Recurrence Patterns After Resection of Sacral Chordoma: Toward an Optimized Postoperative Target Volume Definition
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Characteristics
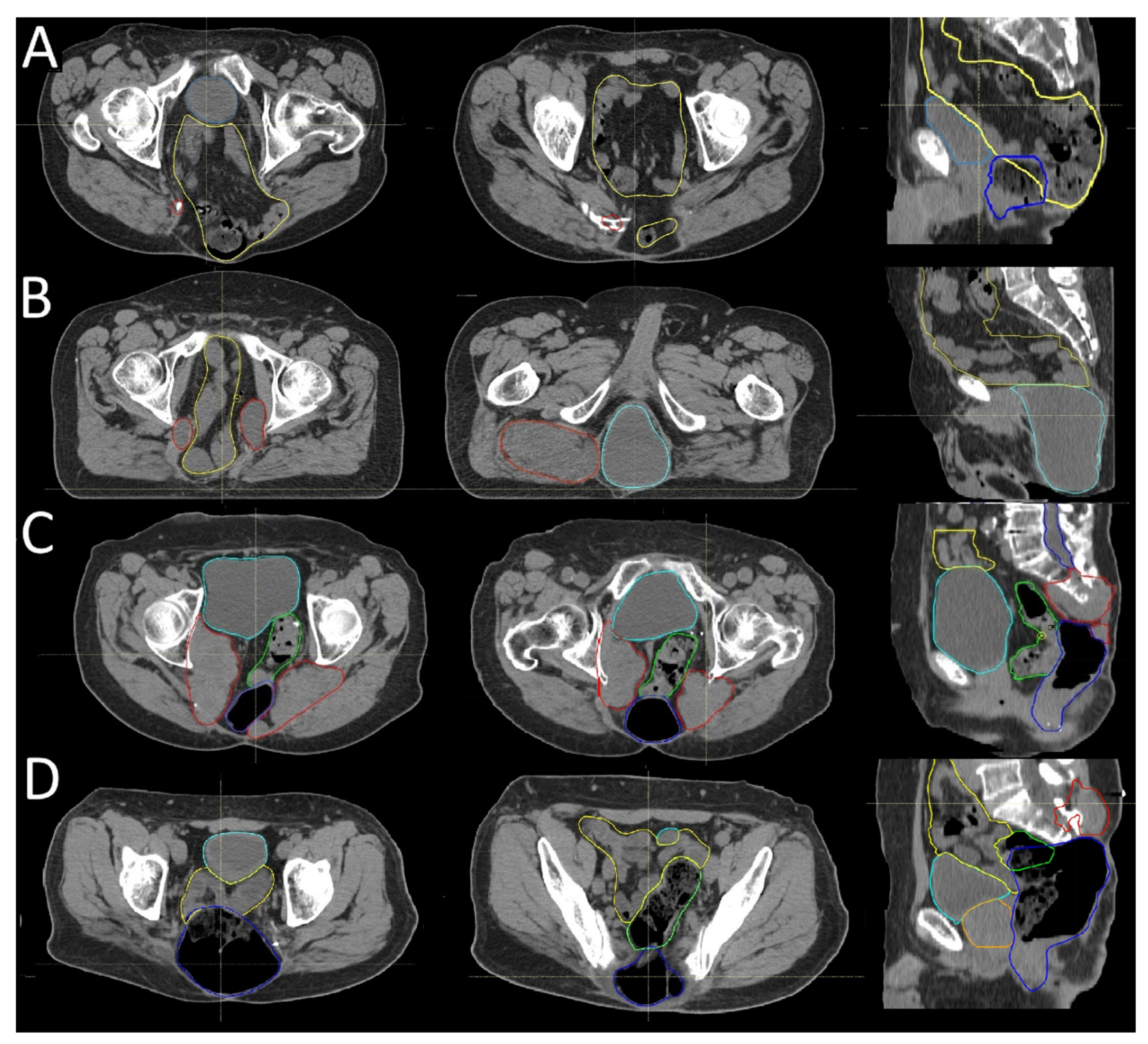
3.2. Recurrence Patterns
3.3. Postoperative Radiotherapy Planning Based on Recurrence Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karpathiou, G.; Dumollard, J.M.; Dridi, M.; Dal Col, P.; Barral, F.-G.; Boutonnat, J.; Peoc’h, M. Chordomas: A review with emphasis on their pathophysiology, pathology, molecular biology, and genetics. Pathol.-Res. Pract. 2020, 216, 153089. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Sommer, J. Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kou, C.; Bai, W.; Yu, W.; Zhu, B.; Zhang, M.; Hua, W.; Li, Y.; Duan, R.; Yin, F. Comparison of Wide Margin and Inadequate Margin for Recurrence in Sacral Chordoma: A Meta-Analysis. Spine 2020, 45, 814–819. [Google Scholar] [CrossRef]
- Branco e Silva, M.; Branco e Silva, M.; Conceição Maia Martins, S.; Voltan Garofo, K.; Eduardo Hideo Hanasilo, C.; Etchebehere, M. Analysis of morbidity and mortality in patients with primary bone tumors who underwent sacrectomy: A systematic review. J. Bone Oncol. 2022, 35, 100445. [Google Scholar] [CrossRef] [PubMed]
- York, J.E.; Kaczaraj, A.; Abi-Said, D.; Fuller, G.N.; Skibber, J.M.; Janjan, N.A.; Gokaslan, Z.L. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery 1999, 44, 74–79; discussion 79–80. [Google Scholar] [CrossRef]
- Ruggieri, P.; Angelini, A.; Ussia, G.; Montalti, M.; Mercuri, M. Surgical margins and local control in resection of sacral chordomas. Clin. Orthop. Relat. Res. 2010, 468, 2939–2947. [Google Scholar] [CrossRef]
- Fuchs, B.; Dickey, I.D.; Yaszemski, M.J.; Inwards, C.Y.; Sim, F.H. Operative management of sacral chordoma. J. Bone Jt. Surg. Am. Vol. 2005, 87, 2211–2216. [Google Scholar] [CrossRef]
- Colangeli, S.; Muratori, F.; Bettini, L.; Frenos, F.; Totti, F.; D’Arienzo, A.; Campo, F.R.; Scoccianti, G.; Beltrami, G.; Campanacci, D.A.; et al. Surgical Treatment of Sacral Chordoma: En Bloc Resection with Negative Margins is a Determinant of the Long-Term Outcome. Surg. Technol. Int. 2018, 33, 343–348. [Google Scholar]
- Kayani, B.; Hanna, S.A.; Sewell, M.D.; Saifuddin, A.; Molloy, S.; Briggs, T.W.R. A review of the surgical management of sacral chordoma. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, X.; Li, Y.; Liu, W.; Xu, H. Recurrence and survival factors analysis of 171 cases of sacral chordoma in a single institute. Eur. Spine J. 2017, 26, 1910–1916. [Google Scholar] [CrossRef]
- Varga, P.P.; Szövérfi, Z.; Fisher, C.G.; Boriani, S.; Gokaslan, Z.L.; Dekutoski, M.B.; Chou, D.; Quraishi, N.A.; Reynolds, J.J.; Luzzati, A.; et al. Surgical treatment of sacral chordoma: Prognostic variables for local recurrence and overall survival. Eur. Spine J. 2015, 24, 1092–1101. [Google Scholar] [CrossRef]
- Radaelli, S.; Fossati, P.; Stacchiotti, S.; Akiyama, T.; Asencio, J.M.; Bandiera, S.; Boglione, A.; Boland, P.; Bolle, S.; Bruland, Ø.; et al. The sacral chordoma margin. Eur. J. Surg. Oncol. 2020, 46, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Delaney, T.F.; Liebsch, N.J.; Hornicek, F.J.; Goldberg, S.; Mankin, H.; Rosenberg, A.E.; Rosenthal, D.I.; Suit, H.D. Sacral chordomas: Impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1514–1521. [Google Scholar] [CrossRef]
- Pennicooke, B.; Laufer, I.; Sahgal, A.; Varga, P.P.; Gokaslan, Z.L.; Bilsky, M.H.; Yamada, Y.J. Safety and Local Control of Radiation Therapy for Chordoma of the Spine and Sacrum: A Systematic Review. Spine 2016, 41 (Suppl. 20), S186–S192. [Google Scholar] [CrossRef]
- Hug, E.B.; Fitzek, M.M.; Liebsch, N.J.; Munzenrider, J.E. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: Results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 467–476. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Liebsch, N.J.; Pedlow, F.X.; Adams, J.; Weyman, E.A.; Yeap, B.Y.; Depauw, N.; Nielsen, G.P.; Harmon, D.C.; Yoon, S.S.; et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J. Surg. Oncol. 2014, 110, 115–122. [Google Scholar] [CrossRef]
- Seidensaal, K.; Froehlke, A.; Lentz-Hommertgen, A.; Lehner, B.; Geisbuesch, A.; Meis, J.; Liermann, J.; Kudak, A.; Stein, K.; Uhl, M.; et al. Hypofractionated proton and carbon ion beam radiotherapy for sacrococcygeal chordoma (ISAC): An open label, randomized, stratified, phase II trial. Radiother. Oncol. 2024, 198, 110418. [Google Scholar] [CrossRef]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma-Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, D.M.; Chi, J.H.; Rhines, L.D.; Gokaslan, Z.L. Chordoma of the spinal column. Neurosurg. Clin. N. Am. 2008, 19, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Fourney, D.R.; Rhines, L.D.; Hentschel, S.J.; Skibber, J.M.; Wolinsky, J.P.; Weber, K.L.; Suki, D.; Gallia, G.L.; Garonzik, I.; Gokaslan, Z.L. En bloc resection of primary sacral tumors: Classification of surgical approaches and outcome. J. Neurosurg. Spine 2005, 3, 111–122. [Google Scholar] [CrossRef]
- Cini, C.; Asunis, E.; Griffoni, C.; Evangelisti, G.; Tedesco, G.; Ghermandi, R.; Girolami, M.; Pipola, V.; Terzi, S.; Barbanti Brodano, G.; et al. Surgical Management of Sacral Bone Tumors: A Retrospective Analysis of Outcomes, Complications, and Survival. Diagnostics 2025, 15, 917. [Google Scholar] [CrossRef]
- Hulen, C.A.; Temple, H.T.; Fox, W.P.; Sama, A.A.; Green, B.A.; Eismont, F.J. Oncologic and Functional Outcome Following Sacrectomy for Sacral Chordoma. J. Bone Jt. Surg. Am. 2006, 88, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, A.; Schaser, K.D.; Weitz, J.; Kirchberg, J.; Fritzmann, J.; Reeps, C.; Schwabe, P.; Melcher, I.; Disch, A.; Dragu, A.; et al. Surgical and Oncologic Outcome following Sacrectomy for Primary Malignant Bone Tumors and Locally Recurrent Rectal Cancer. Cancers 2024, 16, 2334. [Google Scholar] [CrossRef]
- Hanna, S.A.; Aston, W.J.; Briggs, T.W.; Cannon, S.R.; Saifuddin, A. Sacral chordoma: Can local recurrence after sacrectomy be predicted? Clin. Orthop. Relat. Res. 2008, 466, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Imai, R.; Kiyohara, H.; Matsunobu, A.; Okamoto, M.; Okimoto, T.; Tsuji, H.; Ohno, T.; Shioyama, Y.; Nemoto, K.; et al. Carbon ion radiotherapy for sacral chordoma: A retrospective nationwide multicentre study in Japan. Radiother. Oncol. 2021, 154, 1–5. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Welzel, T.; Jensen, A.; Ellerbrock, M.; Haberer, T.; Jakel, O.; Herfarth, K.; Debus, J. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlenther. Onkol. 2015, 191, 597–603. [Google Scholar] [CrossRef]
- Bostel, T.; Mattke, M.; Nicolay, N.H.; Welzel, T.; Wollschlager, D.; Akbaba, S.; Mayer, A.; Sprave, T.; Debus, J.; Uhl, M. High-dose carbon-ion based radiotherapy of primary and recurrent sacrococcygeal chordomas: Long-term clinical results of a single particle therapy center. Radiat. Oncol. 2020, 15, 206. [Google Scholar] [CrossRef]
- Nishida, Y.; Kamada, T.; Imai, R.; Tsukushi, S.; Yamada, Y.; Sugiura, H.; Shido, Y.; Wasa, J.; Ishiguro, N. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 110–116. [Google Scholar] [CrossRef]
- Yolcu, Y.U.; Zreik, J.; Wahood, W.; Bhatti, A.U.R.; Bydon, M.; Houdek, M.T.; Rose, P.S.; Mahajan, A.; Petersen, I.A.; Haddock, M.G.; et al. Comparison of Oncologic Outcomes and Treatment-Related Toxicity of Carbon Ion Radiotherapy and En Bloc Resection for Sacral Chordoma. JAMA Netw. Open 2022, 5, e2141927. [Google Scholar] [CrossRef]

| N | 31 |
|---|---|
| Sex (N, %) | |
| Male | 18 (58) |
| Female | 13 (42) |
| Age at first diagnosis (years) | |
| Median (Range) | 60 (26–78) |
| Brachyury-staining (N, %) | |
| Positive | 11 (36) |
| Negative | 0 |
| Missing | 20 (64) |
| Highest infiltrated sacrococcygeal vertebra at first diagnosis (N, %) | |
| S1 | 1 (3) |
| S2 | 3 (10) |
| S3 | 11 (35) |
| S4 | 7 (23) |
| S5 | 3 (10) |
| Coccygeal | 6 (19) |
| Volume of initial tumor (gross tumor volume, GTV, in mL) (Median, range) | |
| Median (Range) | 113 (31–419) |
| Missing (N, %) | 10 (32) |
| Pathological resection status of the first resection (N, %) | |
| R0 | 7 (22) |
| R0 (close) | 10 (32) |
| R1 | 8 (26) |
| RX (fragmented) | 3 (10) |
| Missing | 3 (10) |
| Time from first resection to first recurrence (months) | |
| Median (Range) | 15 (2–68) |
| Time from first diagnosis to first recurrence (months) | |
| Median (Range) | 17 (2–70) |
| Time from first diagnosis to first presentation at the HIT (months) | |
| Median (Range) | 30 (9–167) |
| Resection of recurrence before irradiation (N, %) | |
| Yes | 11 (36) |
| No | 20 (64) |
| Extension on recurrence on MRI (N, %) | |
| Unifocal lesion | 3 (10) |
| Multifocal lesions | 28 (90) |
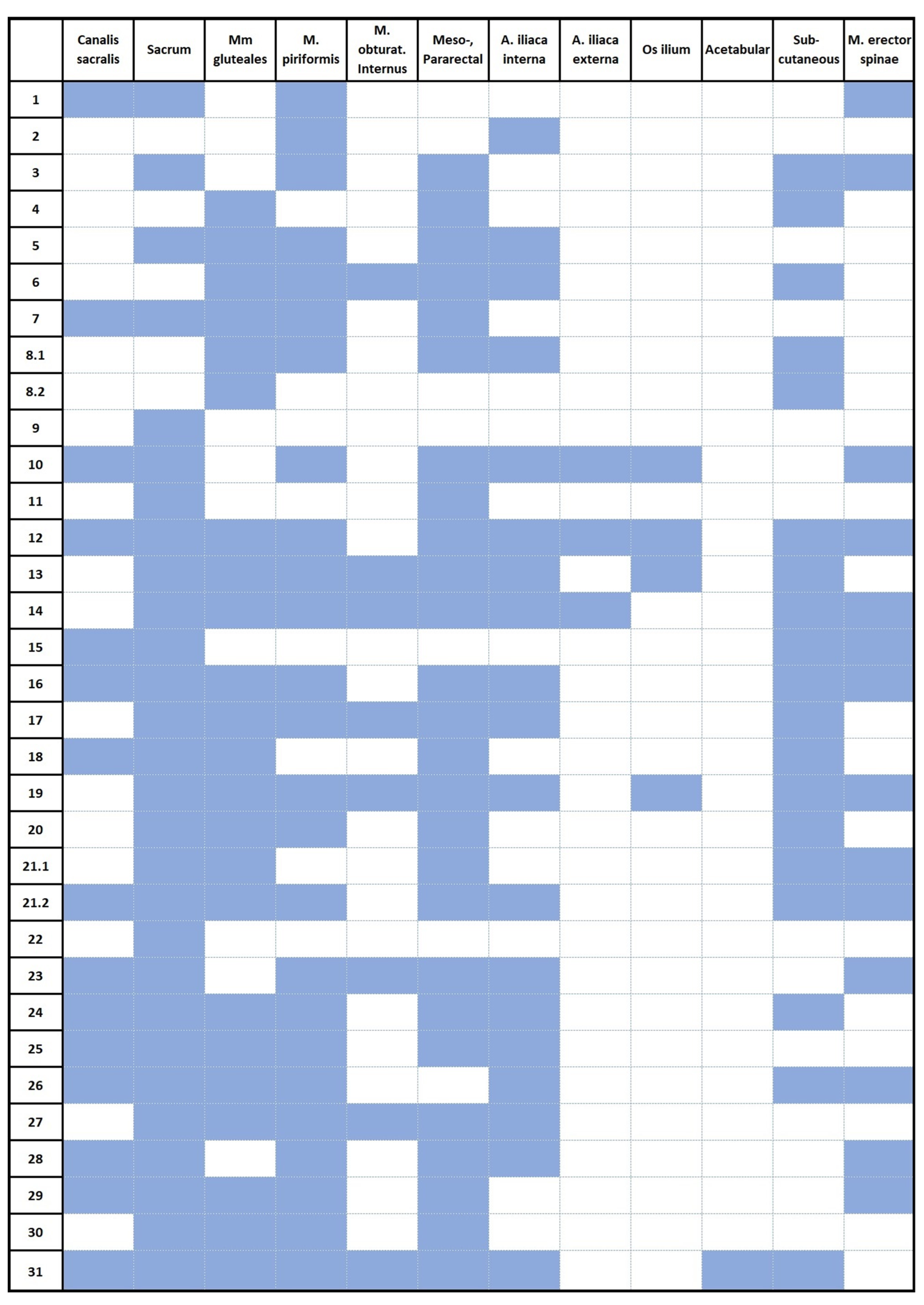
| Total (N) | 31 | |
|---|---|---|
| Localization | Recurrence (N) | Percentage |
| Sacrum | 27 | 87.1% |
| Meso-, Pararectal | 25 | 80.6% |
| M. piriformis | 25 | 80.6% |
| Mm. gluteales | 21 | 67.7% |
| A. Iliaca interna | 19 | 61.3% |
| Subcutaneous | 17 | 54.8% |
| Canalis sacralis | 15 | 48.4% |
| M. erector spinae | 13 | 41.9% |
| M. obturatorius internus | 8 | 25.8% |
| Os ilium | 4 | 12.9% |
| A. Iliaca externa | 3 | 9.7% |
| Acetabular | 1 | 3.2% |
| N = 18 | |||
|---|---|---|---|
| Expansion | Full Coverage of All Recurrence Origins (N, %) | At Least Partial Coverage of All Recurrence Origins (N, %) | At Least One Recurrence Origin Outside the Margin (N, %) |
| GTVinit + 2 cm | 0 (0) | 5 (28) | 13 (72) |
| GTVinit + 3 cm | 2 (11) | 8 (44.5) | 8 (44.5) |
| GTVinit + 5 cm | 10 (56) | 4 (22) | 4 (22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldsperger, H.; Lehner, B.; Geisbuesch, A.; Jotzo, F.; Meixner, E.; König, L.; Regnery, S.; Kozyra, K.; Wessel, L.; Krieg, S.; et al. Recurrence Patterns After Resection of Sacral Chordoma: Toward an Optimized Postoperative Target Volume Definition. Cancers 2025, 17, 2521. https://doi.org/10.3390/cancers17152521
Waldsperger H, Lehner B, Geisbuesch A, Jotzo F, Meixner E, König L, Regnery S, Kozyra K, Wessel L, Krieg S, et al. Recurrence Patterns After Resection of Sacral Chordoma: Toward an Optimized Postoperative Target Volume Definition. Cancers. 2025; 17(15):2521. https://doi.org/10.3390/cancers17152521
Chicago/Turabian StyleWaldsperger, Hanna, Burkhard Lehner, Andreas Geisbuesch, Felix Jotzo, Eva Meixner, Laila König, Sebastian Regnery, Katharina Kozyra, Lars Wessel, Sandro Krieg, and et al. 2025. "Recurrence Patterns After Resection of Sacral Chordoma: Toward an Optimized Postoperative Target Volume Definition" Cancers 17, no. 15: 2521. https://doi.org/10.3390/cancers17152521
APA StyleWaldsperger, H., Lehner, B., Geisbuesch, A., Jotzo, F., Meixner, E., König, L., Regnery, S., Kozyra, K., Wessel, L., Krieg, S., Herfarth, K., Debus, J., & Seidensaal, K. (2025). Recurrence Patterns After Resection of Sacral Chordoma: Toward an Optimized Postoperative Target Volume Definition. Cancers, 17(15), 2521. https://doi.org/10.3390/cancers17152521







