study) PMID 39083724 | ~40 | 10 mg/kg once on day 1 and day 8 of a 21-day cycle until disease progress, unacceptable toxicity, study withdrawal, or death | None reported | None reported | None reported | n/a | n/a |
| Tarlatamab (BsAbs) | DeLLphi-300 [28] (phase 1 study) PMID 36689692 | 107 | Dose exploration (0.003–100 mg every 2 weeks) Expansion dose: 100 mg | 4.7% | 1.8% | 0.9% | Not reported | Not reported |
| Tarlatamab (BsAb) | DeLLphi-301 [29] (phase 2 study) PMID 39876075 | 220 | 10 mg every 2 weeks | 1.4% | 1.4% | None reported | Not reported | Not reported |
| Zanidatamab (BsAb) | Meric-Bernstam et al. [30] (phase 1 study) PMID 36400106 | 46 | 3 + 3 dose escalation (5–30 mg/kg every 1, 2, or 3 weeks) | None reported | None reported | None reported | n/a | n/a |
| Amivantamab (BsAb) | CHRYSALIS trial [31] (phase 1 study) PMID 34339292 | 352 | 1050 mg (1400 mg, ≥80 kg) once a week for the first 4 weeks and then once every 2 weeks starting at week 5 | 3.3% | 0.7% | None reported | Not reported | Not reported |
| BL-B01D1 (BsAb) | Ma et al. [32] (phase 1 study) PMID 38823410 | Total: 195 NSCLC: 113 | 2.5–3.5 mg/kg (days 1 and 8 every 3 weeks) or 4.5–6.0 mg/kg (once every 3 weeks) | 0.5% | Not reported | Not reported | Not reported | Not reported |
| Izalontamab (BsAb) | Xue et al. [33] (phase 1 study) PMID 40260627 | 60 | 3 + 3 dose escalation (nine dose levels from 0.4 to 28.0 mg/kg) and dose expansion (five dose levels from 6.0 to 21.0 mg/kg). Izalontamab was administered weekly or every 2 weeks in a 4-week cycle | None reported | None reported | None reported | n/a | n/a |
| Biomarker Category | Predictive Biomarker(s) Associated with Pneumonitis | Associated Agent(s) | References |
|---|---|---|---|
| Tissue-Based | Single-Nucleotide Polymorphism | Radiotherapy | [75,76,77,78,79] |
| PD-L1 expression | ICIs | [74] | |
| Blood-Based | Cytokines | Radiotherapy, ICIs | [80,81,82] |
| Specific HLA subtypes | ICIs | [79,83,84] | |
| Autoantibodies | ICIs | [85,86] | |
| TCR metrics | ICIs | [87] | |
| White Blood Cell Counts | ICIs | [88] | |
| Imaging-Based | Radiomics | ICIs, Radiotherapy | [89,90,91,92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, D.; Robinson, A.; Mates, M.; Li, Y.; Chooback, N.; Gaudreau, P.-O.; Digby, G.C.; Fung, A.S.; Genta, S. Balancing Innovation and Safety: Prediction, Prevention, and Management of Pneumonitis in Lung Cancer Patients Receiving Novel Anti-Cancer Agents. Cancers 2025, 17, 2522. https://doi.org/10.3390/cancers17152522
Liu S, Wang D, Robinson A, Mates M, Li Y, Chooback N, Gaudreau P-O, Digby GC, Fung AS, Genta S. Balancing Innovation and Safety: Prediction, Prevention, and Management of Pneumonitis in Lung Cancer Patients Receiving Novel Anti-Cancer Agents. Cancers. 2025; 17(15):2522. https://doi.org/10.3390/cancers17152522
Chicago/Turabian StyleLiu, Sarah, Daniel Wang, Andrew Robinson, Mihaela Mates, Yuchen Li, Negar Chooback, Pierre-Olivier Gaudreau, Geneviève C. Digby, Andrea S. Fung, and Sofia Genta. 2025. "Balancing Innovation and Safety: Prediction, Prevention, and Management of Pneumonitis in Lung Cancer Patients Receiving Novel Anti-Cancer Agents" Cancers 17, no. 15: 2522. https://doi.org/10.3390/cancers17152522
APA StyleLiu, S., Wang, D., Robinson, A., Mates, M., Li, Y., Chooback, N., Gaudreau, P.-O., Digby, G. C., Fung, A. S., & Genta, S. (2025). Balancing Innovation and Safety: Prediction, Prevention, and Management of Pneumonitis in Lung Cancer Patients Receiving Novel Anti-Cancer Agents. Cancers, 17(15), 2522. https://doi.org/10.3390/cancers17152522








