Recurrence Patterns, Treatment Outcomes, and Prognostic Factors of Thymic Carcinoma: A Multicenter Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment Characteristics
2.2.1. Surgery
2.2.2. Radiotherapy
2.2.3. Chemotherapy
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Outcomes and Relapse Patterns
3.3. Prognostic Factors for LRFS, PFS, and OS
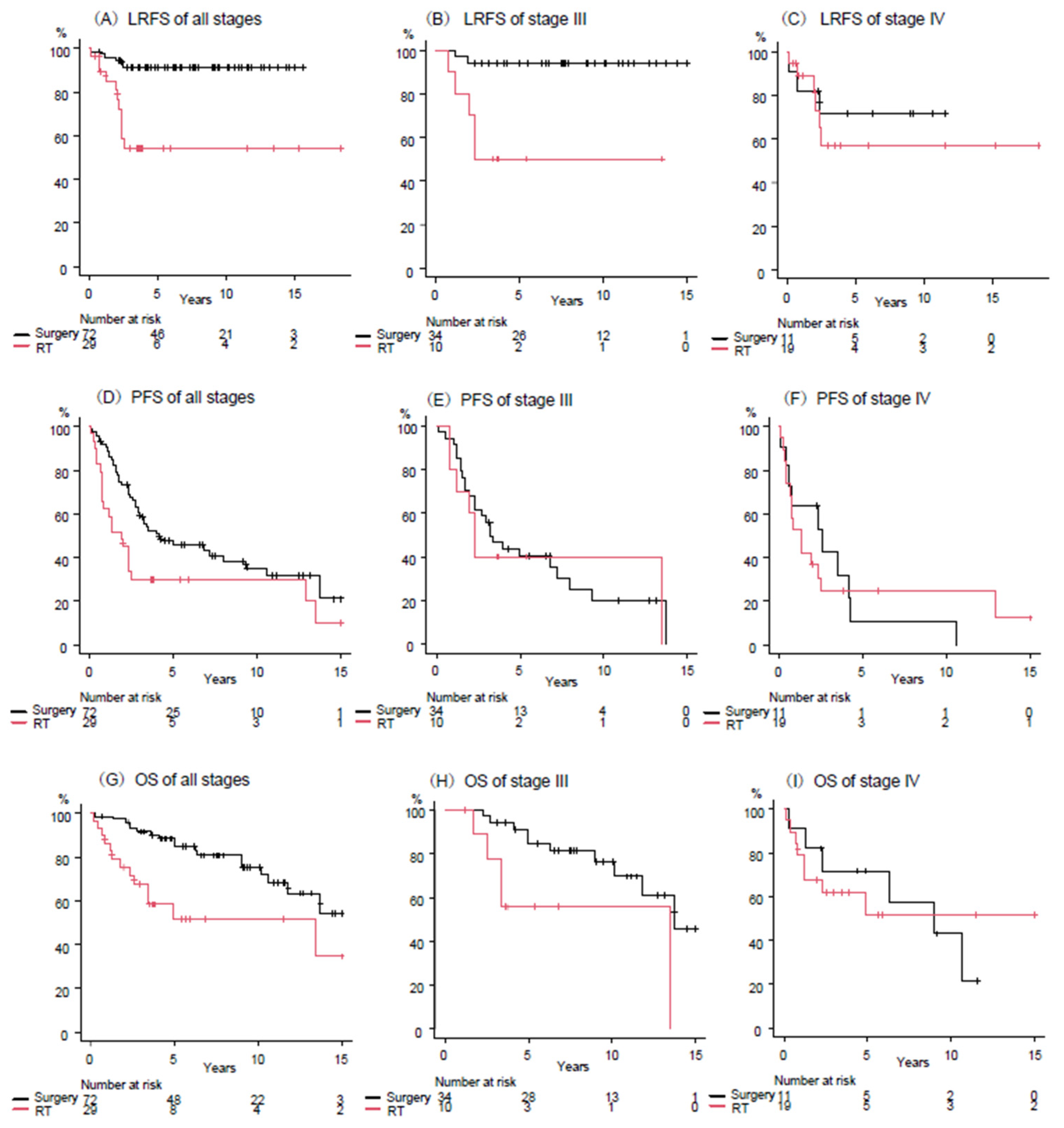
3.4. Outcomes by Treatment Modality According to Stage
3.5. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3DCRT | three-dimensional conformal radiotherapy |
| AEs | adverse events |
| CRT | Chemoradiotherapy |
| CT | computed tomography |
| CTV | clinical target volume |
| ENI | elective nodal irradiation |
| EQD2 | equivalent dose at 2-Gy |
| FDG-PET | 18F-fluorodeoxyglucose positron emission tomography |
| GTV | gross tumor volume |
| IMRT | intensity-modulated radiotherapy |
| ITMIG | International Thymic Malignancy Interest Group |
| LRFS | local recurrence-free survival |
| LQ model | linear-quadratic model |
| OS | overall survival |
| PD-L1 | programmed death ligand 1 |
| PFS | progression-free survival |
| PS | performance status |
| PTV | planning target volume |
| TETs | thymic epithelial tumors |
| RT | radiation therapy |
| UICC | Union for International Cancer Control |
References
- Gatta, G.; van der Zwan, J.M.; Casali, P.G.; Siesling, S.; Dei Tos, A.P.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef]
- Hishida, T.; Nomura, S.; Yano, M.; Asamura, H.; Yamashita, M.; Ohde, Y.; Kondo, K.; Date, H.; Okumura, M.; Nagai, K.; et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: Results of 306 cases from a Japanese Nationwide Database Study. Eur. J. Cardiothorac. Surg. 2016, 49, 835–841. [Google Scholar] [CrossRef]
- Jackson, M.W.; Palma, D.A.; Camidge, D.R.; Jones, B.L.; Robin, T.P.; Sher, D.J.; Koshy, M.; Kavanagh, B.D.; Gaspar, L.E.; Rusthoven, C.G. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2017, 12, 734–744. [Google Scholar] [CrossRef]
- Ahmad, U.; Yao, X.; Detterbeck, F.; Huang, J.; Antonicelli, A.; Filosso, P.L.; Ruffini, E.; Travis, W.; Jones, D.R.; Zhan, Y.; et al. Thymic carcinoma outcomes and prognosis: Results of an international analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 95–101.e2. [Google Scholar] [CrossRef]
- Lim, Y.J.; Song, C.; Kim, J.-S. Improved survival with postoperative radiotherapy in thymic carcinoma: A propensity-matched analysis of Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2017, 108, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bull, D.A.; Hsu, C.-H.; Hsu, C.C. The Role of Adjuvant Therapy in Advanced Thymic Carcinoma: A National Cancer Database Analysis. Ann. Thorac. Surg. 2020, 109, 1095–1103. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN) Guidelines. Thymomas and Thymic Carcinomas Version 1.2025. Available online: https://www.nccn.org (accessed on 7 April 2025).
- Süveg, K.; Putora, P.M.; Joerger, M.; Iseli, T.; Fischer, G.F.; Ammann, K.; Glatzer, M. Radiotherapy for thymic epithelial tumours: A review. Transl. Lung Cancer Res. 2021, 10, 2088–2100. [Google Scholar] [CrossRef]
- Omasa, M.; Date, H.; Sozu, T.; Sato, T.; Nagai, K.; Yokoi, K.; Okamoto, T.; Ikeda, N.; Tanaka, F.; Maniwa, Y.; et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: The Japanese Association for Research on the Thymus Database Study. Cancer 2015, 121, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, C.Y.; Kim, E.Y.; Lee, C.G.; Hong, M.H.; Park, B.J.; Yoon, H.I.; Kim, K.H.; Lee, S.H.; Byun, H.K.; et al. Clinical Outcomes of Thymic Carcinoma: The Role of Radiotherapy Combined with Multimodal Treatments. Cancers 2023, 15, 2262. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Okuda, K.; Ogawa, Y.; Iida, M.; Eguchi, Y.; Kitagawa, Y.; Uchiyama, K.; Takaoka, T.; Nakanishi, R.; Shibamoto, Y. Relationship between radiation doses to heart substructures and radiation pneumonitis in patients with thymic epithelial tumors. Sci. Rep. 2020, 10, 11191. [Google Scholar] [CrossRef]
- Speirs, C.K.; DeWees, T.A.; Rehman, S.; Molotievschi, A.; Velez, M.A.; Mullen, D.; Fergus, S.; Trovo, M.; Bradley, J.D.; Robinson, C.G. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 293–301. [Google Scholar] [CrossRef]
- Niwa, M.; Tomita, N.; Ishiyama, H.; Kaneko, H.; Oshima, Y.; Takano, H.; Matsuo, M.; Kuno, M.; Miyakawa, A.; Otsuka, S.; et al. Clinical Outcomes and Prognostic Factors for Extramammary Paget’s Disease Treated with Radiation Therapy: A Multi-Institutional Observational Study. Cancers 2025, 17, 1507. [Google Scholar] [CrossRef]
- Niwa, M.; Tomita, N.; Takaoka, T.; Takano, H.; Makita, C.; Matsuo, M.; Adachi, S.; Oshima, Y.; Yamamoto, S.; Kuno, M.; et al. Clinical Outcomes of Radiation Therapy for Angiosarcoma of the Scalp and Face: A Multi-Institutional Observational Study. Cancers 2023, 15, 3696. [Google Scholar] [CrossRef]
- Okumura, M.; Marino, M.; Cilento, V.; Goren, E.; Ruffini, E.; Dibaba, D.; Ahmad, U.; Appel, S.; Bille, A.; Boubia, S.; et al. The International Association for the Study of Lung Cancer Thymic Epithelial Tumor Staging Project: Proposal for the T Component for the Forthcoming (Ninth) Edition of the TNM Classification of Malignant Tumors. J. Thorac. Oncol. 2023, 18, 1638–1654. [Google Scholar] [CrossRef]
- Huang, J.; Detterbeck, F.C.; Wang, Z.; Loehrer, P.J., Sr. Standard outcome measures for thymic malignancies. J. Thorac. Oncol. 2010, 5, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Detterbeck, F.; Van Raemdonck, D.; Rocco, G.; Thomas, P.; Weder, W.; Brunelli, A.; Guerrera, F.; Keshavjee, S.; Altorki, N.; et al. Thymic carcinoma: A cohort study of patients from the European society of thoracic surgeons database. J. Thorac. Oncol. 2014, 9, 541–548. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, D.; Aramini, B.; Yang, F.; Chen, X.; Wang, X.; Wu, L.; Huang, W.; Fan, J. Thymoma and Thymic Carcinoma: Surgical Resection and Multidisciplinary Treatment. Cancers 2023, 15, 1953. [Google Scholar] [CrossRef]
- Riely, G.J.; Huang, J.; Rimner, A. Multidisciplinary management of thymic carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2012, 32, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.G.; Rimner, A.; Amini, A.; Chang, J.Y.; Donington, J.; Edelman, M.J.; Geng, Y.; Gubens, M.A.; Higgins, K.A.; Iyengar, P.; et al. American Radium Society Appropriate Use Criteria for Radiation Therapy in the Multidisciplinary Management of Thymic Carcinoma. JAMA Oncol. 2023, 9, 971–980. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; Frazier, A.A.; Giaccone, G.; Huang, J.; et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9, S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Van Schil, P.; Detterbeck, F.C.; Okumura, M.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9, S81–S87. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, A.; Monden, Y.; Nakahara, K.; Tanioka, T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981, 48, 2485–2492. [Google Scholar] [CrossRef]
- Koga, K.; Matsuno, Y.; Noguchi, M.; Mukai, K.; Asamura, H.; Goya, T.; Shimosato, Y. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol. Int. 1994, 44, 359–367. [Google Scholar] [CrossRef]
- Hadoux, J.; Girard, N.; Besse, B. Thymic epithelial neoplasms: Updates on diagnosis, staging, biology and management in France. Bull Cancer 2012, 99, 1045–1055. [Google Scholar] [CrossRef]
- Pinelopi-Theopisti, M.; Aikaterini, P.; Ariadni, K.; Chrysoula, I.; Panagiotis, P.; Martha, C. The Place and the Role of Radiation Therapy in the Treatment of Thymic Carcinoma. World J. Oncol. 2016, 7, 95–97. [Google Scholar] [CrossRef][Green Version]
- Owen, D.; Chu, B.; Lehman, A.M.; Annamalai, L.; Yearley, J.H.; Shilo, K.; Otterson, G.A. Expression Patterns, Prognostic Value, and Intratumoral Heterogeneity of PD-L1 and PD-1 in Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2018, 13, 1204–1212. [Google Scholar] [CrossRef]
- Shukuya, T.; Asao, T.; Goto, Y.; Mimori, T.; Takayama, K.; Kaira, K.; Tanaka, H.; Ko, R.; Amano, Y.; Tachihara, M.; et al. Activity and safety of atezolizumab plus carboplatin and paclitaxel in patients with advanced or recurrent thymic carcinoma (MARBLE): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2025, 26, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Lalani, N.; Brade, A.M. Radiation dose for thymic tumours. Mediastinum 2020, 4, 35. [Google Scholar] [CrossRef]
- Tomita, N.; Uchiyama, K.; Mizuno, T.; Imai, M.; Sugie, C.; Ayakawa, S.; Niwa, M.; Matsui, T.; Otsuka, S.; Manabe, Y.; et al. Impact of advanced radiotherapy techniques and dose intensification on toxicity of salvage radiotherapy after radical prostatectomy. Sci. Rep. 2020, 10, 114. [Google Scholar] [CrossRef]
- Zhang, X.; Schalke, B.; Kvell, K.; Kriegsmann, K.; Kriegsmann, M.; Graeter, T.; Preissler, G.; Ott, G.; Kurz, K.; Bulut, E.; et al. WNT4 overexpression and secretion in thymic epithelial tumors drive an autocrine loop in tumor cells in vitro. Front Oncol. 2022, 12, 920871. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | n = 101 |
|---|---|
| Age (years) | 62 (24–82) |
| Male/female | 50/51 |
| ECOG PS 0/1/2 | 62/37/2 |
| Histology by the WHO classification | |
| Squamous cell carcinoma | 85 |
| Thymic carcinoma, NOS | 10 |
| Neuroendocrine carcinoma | 4 |
| Undifferentiated carcinoma, NOS | 1 |
| Adenocarcinoma | 1 |
| Stage (TNM 9th) | |
| I/II/IIIa/IIIb/IVa/IVb | 14/13/31/13/11/19 |
| T1/2/3/4 | 15/15/46/25 |
| N0/1/2 | 72/10/19 |
| M0/1a/1b | 98/3/0 |
| Maximum tumor diameter (cm) | 6.0 (1.8–12.0) |
| Treatment methods | |
| Surgery after preoperative RT | 17 |
| Surgery followed by postoperative RT | 55 |
| Definitive RT | 29 |
| Chemotherapy | |
| Use/non-use | 57/44 |
| Concurrent/non-concurrent | 28/29 |
| RT technique | |
| 3DCRT/IMRT | 86/15 |
| Non-IGRT/IGRT | 35/66 |
| ENI/non-use | 23/78 |
| RT dose (Gy) * | 54 (36–70) |
| Preoperative RT | 40 (36–50) |
| Postoperative RT | 50 (40–64) |
| Definitive RT | 60 (40–70) |
| Treatment Modality | Surgical Margin Status | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-RT and Surgery | Surgery and Post-RT | Definitive RT | R0 | R1 | R2 | ||||
| I–II | (n = 27) | 1 (4%) | 26 (96%) | 0 (0%) | I–II | (n = 27) | 20 (74%) | 7 (26%) | 0 (0%) |
| III | (n = 44) | 13 (30%) | 21 (48%) | 10 (23%) | III | (n = 34) | 22 (65%) | 8 (24%) | 4 (12%) |
| IVa | (n = 11) | 1 (9%) | 5 (45%) | 5 (45%) | IVa | (n = 6) | 3 (50%) | 2 (33%) | 1 (17%) |
| IVb | (n = 19) | 2 (11%) | 3 (16%) | 14 (74%) | IVb | (n = 5) | 3 (60%) | 1 (20%) | 1 (20%) |
| LRFS | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Predictor | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age (>65 years vs. ≤65) | 1.1 (0.41–3.0) | 0.84 | 0.76 (0.41–1.4) | 0.38 | 1.0 (0.49–2.2) | 0.93 |
| TNM stage (IVb, IVa, III, I–II) | 2.1 (1.3–3.3) | 0.001 | 1.7 (1.4–2.2) | <0.001 | 1.8 (1.3–2.4) | <0.001 |
| Treatment modality * | 6.3 (2.3–17) | <0.001 | 1.9 (1.1–3.2) | 0.017 | 2.6 (1.3–5.4) | 0.008 |
| Chemotherapy ** | 0.72 (0.41–1.3) | 0.26 | 0.67 (0.51–0.89) | 0.005 | 0.73 (0.49–1.1) | 0.13 |
| RT dose (≤54 Gy vs. >54 Gy) | 0.76 (0.29–2.0) | 0.58 | 0.74 (0.46–1.2) | 0.24 | 1.2 (0.58–2.4) | 0.66 |
| RT technique (3DCRT vs. IMRT) | 0.49 (0.16–1.5) | 0.21 | 1.2 (0.53–2.6) | 0.71 | 0.60 (0.21–1.8) | 0.36 |
| IGRT (no use vs. use) | 1.9 (0.74–5.0) | 0.18 | 1.2 (0.70–2.0) | 0.53 | 1.2 (0.57–2.6) | 0.62 |
| ENI (no use vs. use) | 2.0 (0.45–8.6) | 0.37 | 1.0 (0.56–1.9) | 0.94 | 0.51 (0.24–1.1) | 0.070 |
| LRFS | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Predictor | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age (>65 years vs. ≤65) | 0.51 (0.16–1.7) | 0.28 | 0.75 (0.41–1.4) | 0.37 | 0.68 (0.28–1.7) | 0.41 |
| TNM stage (IVb, IVa, III, I–II) | 2.1 (1.0–4.1) | 0.040 | 1.9 (1.4–2.7) | <0.001 | 1.6 (1.0–2.5) | 0.048 |
| Treatment modality * | 5.8 (1.4–24) | 0.015 | 1.1 (0.56–2.2) | 0.77 | 1.9 (0.68–5.3) | 0.22 |
| Chemotherapy ** | 0.96 (0.46–2.0) | 0.92 | 0.86 (0.62–1.2) | 0.39 | 0.94 (0.58–1.5) | 0.79 |
| RT dose (≤54 Gy vs. >54 Gy) | 1.7 (0.52–5.7) | 0.37 | 0.88 (0.50–1.5) | 0.65 | 2.2 (0.91–5.2) | 0.082 |
| RT technique (3DCRT vs. IMRT) | 0.27 (0.06–1.2) | 0.083 | 1.3 (0.52–3.1) | 0.59 | 0.47 (0.14–1.6) | 0.24 |
| IGRT (no use vs. use) | 9.9 (2.3–43) | 0.002 | 1.6 (0.86–2.9) | 0.14 | 1.7 (0.71–4.2) | 0.23 |
| ENI (no use vs. use) | 8.3 (1.6–43) | 0.013 | 2.4 (1.1–4.9) | 0.023 | 0.84 (0.33–2.1) | 0.71 |
| Characteristic | Surgery with RT | Definitive RT | p-Value |
|---|---|---|---|
| Age (years) | 60 (24–82) | 64 (27–82) | 0.044 |
| Male/female | 35/37 | 15/14 | 0.78 |
| ECOG PS 0/1/2 | 50/21/1 | 12/16/1 | 0.008 |
| Histology | |||
| Squamous cell carcinoma | 61 | 24 | 1 |
| Others | 11 | 5 | |
| Stage I–II/III/IVa/IVb | 27/34/6/5 | 0/10/5/14 | <0.001 |
| Maximum tumor diameter (cm) | 5.1 (1.8–12.0) | 8.0 (3.5–12.0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, N.; Ishihara, S.; Nomoto, Y.; Takada, A.; Nakamura, K.; Konishi, K.; Wakabayashi, K.; Ohshima, Y.; Yamada, M.; Matsuo, M.; et al. Recurrence Patterns, Treatment Outcomes, and Prognostic Factors of Thymic Carcinoma: A Multicenter Study. Cancers 2025, 17, 2513. https://doi.org/10.3390/cancers17152513
Tomita N, Ishihara S, Nomoto Y, Takada A, Nakamura K, Konishi K, Wakabayashi K, Ohshima Y, Yamada M, Matsuo M, et al. Recurrence Patterns, Treatment Outcomes, and Prognostic Factors of Thymic Carcinoma: A Multicenter Study. Cancers. 2025; 17(15):2513. https://doi.org/10.3390/cancers17152513
Chicago/Turabian StyleTomita, Natsuo, Shunichi Ishihara, Yoshihito Nomoto, Akinori Takada, Katsumasa Nakamura, Kenta Konishi, Kohei Wakabayashi, Yukihiko Ohshima, Maho Yamada, Masayuki Matsuo, and et al. 2025. "Recurrence Patterns, Treatment Outcomes, and Prognostic Factors of Thymic Carcinoma: A Multicenter Study" Cancers 17, no. 15: 2513. https://doi.org/10.3390/cancers17152513
APA StyleTomita, N., Ishihara, S., Nomoto, Y., Takada, A., Nakamura, K., Konishi, K., Wakabayashi, K., Ohshima, Y., Yamada, M., Matsuo, M., Ito, M., Okuda, K., Takaoka, T., Okazaki, D., Kita, N., Takano, S., & Hiwatashi, A. (2025). Recurrence Patterns, Treatment Outcomes, and Prognostic Factors of Thymic Carcinoma: A Multicenter Study. Cancers, 17(15), 2513. https://doi.org/10.3390/cancers17152513







