Critical Care Management of Surgically Treated Gynecological Cancer Patients: Current Concepts and Future Directions
Simple Summary
Abstract
1. Introduction
2. Review Methods
3. Major Physiological Changes Supporting Critical Care Support
3.1. Cardiovascular System
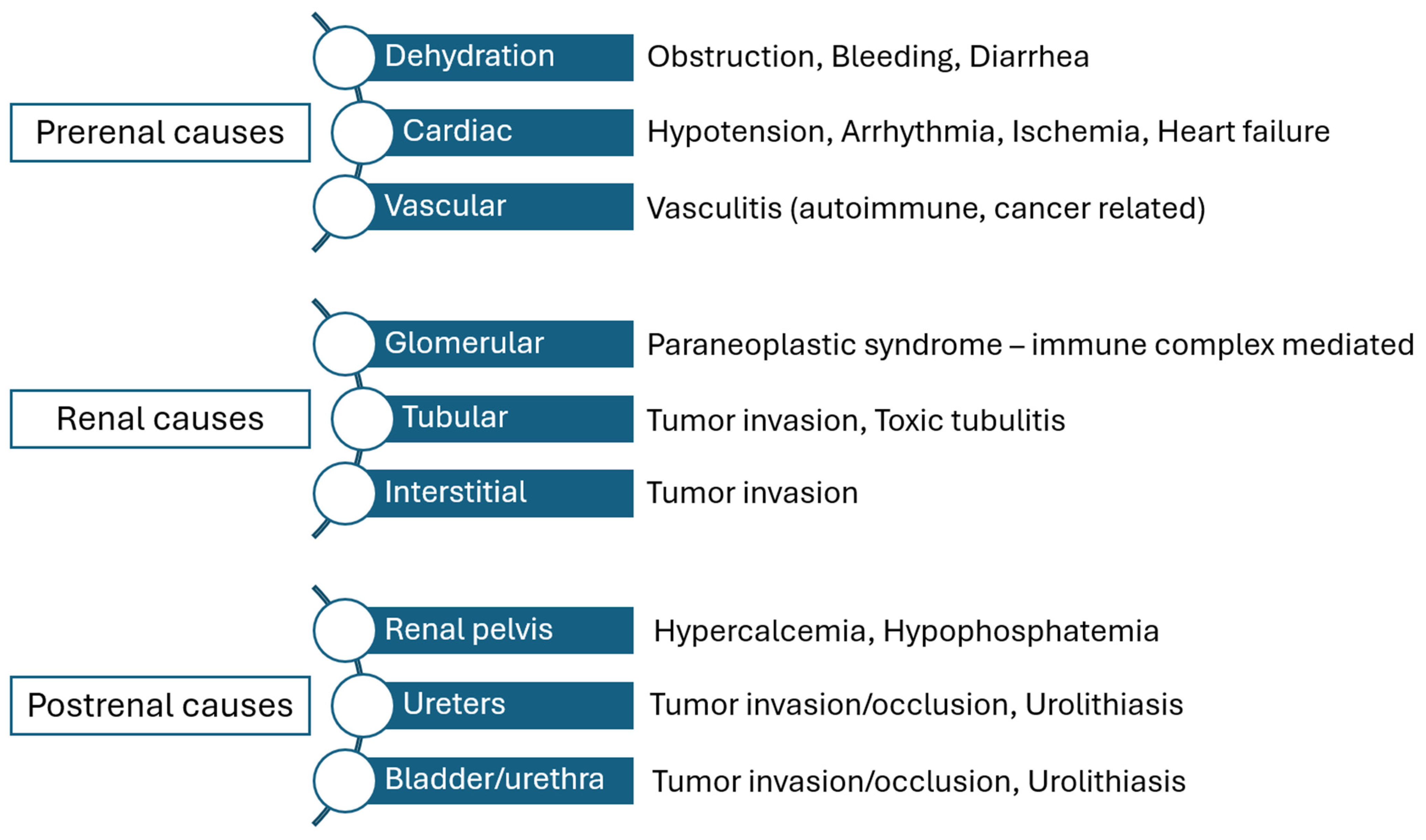
3.2. Renal Function
3.3. Pulmonary Function
4. Importance and Structure of the Surgical Critical Care Units
4.1. Organization of Critical Care Units
4.2. Intensive Care Units in Gynecologic Oncology
4.2.1. PACU
4.2.2. High Dependency Unit
4.2.3. Intensive Care Unit
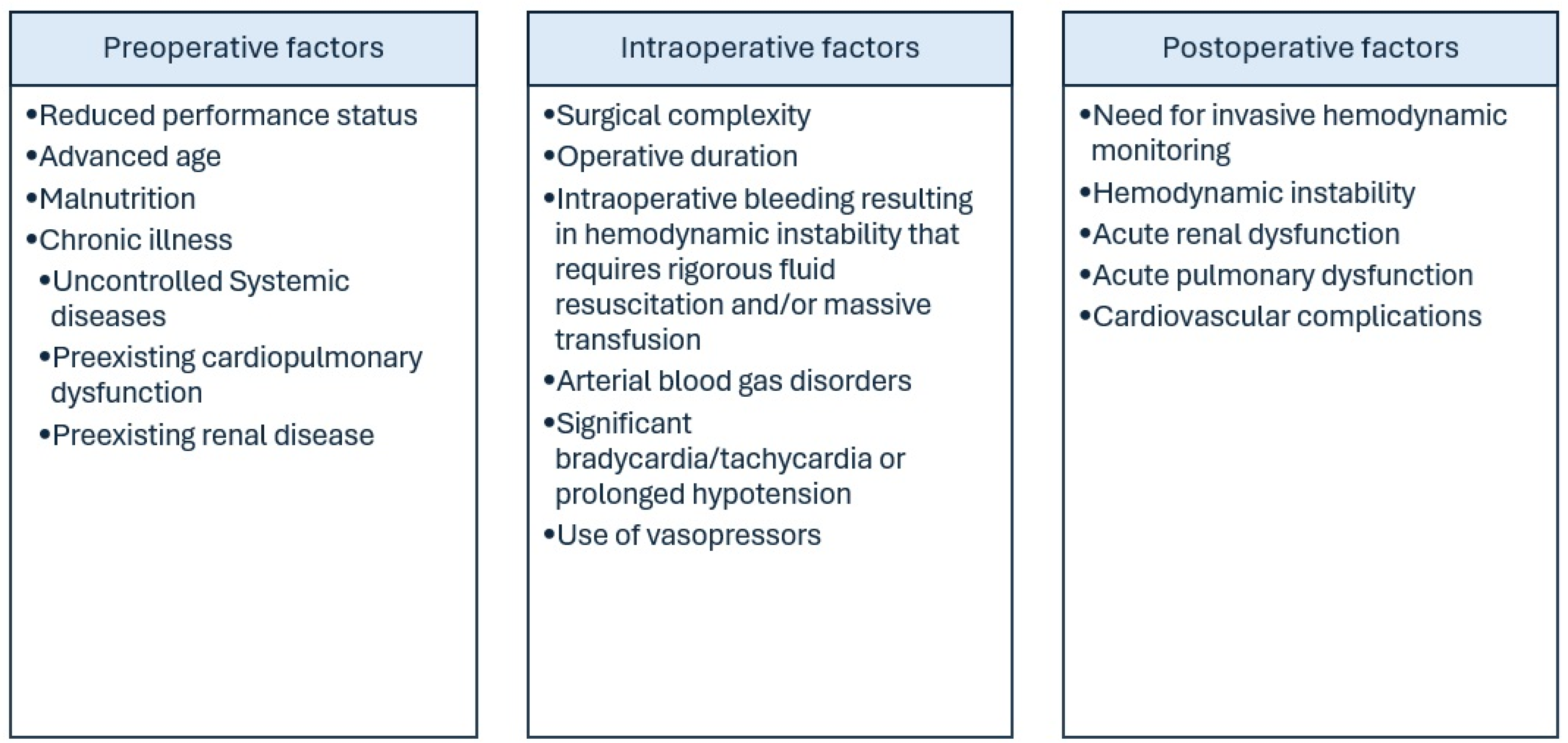
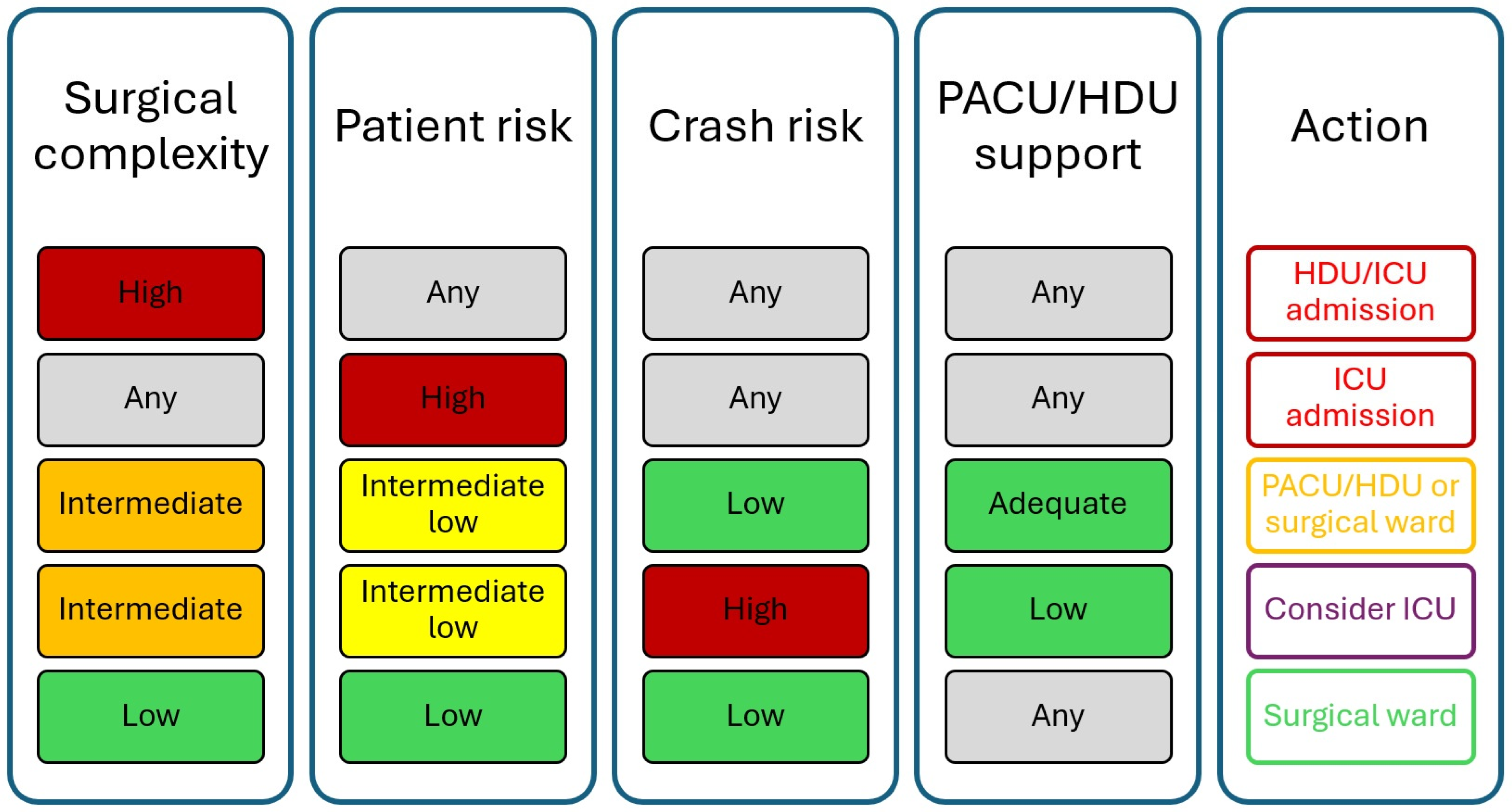
5. Criteria for Admission and Discharge from Critical Care Facilities
6. Clinical Scenarios of Intensive Care Support in Gynecologic Malignancies
6.1. Ovarian Cancer
6.2. Endometrial Cancer
6.3. Vulvar Cancer and Cervical Cancer
6.4. Cost-Effectiveness of Critical Care Support
7. Special Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Priyadarshini, S.; Swain, P.K.; Agarwal, K.; Jena, D.; Padhee, S. Trends in gynecological cancer incidence, mortality, and survival among elderly women: A SEER study. Aging Med. 2024, 7, 179–188. [Google Scholar] [CrossRef]
- Ør Knudsen, A.; Schledermann, D.; Nyvang, G.B.; Mogensen, O.; Herrstedt, J. Trends in gynecologic cancer among elderly women in Denmark, 1980–2012. Acta Oncol. 2016, 55 (Suppl. S1), 65–73. [Google Scholar] [CrossRef]
- Dumas, L.; Ring, A.; Butler, J.; Kalsi, T.; Harari, D.; Banerjee, S. Improving outcomes for older women with gynaecological malignancies. Cancer Treat. Rev. 2016, 50, 99–108. [Google Scholar] [CrossRef]
- Ogle, K.S.; Swanson, G.M.; Woods, N.; Azzouz, F. Cancer and comorbidity: Redefining chronic diseases. Cancer 2000, 88, 653–663. [Google Scholar] [CrossRef]
- Bell, C.F.; Lei, X.; Haas, A.; Baylis, R.A.; Gao, H.; Luo, L.; Giordano, S.H.; Wehner, M.R.; Nead, K.T.; Leeper, N.J. Risk of Cancer After Diagnosis of Cardiovascular Disease. JACC CardioOncol. 2023, 5, 431–440. [Google Scholar] [CrossRef]
- Kitson, S.J.; Lindsay, J.; Sivalingam, V.N.; Lunt, M.; Ryan, N.A.J.; Edmondson, R.J.; Rutter, M.K.; Crosbie, E.J. The unrecognized burden of cardiovascular risk factors in women newly diagnosed with endometrial cancer: A prospective case control study. Gynecol. Oncol. 2018, 148, 154–160. [Google Scholar] [CrossRef]
- Anderson, C.; Olshan, A.F.; Bae-Jump, V.L.; Brewster, W.R.; Lund, J.L.; Nichols, H.B. Cardiovascular disease diagnoses among older women with endometrial cancer. Gynecol. Oncol. 2022, 167, 51–57. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Yuan, Y.-X.; Xia, M.-Y.; Li, Y.; Yang, Y.; Wang, S.-N.; Meng, X.-Z.; Sun, M.-Y.; Wang, N. Cardiovascular mortality risk in patients with ovarian cancer: A population-based study. J. Ovarian Res. 2024, 17, 88. [Google Scholar] [CrossRef]
- Shinn, E.H.; Lenihan, D.J.; Urbauer, D.L.; Basen-Engquist, K.M.; Valentine, A.; Palmero, L.; Woods, M.L.; Patel, P.; Nick, A.M.; Shahzad, M.M.; et al. Impact of cardiovascular comorbidity on ovarian cancer mortality. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2102–2109. [Google Scholar] [CrossRef]
- Al-Badawi, I.A.; Alomar, O.; Alsehaimi, S.O.; Jamjoom, M.Z.; Abdulmalik, N.A.; Bukhari, I.A.; Alyousef, A.; Alabdrabalamir, S.; Baradwan, S.; Sayasneh, A.; et al. Cardiovascular Mortality in Ovarian Cancer Patients: An Analysis of Patient Characteristics Using the SEER Database. Medicina 2023, 59, 1476. [Google Scholar] [CrossRef]
- Machida, H.; Hom, M.S.; Maeda, M.; Yeo, J.J.; Ghattas, C.S.; Grubbs, B.H.; Matsuo, K. Signs and Symptoms of Venous Thromboembolism and Survival Outcome of Endometrial Cancer. Int. J. Gynecol. Cancer 2016, 26, 924–932. [Google Scholar] [CrossRef]
- Soisson, S.; Ganz, P.A.; Gaffney, D.; Rowe, K.; Snyder, J.; Wan, Y.; Deshmukh, V.; Newman, M.; Fraser, A.; Smith, K.; et al. Long-term Cardiovascular Outcomes Among Endometrial Cancer Survivors in a Large, Population-Based Cohort Study. J. Natl. Cancer Inst. 2018, 110, 1342–1351. [Google Scholar] [CrossRef]
- Parashar, S.; Akhter, N.; Paplomata, E.; Elgendy, I.Y.; Upadhyaya, D.; Scherrer-Crosbie, M.; Okwuosa, T.M.; Sanghani, R.M.; Chalas, E.; Lindley, K.J.; et al. Cancer Treatment-Related Cardiovascular Toxicity in Gynecologic Malignancies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2023, 5, 159–173. [Google Scholar] [CrossRef]
- Oikonomou, E.; Anastasiou, Μ.; Siasos, G.; Androulakis, E.; Psyrri, A.; Toutouzas, K.; Tousoulis, D. Cancer Therapeutics-Related Cardiovascular Complications. Mechanisms, Diagnosis and Treatment. Curr. Pharm. Des. 2018, 24, 4424–4435. [Google Scholar] [CrossRef]
- STARSurg Collaborative; The EuroSurg Collaborative. Impact of postoperative cardiovascular complications on 30-day mortality after major abdominal surgery: An international prospective cohort study. Anaesthesia 2024, 79, 715–724. [Google Scholar] [CrossRef]
- Puelacher, C.; Lurati Buse, G.; Seeberger, D.; Sazgary, L.; Marbot, S.; Lampart, A.; Espinola, J.; Kindler, C.; Hammerer, A.; Seeberger, E.; et al. Perioperative Myocardial Injury After Noncardiac Surgery: Incidence, Mortality, and Characterization. Circulation 2018, 137, 1221–1232. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Szczeklik, W. Myocardial injury after non-cardiac surgery: Diagnosis and management. Eur. Heart J. 2020, 41, 3083–3091. [Google Scholar] [CrossRef]
- Bashar, H.; Kobo, O.; Curzen, N.; Mamas, M.A. Association of myocardial injury with adverse long-term survival among cancer patients. Eur. J. Prev. Cardiol. 2024, zwae116. [Google Scholar] [CrossRef]
- Landesberg, G.; Beattie, W.S.; Mosseri, M.; Jaffe, A.S.; Alpert, J.S. Perioperative myocardial infarction. Circulation 2009, 119, 2936–2944. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Hiro, T.; Fujii, T.; Hashimoto, G.; Fujimura, T.; Yamada, J.; Okamura, T.; Matsuzaki, M. Localized elevation of shear stress is related to coronary plaque rupture: A 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J. Am. Coll. Cardiol. 2008, 51, 645–650. [Google Scholar] [CrossRef]
- Bursi, F.; Babuin, L.; Barbieri, A.; Politi, L.; Zennaro, M.; Grimaldi, T.; Rumolo, A.; Gargiulo, M.; Stella, A.; Modena, M.G.; et al. Vascular surgery patients: Perioperative and long-term risk according to the ACC/AHA guidelines, the additive role of post-operative troponin elevation. Eur. Heart J. 2005, 26, 2448–2456. [Google Scholar] [CrossRef]
- Hasselgren, E.; Hertzberg, D.; Camderman, T.; Björne, H.; Salehi, S. Perioperative fluid balance and major postoperative complications in surgery for advanced epithelial ovarian cancer. Gynecol. Oncol. 2021, 161, 402–407. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.; Yoo, K.; Kim, M.; Kang, S.S.; Kwon, Y.S.; Lee, J.J. Prediction of Postoperative Pulmonary Edema Risk Using Machine Learning. J. Clin. Med. 2023, 12, 1804. [Google Scholar] [CrossRef]
- Sanaiha, Y.; Juo, Y.Y.; Aguayo, E.; Seo, Y.J.; Dobaria, V.; Ziaeian, B.; Benharash, P. Incidence and trends of cardiac complications in major abdominal surgery. Surgery 2018, 164, 539–545. [Google Scholar] [CrossRef]
- Basaran, D.; Boerner, T.; Suhner, J.; Sassine, D.; Liu, Y.; Grisham, R.N.; Tew, W.P.; Gardner, G.J.; Zivanovic, O.; Sonoda, Y.; et al. Risk of venous thromboembolism in ovarian cancer patients receiving neoadjuvant chemotherapy. Gynecol. Oncol. 2021, 163, 36–40. [Google Scholar] [CrossRef]
- Chokshi, S.K.; Gaughan, J.P.; Krill, L. Incidence and patient characteristics of venous thromboembolism during neoadjuvant chemotherapy for ovarian cancer. J. Thromb. Thrombolysis 2022, 53, 202–207. [Google Scholar] [CrossRef]
- Pin, S.; Mateshaytis, J.; Ghosh, S.; Batuyong, E.; Easaw, J.C. Risk factors for venous thromboembolism in endometrial cancer. Curr. Oncol. 2020, 27, 198–203. [Google Scholar] [CrossRef]
- Tsai, S.J.; Ruan, Y.X.; Lee, C.C.; Lee, M.S.; Chiou, W.Y.; Lin, H.Y.; Hsu, F.C.; Su, Y.C.; Hung, S.K. The incidence of venous thromboembolism in cervical cancer: A nationwide population-based study. BMC Res. Notes 2012, 5, 316. [Google Scholar] [CrossRef]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef]
- Oxley, S.G.; Achampong, Y.A.; Sambandan, N.; Hughes, D.J.; Thomas, M.; Lockley, M.; Olaitan, A. Venous thromboembolism in women with ovarian cancer undergoing neoadjuvant chemotherapy prior to cytoreductive surgery: A retrospective study. Acta Obstet. Gynecol. Scand. 2021, 100, 2091–2096. [Google Scholar] [CrossRef]
- Greco, P.S.; Bazzi, A.A.; McLean, K.; Reynolds, R.K.; Spencer, R.J.; Johnston, C.M.; Liu, J.R.; Uppal, S. Incidence and Timing of Thromboembolic Events in Patients With Ovarian Cancer Undergoing Neoadjuvant Chemotherapy. Obstet. Gynecol. 2017, 129, 979–985. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Haidopoulos, D.; Tzortzis, A.S.; Antonopoulos, I.; Thomakos, N.; Rodolakis, A. The impact of waiting intervals on survival outcomes of patients with endometrial cancer: A systematic review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 1–6. [Google Scholar] [CrossRef]
- Mei, R.; Wang, G.; Chen, R.; Wang, H. The ICU-venous thromboembolism score and tumor grade can predict inhospital venous thromboembolism occurrence in critical patients with tumors. World J. Surg. Oncol. 2022, 20, 245. [Google Scholar] [CrossRef]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef]
- Barrantes, F.; Tian, J.; Vazquez, R.; Amoateng-Adjepong, Y.; Manthous, C.A. Acute kidney injury criteria predict outcomes of critically ill patients. Crit. Care Med. 2008, 36, 1397–1403. [Google Scholar] [CrossRef]
- Abelha, F.J.; Botelho, M.; Fernandes, V.; Barros, H. Determinants of postoperative acute kidney injury. Crit. Care 2009, 13, R79. [Google Scholar] [CrossRef]
- Kheterpal, S.; Tremper, K.K.; Englesbe, M.J.; O’Reilly, M.; Shanks, A.M.; Fetterman, D.M.; Rosenberg, A.L.; Swartz, R.D. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007, 107, 892–902. [Google Scholar] [CrossRef]
- Acute Kidney Injury (AKI). Available online: https://kdigo.org/guidelines/acute-kidney-injury/ (accessed on 11 July 2025).
- Boyer, N.; Eldridge, J.; Prowle, J.R.; Forni, L.G. Postoperative Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2022, 17, 1535–1545. [Google Scholar] [CrossRef]
- O’Connor, M.E.; Hewson, R.W.; Kirwan, C.J.; Ackland, G.L.; Pearse, R.M.; Prowle, J.R. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br. J. Surg. 2017, 104, 868–876. [Google Scholar] [CrossRef]
- Long, T.E.; Helgason, D.; Helgadottir, S.; Palsson, R.; Gudbjartsson, T.; Sigurdsson, G.H.; Indridason, O.S.; Sigurdsson, M.I. Acute Kidney Injury After Abdominal Surgery: Incidence, Risk Factors, and Outcome. Anesth. Analg. 2016, 122, 1912–1920. [Google Scholar] [CrossRef]
- Prowle, J.R.; Forni, L.G.; Bell, M.; Chew, M.S.; Edwards, M.; Grams, M.E.; Grocott, M.P.W.; Liu, K.D.; McIlroy, D.; Murray, P.T.; et al. Postoperative acute kidney injury in adult non-cardiac surgery: Joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative. Nat. Rev. Nephrol. 2021, 17, 605–618. [Google Scholar] [CrossRef]
- Kheterpal, S.; Tremper, K.K.; Heung, M.; Rosenberg, A.L.; Englesbe, M.; Shanks, A.M.; Campbell, D.A., Jr. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: Results from a national data set. Anesthesiology 2009, 110, 505–515. [Google Scholar] [CrossRef]
- Habas, E.; Akbar, R.; Farfar, K.; Arrayes, N.; Habas, A.; Rayani, A.; Alfitori, G.; Habas, E.; Magassabi, Y.; Ghazouani, H.; et al. Malignancy diseases and kidneys: A nephrologist prospect and updated review. Medicine 2023, 102, e33505. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Soiffer, R.J.; Magee, C.C. Renal Failure Associated with Cancer and Its Treatment: An Update. J. Am. Soc. Nephrol. 2005, 16, 151–161. [Google Scholar] [CrossRef]
- Salahudeen, A.K.; Doshi, S.M.; Pawar, T.; Nowshad, G.; Lahoti, A.; Shah, P. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin. J. Am. Soc. Nephrol. 2013, 8, 347–354. [Google Scholar] [CrossRef]
- Lameire, N.H.; Flombaum, C.D.; Moreau, D.; Ronco, C. Acute renal failure in cancer patients. Ann. Med. 2005, 37, 13–25. [Google Scholar] [CrossRef]
- Naganuma, M.; Motooka, Y.; Sasaoka, S.; Hatahira, H.; Hasegawa, S.; Fukuda, A.; Nakao, S.; Shimada, K.; Hirade, K.; Mori, T.; et al. Analysis of adverse events of renal impairment related to platinum-based compounds using the Japanese Adverse Drug Event Report database. SAGE Open Med. 2018, 6, 2050312118772475. [Google Scholar] [CrossRef]
- Rabah, S.O. Acute Taxol nephrotoxicity: Histological and ultrastructural studies of mice kidney parenchyma. Saudi J. Biol. Sci. 2010, 17, 105–114. [Google Scholar] [CrossRef]
- Superfin, D.; Iannucci, A.A.; Davies, A.M. Commentary: Oncologic drugs in patients with organ dysfunction: A summary. Oncologist 2007, 12, 1070–1083. [Google Scholar] [CrossRef]
- Gupta, S.; Hanna, P.E.; Ouyang, T.; Yamada, K.S.; Sawtell, R.; Wang, Q.; Katz-Agranov, N.; Feghali, L.; Krasner, C.N.; Bouberhan, S.; et al. Kidney function in patients with ovarian cancer treated with poly (ADP-ribose) polymerase (PARP) inhibitors. J. Natl. Cancer Inst. 2023, 115, 831–837. [Google Scholar] [CrossRef]
- Wu, S.; Kim, C.; Baer, L.; Zhu, X. Bevacizumab increases risk for severe proteinuria in cancer patients. J. Am. Soc. Nephrol. 2010, 21, 1381–1389. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Bellos, I.; Thomakos, N.; Haidopoulos, D.; Perrea, D.N.; Kontzoglou, K.; Daskalakis, G.; Rodolakis, A. Survival outcomes of patients with cervical cancer and accompanying hydronephrosis: A systematic review of the literature. Oncol. Rev. 2019, 13, 387. [Google Scholar] [CrossRef]
- Yang, C.K.; Teng, A.; Lee, D.Y.; Rose, K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J. Surg. Res. 2015, 198, 441–449. [Google Scholar] [CrossRef]
- Alvarez, M.P.; Samayoa-Mendez, A.X.; Naglak, M.C.; Yuschak, J.V.; Murayama, K.M. Risk Factors for Postoperative Unplanned Intubation: Analysis of a National Database. Am. Surg. 2015, 81, 820–825. [Google Scholar] [CrossRef]
- Mannino, D.M.; Ford, E.S.; Redd, S.C. Obstructive and restrictive lung disease and functional limitation: Data from the Third National Health and Nutrition Examination. J. Intern. Med. 2003, 254, 540–547. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Zheng, X.; Peng, J.; Chen, Y.; Yu, K.; Yang, Y.; Wang, X.; Yang, X.; Qian, J.; et al. Deaths from COPD in patients with cancer: A population-based study. Aging 2021, 13, 12641–12659. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Gao, C.; Zhou, Y.; Xie, Y. Postoperative pulmonary complications and outcomes in cytoreductive surgery for ovarian cancer: A propensity-matched analysis. BMC Anesthesiol. 2022, 22, 120. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Feroussis, L.; Papadatou, K.; Vlachos, D.E.; Aggelou, K.; Rodolakis, I.; Alexakis, N.; Bramis, K.; Daskalakis, G.; Thomakos, N.; et al. Diaphragmatic stripping in epithelial ovarian cancer at first diagnosis: Impact on morbidity and survival outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 299, 225–230. [Google Scholar] [CrossRef]
- Hudry, D.; Longuepée-Bourdon, L.; Scarpelli, E.; Barthoulot, M.; Risbourg, S.; Duchatelet, M.; Codt, M.D.; Lefebvre, M.; Ovaere, C.; Gomez, C.M.; et al. #444 Verification of the association of the surgical complexity score (aletti score) with postoperative complications in a cohort of patients operated for advanced ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, A280–A281. [Google Scholar] [CrossRef]
- Haidopoulos, D.; Pergialiotis, V.; Zachariou, E.; Sapantzoglou, I.; Thomakos, N.; Stamatakis, E.; Alexakis, N. Maximal Effort Cytoreduction in Epithelial Ovarian Cancer: Perioperative Complications and Survival Outcomes from a Retrospective Cohort. J. Clin. Med. 2023, 12, 622. [Google Scholar] [CrossRef]
- So, K.A.; Shim, S.H.; Lee, S.J.; Kim, T.J. Surgical Treatment Outcomes of Gynecologic Cancer in Older Patients: A Retrospective Study. J. Clin. Med. 2023, 12, 2518. [Google Scholar] [CrossRef]
- Luzarraga-Aznar, A.; Teixeira, N.; Luna-Guibourg, R.; Español, P.; Soler-Moreno, C.; Rovira, R. Surgical treatment in older patients with endometrial cancer: A retrospective study. Surg. Oncol. 2022, 44, 101852. [Google Scholar] [CrossRef]
- Morton, M.; Patterson, J.; Sciuva, J.; Perni, J.; Backes, F.; Nagel, C.; O’Malley, D.M.; Chambers, L.M. Malnutrition, sarcopenia, and cancer cachexia in gynecologic cancer. Gynecol. Oncol. 2023, 175, 142–155. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, L.; Liu, T.; Ding, J.S.; Zhang, X.; Song, M.M.; Wang, Z.W.; Ge, Y.Z.; Hu, C.L.; Li, X.R.; et al. Prevalence and Prognostic Value of Malnutrition Among Elderly Cancer Patients Using Three Scoring Systems. Front. Nutr. 2021, 8, 738550. [Google Scholar] [CrossRef]
- Horner, W.; Peng, K.; Pleasant, V.; Brackmann, M.; Ebott, J.; Gutfreund, R.; McLean, K.; Reynolds, R.K.; Uppal, S. Trends in surgical complexity and treatment modalities utilized in the management of ovarian cancer in an era of neoadjuvant chemotherapy. Gynecol. Oncol. 2019, 154, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Emerson, P.; Flabouris, A.; Thomas, J.; Fernando, J.; Senthuran, S.; Knowles, S.; Hammond, N.; Sundararajan, K. Intensive care utilisation after elective surgery in Australia and New Zealand: A point prevalence study. Crit. Care Resusc. 2024, 26, 1–7. [Google Scholar] [CrossRef]
- The International Surgical Outcomes Study Group. Global patient outcomes after elective surgery: Prospective cohort study in 27 low-, middle- and high-income countries. Br. J. Anaesth. 2016, 117, 601–609. [Google Scholar] [CrossRef]
- Zammert, M.; Carpenter, A.J.; Zwischenberger, J.B.; Sade, R.M. Surgeon or Intensivist: Who Should Be in Charge of Postoperative Intensive Care? Ann. Thorac. Surg. 2023, 116, 679–683. [Google Scholar] [CrossRef]
- Penkoske, P.A.; Buchman, T.G. The Relationship between the Surgeon and the Intensivist in the Surgical Intensive Care Unit. Surg. Clin. N. Am. 2006, 86, 1351–1357. [Google Scholar] [CrossRef]
- Hui, D.S.; Eastman, A.L.; Lang, J.L.; Frankel, H.L.; O’Keeffe, T. A Survey of Critical Care Training Amongst Surgical Residents: Will They be Ready? J. Surg. Res. 2010, 163, 132–141. [Google Scholar] [CrossRef]
- Turner, C.J.; Haas, B.; Lee, C.; Brar, S.; Detsky, M.E.; Munshi, L. Improving Communication Between Surgery and Critical Care Teams: Beyond the Handover. Am. J. Crit. Care 2018, 27, 392–397. [Google Scholar] [CrossRef]
- Oh, T.K.; Song, I.A.; Jeon, Y.T. Admission to the surgical intensive care unit during intensivist coverage is associated with lower incidence of postoperative acute kidney injury and shorter ventilator time. J. Anesth. 2019, 33, 647–655. [Google Scholar] [CrossRef]
- Tak Kyu, O.; Ji, E.; Ahn, S.; Kim, D.J.; Song, I.A. Admission to surgical intensive care unit in time with intensivist coverage and its association with postoperative 30-day mortality: The role of intensivists in a surgical intensive care unit. Anaesth. Crit. Care Pain Med. 2019, 38, 259–263. [Google Scholar] [CrossRef]
- Yesilyaprak, T.; Demir Korkmaz, F. The relationship between surgical intensive care unit nurses’ patient safety culture and adverse events. Nurs. Crit. Care 2023, 28, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Urisman, T.; Garcia, A.; Harris, H.W. Impact of surgical intensive care unit interdisciplinary rounds on interprofessional collaboration and quality of care: Mixed qualitative-quantitative study. Intensive Crit. Care Nurs. 2018, 44, 18–23. [Google Scholar] [CrossRef]
- Whitaker Chair, D.K.; Booth, H.; Clyburn, P.; Harrop-Griffiths, W.; Hosie, H.; Kilvington, B.; Macmahon, M.; Smedley, P.; Verma, R. Immediate post-anaesthesia recovery 2013: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2013, 68, 288–297. [Google Scholar] [CrossRef]
- Ding, D.; Ishag, S. Aldrete Scoring System. In StatPearls; Updated 8 July 2023; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK594237/ (accessed on 3 May 2025).
- Flanigan, M.R.; Bell, S.G.; Donovan, H.S.; Zhao, J.; Holder-Murray, J.M.; Esper, S.A.; Ficerai-Garland, G.; Taylor, S.E. Variables impacting prolonged post-anesthesia care unit length of stay in gynecologic cancer patients in the era of same day minimally invasive hysterectomy. Gynecol. Oncol. 2024, 186, 211–215. [Google Scholar] [CrossRef]
- Bing-Hua, Y.U. Delayed admission to intensive care unit for critically surgical patients is associated with increased mortality. Am. J. Surg. 2014, 208, 268–274. [Google Scholar] [CrossRef]
- Reuter, S.; Schmalfeldt, B.; Haas, S.A.; Zapf, A.; Cevirme, S.; Prieske, K.; Wölber, L.; Müller, V.; Zöllner, C.; Jaeger, A. Impact of Introducing a PACU24 Concept on the Perioperative Outcome of Patients with Advanced Ovarian Cancer Treated with Cytoreductive Surgery. Geburtshilfe Frauenheilkd. 2023, 83, 1022–1030. [Google Scholar] [CrossRef]
- Koning, N.J.; Lokin, J.L.C.; Roovers, L.; Kallewaard, J.W.; van Harten, W.H.; Kalkman, C.J.; Preckel, B. Introduction of a Post-Anaesthesia Care Unit in a Teaching Hospital Is Associated with a Reduced Length of Hospital Stay in Noncardiac Surgery: A Single-Centre Interrupted Time Series Analysis. J. Clin. Med. 2024, 13, 534. [Google Scholar] [CrossRef]
- Kastrup, M.; Seeling, M.; Barthel, S.; Bloch, A.; le Claire, M.; Spies, C.; Scheller, M.; Braun, J. Effects of intensivist coverage in a post-anaesthesia care unit on surgical patients’ case mix and characteristics of the intensive care unit. Crit. Care 2012, 16, R126. [Google Scholar] [CrossRef]
- Jones, H.J.; Coggins, R.; Lafuente, J.; de Cossart, L. Value of a surgical high-dependency unit. Br. J. Surg. 1999, 86, 1578–1582. [Google Scholar] [CrossRef]
- Lefstad, L.; Rist, M. The Need for Treatment in a High Dependency Unit Following Major Abdominal Surgery. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2015. Available online: http://hdl.handle.net/11250/2417763 (accessed on 3 May 2025).
- McIlroy, D.R.; Coleman, B.D.; Myles, P.S. Outcomes following a shortage of high dependency unit beds for surgical patients. Anaesth. Intensive Care 2006, 34, 457–463. [Google Scholar] [CrossRef]
- Prin, M.; Harrison, D.; Rowan, K.; Wunsch, H. Epidemiology of admissions to 11 stand-alone high-dependency care units in the UK. Intensive Care Med. 2015, 41, 1903–1910. [Google Scholar] [CrossRef]
- Wunsch, H.; Harrison, D.A.; Jones, A.; Rowan, K. The impact of the organization of high-dependency care on acute hospital mortality and patient flow for critically ill patients. Am. J. Respir. Crit. Care Med. 2015, 191, 186–193. [Google Scholar] [CrossRef]
- Thomakos, N.; Zacharakis, D.; Rodolakis, A.; Zagouri, F.; Papadimitriou, C.A.; Bamias, A.; Dimopoulos, M.A.; Haidopoulos, D.; Vlahos, G.; Antsaklis, A. Gynecologic oncology patients in the surgical high dependency unit: An analysis of indications. Arch. Gynecol. Obstet. 2014, 290, 335–339. [Google Scholar] [CrossRef]
- Knoedler, S.; Matar, D.Y.; Friedrich, S.; Knoedler, L.; Haug, V.; Hundeshagen, G.; Kauke-Navarro, M.; Kneser, U.; Pomahac, B.; Orgill, D.P.; et al. The surgical patient of yesterday, today, and tomorrow-a time-trend analysis based on a cohort of 8.7 million surgical patients. Int. J. Surg. 2023, 109, 2631–2640. [Google Scholar] [CrossRef]
- Dhillon, N.K.; Ko, A.; Smith, E.J.T.; Kharabi, M.; Castongia, J.; Nurok, M.; Gewertz, B.L.; Ley, E.J. Potentially Avoidable Surgical Intensive Care Unit Admissions and Disposition Delays. JAMA Surg. 2017, 152, 1015–1022. [Google Scholar] [CrossRef]
- Pepin, K.; Bregar, A.; Davis, M.; Melamed, A.; Hinchcliff, E.; Gockley, A.; Horowitz, N.; Del Carmen, M.G. Intensive care admissions among ovarian cancer patients treated with primary debulking surgery and neoadjuvant chemotherapy-interval debulking surgery. Gynecol. Oncol. 2017, 147, 612–616. [Google Scholar] [CrossRef]
- Davidovic-Grigoraki, M.; Thomakos, N.; Haidopoulos, D.; Vlahos, G.; Rodolakis, A. Do critical care units play a role in the management of gynaecological oncology patients? The contribution of gynaecologic oncologist in running critical care units. Eur. J. Cancer Care 2017, 26, e12438. [Google Scholar] [CrossRef]
- Ruskin, R.; Urban, R.R.; Sherman, A.E.; Chen, L.L.; Powell, C.B.; Burkhardt, D.H., 3rd; Chen, L.M. Predictors of intensive care unit utilization in gynecologic oncology surgery. J. Gynecol. Cancer 2011, 21, 1336–1342. [Google Scholar] [CrossRef]
- Krawczyk, P.; Trojnarska, D.; Baran, R.; Lonc, T.; Swistek, R.; Tyszecki, P.; Jach, R. Postoperative gynecologic oncology admissions to intensive care unit in the tertiary care center: An eight-year retrospective study. Ginekol. Pol. 2022, 94, 599–604. [Google Scholar] [CrossRef]
- Abbas, F.M.; Sert, M.B.; Rosenshein, N.B.; Zahyrak, M.L.; Currie, J.L. Gynecologic oncology patients in the surgical ICU. Impact on outcome. J. Reprod. Med. 1997, 42, 173–178. [Google Scholar]
- Gao, W.; Jin, J. Expanding the scope of prehabilitation: Reducing critical illness weakness across elective surgical patients scheduled for postoperative ICU care. Crit. Care 2024, 28, 3. [Google Scholar] [CrossRef]
- Sompratthana, T.; Phoolcharoen, N.; Schmeler, K.M.; Lertkhachonsuk, R. End-of-life symptoms and interventions among women with gynecologic cancers in a tertiary-care hospital in Thailand. Int. J. Gynecol. Cancer 2019, 29, 951–955. [Google Scholar] [CrossRef]
- Stein, R.V.; Aronson, L.; Hendriks, F.; Houwink, A.; Schutte, P.; Kroon, C.D.; Sonke, G.; Driel, W.V. EP297/#520 Is routine admission to a critical care unit following HIPEC for ovarian cancer necessary? Int. J. Gynecol. Cancer 2022, 32, A173. [Google Scholar] [CrossRef]
- Koutsoukou, A. Admission of critically ill patients with cancer to the ICU: Many uncertainties remain. ESMO Open 2017, 2, e000105. [Google Scholar] [CrossRef]
- Nitecki, R.; Bercow, A.S.; Gockley, A.A.; Lee, H.; Penson, R.T.; Growdon, W.B. Clinical trial participation and aggressive care at the end of life in patients with ovarian cancer. Int. J. Gynecol. Cancer 2020, 30, 201–206. [Google Scholar] [CrossRef]
- Lee, M.T.; Hu, P.; Hsi, S.C.; Liu, K.Y.; Chao, H.M.; Chen, Y.Q. Mortality rates under the care of junior and senior surgery residents in a surgical intensive care unit/neurologic intensive care unit: A 5-year retrospective cohort study at Taoyuan Armed Forces General Hospital. J. Crit. Care 2008, 23, 550–555. [Google Scholar] [CrossRef]
- Park, C.M.; Chun, H.K.; Lee, D.S.; Jeon, K.; Suh, G.Y.; Jeong, J.C. Impact of a surgical intensivist on the clinical outcomes of patients admitted to a surgical intensive care unit. Ann. Surg. Treat. Res. 2014, 86, 319–324. [Google Scholar] [CrossRef][Green Version]
- Frountzas, M.; Liatsou, E.; Schizas, D.; Pergialiotis, V.; Vailas, M.; Kritikos, N.; Toutouzas, K.G. The impact of surgery delay on survival of resectable pancreatic cancer: A systematic review of observational studies. Surg. Oncol. 2022, 45, 101855. [Google Scholar] [CrossRef]
- Zouzoulas, D.; Karalis, T.; Sofianou, I.; Anthoulakis, C.; Tzika, K.; Zafrakas, M.; Timotheadou, E.; Grimbizis, G.; Tsolakidis, D. The Impact of Treatment Delay on Endometrial and Ovarian Cancer Patients: A Systematic Review. Cancers 2025, 21, 2076. [Google Scholar] [CrossRef]
- Dai, Z.; Perera, S.C.; Wang, J.-J.; Mangla, S.K.; Li, G. Elective surgery scheduling under uncertainty in demand for intensive care unit and inpatient beds during epidemic outbreaks. Comput. Ind. Eng. 2023, 176, 108893. [Google Scholar] [CrossRef]
- Nates, J.L.; Nunnally, M.; Kleinpell, R.; Blosser, S.; Goldner, J.; Birriel, B.; Fowler, C.S.; Byrum, D.; Miles, W.S.; Bailey, H.; et al. ICU Admission, Discharge, and Triage Guidelines: A Framework to Enhance Clinical Operations, Development of Institutional Policies, and Further Research. Crit. Care Med. 2016, 44, 1553–1602. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Lone, N.I.; Bagshaw, S.M. Admission to intensive care unit after major surgery. Intensive Care Med. 2023, 49, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.; Eddlestone, J.; Lees, N. The Higher Risk General Surgical Patient—Towards Improved Care for a Forgotten Group; Royal College of Surgeons of England: London, UK, 2011; Available online: https://www.rcseng.ac.uk/-/media/files/rcs/library-and-publications/non-journal-publications/higher_risk_surgical_patient_2011_web.pdf (accessed on 30 July 2024).
- Linke, G.R.; Mieth, M.; Hofer, S.; Trierweiler-Hauke, B.; Weitz, J.; Martin, E.; Büchler, M.W. Surgical intensive care unit—Essential for good outcome in major abdominal surgery? Langenbecks Arch. Surg. 2011, 396, 417–428. [Google Scholar] [CrossRef]
- Gitas, G.; Proppe, L.; Baum, S.; Kruggel, M.; Rody, A.; Tsolakidis, D.; Zouzoulas, D.; Laganà, A.S.; Guenther, V.; Freytag, D.; et al. A risk factor analysis of complications after surgery for vulvar cancer. Arch. Gynecol. Obstet. 2021, 304, 511–519. [Google Scholar] [CrossRef]
- Zheng, B.; Reardon, P.M.; Fernando, S.M.; Webber, C.; Thavorn, K.; Thompson, L.H.; Tanuseputro, P.; Munshi, L.; Kyeremanteng, K. Costs and Outcomes of Patients Admitted to the Intensive Care Unit With Cancer. J. Intensive Care Med. 2021, 36, 203–210. [Google Scholar] [CrossRef]
- Nazer, L.H.; Lopez-Olivo, M.A.; Brown, A.R.; Cuenca, J.A.; Sirimaturos, M.; Habash, K.; AlQadheeb, N.; May, H.; Milano, V.; Taylor, A.; et al. A Systematic Review and Meta-Analysis Evaluating Geographical Variation in Outcomes of Cancer Patients Treated in ICUs. Crit. Care Explor. 2022, 4, e0757. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Divatia, J.V. A prospective audit of costs of intensive care in cancer patients in India. Indian J. Crit. Care Med. 2013, 17, 292–297. [Google Scholar] [CrossRef][Green Version]
- Jo, M.; Lee, Y.; Kim, T. Medical care costs at the end of life among older adults with cancer: A national health insurance data-based cohort study. BMC Palliat. Care 2023, 22, 76. [Google Scholar] [CrossRef]
- Inoue, S.; Nakanishi, N.; Amaya, F.; Fujinami, Y.; Hatakeyama, J.; Hifumi, T.; Iida, Y.; Kawakami, D.; Kawai, Y.; Kondo, Y.; et al. Post-intensive care syndrome: Recent advances and future directions. Acute Med. Surg. 2024, 11, e929. [Google Scholar] [CrossRef]
- Siesage, K.; Joelsson-Alm, E.; Schandl, A.; Karlsson, E. Extended physiotherapy after Intensive Care Unit (ICU) stay: A prospective pilot study with a before and after design. Physiother. Theory Pract. 2024, 40, 1232–1240. [Google Scholar] [CrossRef]
- Sommers, J.; Engelbert, R.H.; Dettling-Ihnenfeldt, D.; Gosselink, R.; Spronk, P.E.; Nollet, F.; van der Schaaf, M. Physiotherapy in the intensive care unit: An evidence-based, expert driven, practical statement and rehabilitation recommendations. Clin. Rehabil. 2015, 29, 1051–1063. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Walter, A.C.; Ruskin, R.; Ding, K.; Moore, K.N. Post-intensive care unit syndrome in gynecologic oncology patients. Support. Care Cancer 2016, 24, 4627–4632. [Google Scholar] [CrossRef]
- Sharma, G.; Maben, E.V.S.; Kotian, M.S.; Ganaraja, B. Psychological evaluation of patients in critical care/intensive care unit and patients admitted in wards. J. Clin. Diagn. Res. 2014, 8, WC01–WC03. [Google Scholar] [CrossRef]
- Miralpeix, E.; Nick, A.M.; Meyer, L.A.; Cata, J.; Lasala, J.; Mena, G.E.; Gottumukkala, V.; Iniesta-Donate, M.; Salvo, G.; Ramirez, P.T. A call for new standard of care in perioperative gynecologic oncology practice: Impact of enhanced recovery after surgery (ERAS) programs. Gynecol. Oncol. 2016, 141, 371–378. [Google Scholar] [CrossRef]
- Boitano, T.K.L.; Smith, H.J.; Rushton, T.; Johnston, M.C.; Lawson, P.; Leath, C.A., 3rd; Xhaja, A.; Guthrie, M.P.; Straughn, J.M., Jr. Impact of enhanced recovery after surgery (ERAS) protocol on gastrointestinal function in gynecologic oncology patients undergoing laparotomy. Gynecol. Oncol. 2018, 151, 282–286. [Google Scholar] [CrossRef]
- Bisch, S.P.; Wells, T.; Gramlich, L.; Faris, P.; Wang, X.; Tran, D.T.; Thanh, N.X.; Glaze, S.; Chu, P.; Ghatage, P.; et al. Enhanced Recovery After Surgery (ERAS) in gynecologic oncology: System-wide implementation and audit leads to improved value and patient outcomes. Gynecol. Oncol. 2018, 151, 117–123. [Google Scholar] [CrossRef]
- Nelson, G.; Fotopoulou, C.; Taylor, J.; Glaser, G.; Bakkum-Gamez, J.; Meyer, L.A.; Stone, R.; Mena, G.; Elias, K.M.; Altman, A.D.; et al. Enhanced recovery after surgery (ERAS®) society guidelines for gynecologic oncology: Addressing implementation challenges—2023 update. Gynecol. Oncol. 2023, 173, 58–67. [Google Scholar] [CrossRef]
- Lindemann, K.; Kleppe, A.; Eyjólfsdóttir, B.; Heimisdottir Danbolt, S.; Wang, Y.Y.; Heli-Haugestøl, A.G.; Walcott, S.L.; Mjåland, O.; Navestad, G.-A.; Hermanrud, S.; et al. Prospective evaluation of an enhanced recovery after surgery (ERAS) pathway in a Norwegian cohort of patients with suspected or advanced ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 1279. [Google Scholar] [CrossRef]
- Socias, M.C.; Pérez-Benavente, M.A.; Sánchez-Iglesias, J.L.; Manrique-Muñoz, S.; Burgos-Pelaez, R.; Pamies-Serrano, M.; Rubio-Vázquez, D.; Capote, S.; Díaz-Feijoo, B.; Puig-Puig, O.; et al. PROFAST: ERAS in advanced ovarian cancer, a randomised trial. Clin. Nutr. ESPEN 2016, 12, e48–e49. [Google Scholar] [CrossRef][Green Version]
- Reuter, S.; Woelber, L.; Trepte, C.C.; Perez, D.; Zapf, A.; Cevirme, S.; Mueller, V.; Schmalfeldt, B.; Jaeger, A. The impact of Enhanced Recovery after Surgery (ERAS) pathways with regard to perioperative outcome in patients with ovarian cancer. Arch. Gynecol. Obstet. 2022, 306, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, G.; Jakovljević, D.K.; Lukić-Šarkanović, M. Enhanced Recovery in Surgical Intensive Care: A Review. Front. Med. 2018, 5, 256. [Google Scholar] [CrossRef] [PubMed]
| Intensive Care Unit | High Dependency Unit | |
|---|---|---|
| Respiratory | Spontaneous respiration | Spontaneous respiration |
| <81 O2/min | <41 O2/min | |
| CPAP ≤6 h/day | No need for CPAP | |
| Cardiovascular | Noradrenaline infusion ≤0.1 μg/kg/min | No need for vasoactive medication |
| No need for invasive monitoring | ||
| Urinary system | No need for continuous renal impairment support | Adequate urine output (>0.5 mL/kg/h) |
| Neurology | No impact on respiratory function | No relevant neurological impairment |
| Hospital care requirements | No need for nurse–patient care ≤1:2 | No need for nurse–patient care ≤1:4 |
| Additional criteria | No lactic acidosis | Serum lactate at normal levels |
| No acute bleeding | Confined blood loss (<2 mg/dL/24 h) | |
| Adequate patient mobilization | ||
| Adequate pain management |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergialiotis, V.; Morice, P.; Lygizos, V.; Haidopoulos, D.; Thomakos, N. Critical Care Management of Surgically Treated Gynecological Cancer Patients: Current Concepts and Future Directions. Cancers 2025, 17, 2514. https://doi.org/10.3390/cancers17152514
Pergialiotis V, Morice P, Lygizos V, Haidopoulos D, Thomakos N. Critical Care Management of Surgically Treated Gynecological Cancer Patients: Current Concepts and Future Directions. Cancers. 2025; 17(15):2514. https://doi.org/10.3390/cancers17152514
Chicago/Turabian StylePergialiotis, Vasilios, Philippe Morice, Vasilios Lygizos, Dimitrios Haidopoulos, and Nikolaos Thomakos. 2025. "Critical Care Management of Surgically Treated Gynecological Cancer Patients: Current Concepts and Future Directions" Cancers 17, no. 15: 2514. https://doi.org/10.3390/cancers17152514
APA StylePergialiotis, V., Morice, P., Lygizos, V., Haidopoulos, D., & Thomakos, N. (2025). Critical Care Management of Surgically Treated Gynecological Cancer Patients: Current Concepts and Future Directions. Cancers, 17(15), 2514. https://doi.org/10.3390/cancers17152514








