Long-Term Adverse Events Following Early Breast Cancer Treatment with a Focus on the BRCA-Mutated Population
Simple Summary
Abstract
1. Introduction
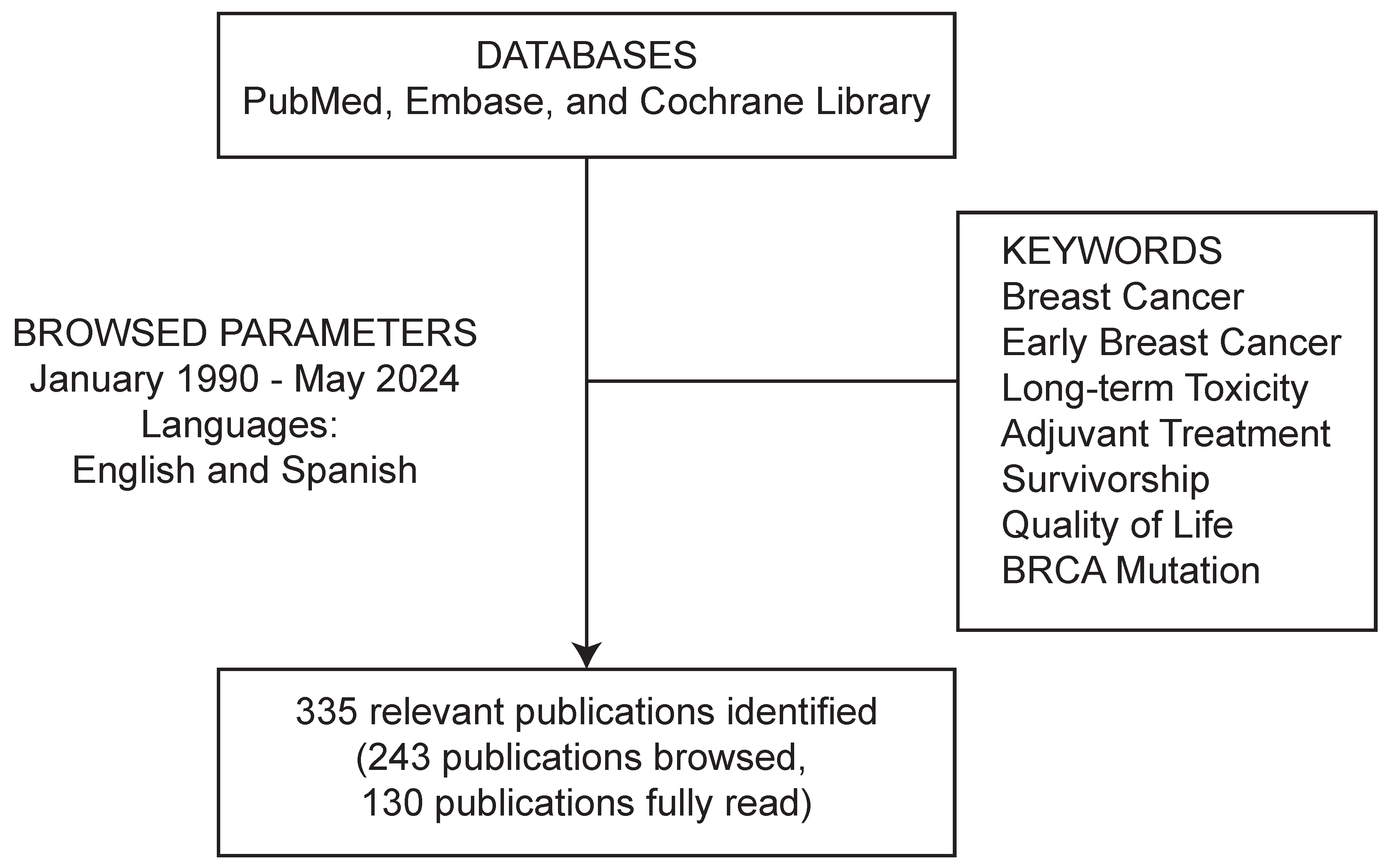
2. Methodology
3. Long-Term Adverse Events in Patients with eBC
3.1. Adverse Events Affecting the Chest Wall and Breast
3.1.1. Post-Mastectomy Pain Syndrome
3.1.2. Lymphoedema
3.1.3. Skin and Soft Tissue Affection
3.2. Cardiotoxicity
3.3. Neurotoxicity
3.3.1. Chemotherapy-Induced Peripheral Neuropathy (CIPN)
3.3.2. Cognitive Dysfunction
3.4. Psychological Alterations
3.5. Fatigue
3.6. Hormonal Alterations
3.7. Sexual Disorders and Fertility
3.8. Gastrointestinal Symptoms
3.9. Endocrine Toxicity
3.10. Osteomuscular Alterations
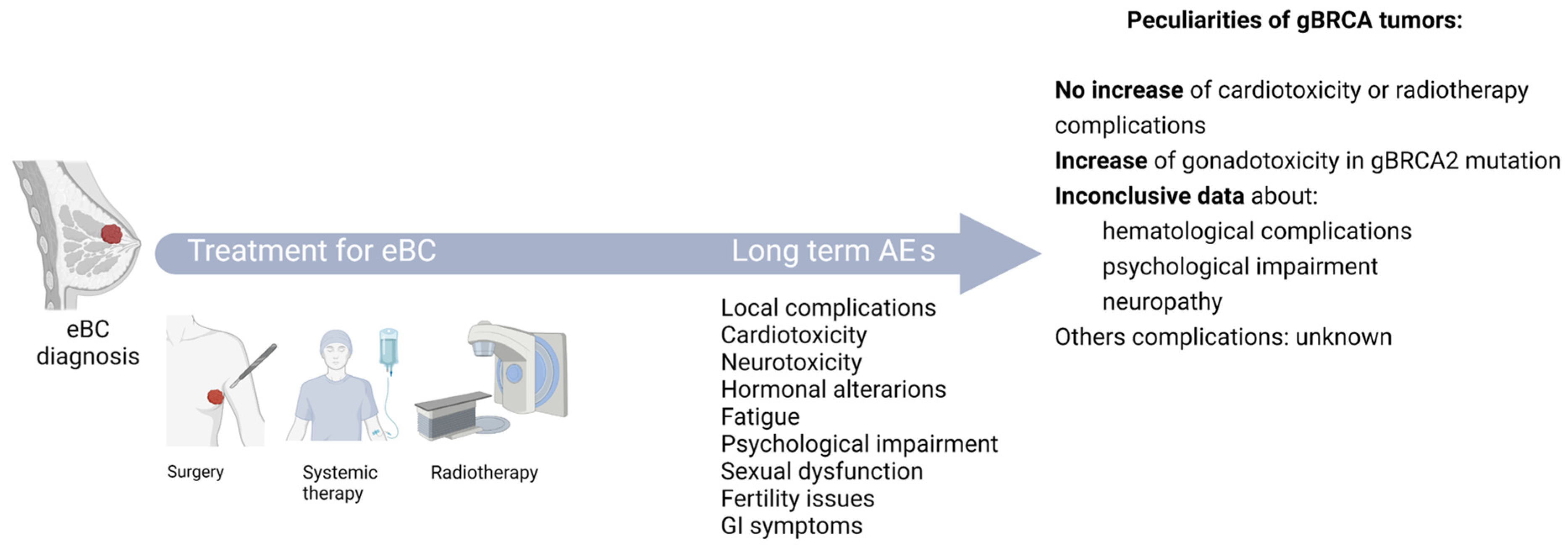
4. Patients with BRCA-Mutated Tumors
5. Current Research Needs in Breast Cancer Survivorship
6. Gaps and Future Directions in the Study of Long-Term Adverse Events in BC Survivors
- Limited integration across affected systems: most studies examine individual domains, such as cardiotoxicity [44,66], CIPN [29,67], or psychological distress [32,55], in isolation. There is a lack of comprehensive frameworks to assess how these symptoms may interact over time and to determine their combined impact on the overall quality of life of BC survivors.
- Inadequate stratification by patient subgroups: while gBRCA1/2 carriers and younger women have been studied in specific contexts [143,156], many studies fail to stratify findings by age, menopausal status, race/ethnicity, or comorbidities. This limits the ability to deliver tailored survivorship care that addresses the distinct risks and experiences of diverse BC patient populations.
- Understudied and emerging adverse events: several important domains remain under-researched in long-term BC survivorship. These include endocrine dysfunction [35,126], sexual health and intimacy issues [36,58], weight gain, and metabolic syndrome [112,113]. These outcomes are rarely included in clinical trials and are inconsistently addressed in follow-up care plans, limiting comprehensive survivorship planning.
- Inconsistency in the definition of long-term adverse events, including time frames: there is considerable variability in how studies define long-term and late adverse effects. Some studies classify them as events persisting beyond 1 year post-treatment, while others only focus on those emerging after 5 years [38,42]. Additionally, the heterogeneity in study endpoints, from clinical indicators to patient-reported outcomes, hampers cross-study comparisons and limits the ability to synthesize findings effectively.
- Opportunity for retrospective studies using electronic health records (EHRs): retrospective analysis of large EHR databases offers a promising avenue to study the impact of long-term adverse events on BC survivorship at scale in real-world clinical practice, beyond a controlled clinical trial setting. Several studies have demonstrated the utility of advanced natural language processing and machine learning to extract and analyze longitudinal data from unstructured clinical notes, enhancing the detection of late toxicities and comorbidities [162,163,164,165]. Leveraging such EHR-based platforms can fill critical gaps, especially in understudied patient subpopulations and rare adverse events, while providing insights into BC survivorship care patterns and outcomes across diverse healthcare systems.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Risk Factors for Breast Cancer. 2023. Available online: https://www.cancerresearchuk.org/about-cancer/breast-cancer/risks-causes/risk-factors (accessed on 28 February 2025).
- Wooster, R.; Weber, B.L. Breast and ovarian cancer. N. Engl. J. Med. 2003, 348, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Parmigiani, G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. 2007, 25, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Malone, K.E.; Daling, J.R.; Doody, D.R.; Hsu, L.; Bernstein, L.; Coates, R.J.; Marchbanks, P.A.; Simon, M.S.; McDonald, J.A.; Norman, S.A.; et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006, 66, 8297–8308. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.N.; Esen, B.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J. Natl. Cancer Inst. 2022, 114, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Tang, G.; Paik, S.; Baehner, F.L.; Liu, Q.; Jeong, J.H.; Kim, S.R.; Butler, S.M.; Jamshidian, F.; Cherbavaz, D.B.; et al. 21-Gene Recurrence Score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: Results from NSABP B-28/NRG Oncology. Breast Cancer Res. Treat. 2018, 168, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Pagani, O.; Francis, P.A.; Fleming, G.F.; Walley, B.A.; Viale, G.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Pinotti, G.; et al. Absolute improvements in freedom from distant recurrence to tailor adjuvant endocrine therapies for premenopausal women: Results from TEXT and SOFT. J. Clin. Oncol. 2020, 38, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.L.; Liu, J.J.; Liu, Y.X.; Yu, S.H.; Liu, X.; Lin, X.Q.; Chen, H.D.; Fang, X.; Ma, T.; Li, Y.Q.; et al. Characteristics of recurrence, predictors for relapse and prognosis of rapid relapse triple-negative breast cancer. Front. Oncol. 2023, 13, 1119611. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Tolaney, S.M.; Yardley, D.A.; Hart, L.; Razavi, P.; Fasching, P.A.; Janni, W.; Schwartzberg, L.; Kim, J.; Akdere, M.; et al. Real-world risk of recurrence and treatment outcomes with adjuvant endocrine therapy in patients with stage II-III HR+/HER2- early breast cancer. Breast 2025, 81, 104437. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kummel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.; Myers, S.; Shen, S.; Downs-Canner, S.; Robson, M.; Norton, L.; Chen, Y.; Traina, T.; Abuhadra, N. Dose dense doxorubicin plus cyclophosphamide in a modified KEYNOTE522 regimen for triple negative breast cancer. npj Breast Cancer 2024, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Lipatov, O.; Nowecki, Z.; McAndrew, N.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; Fasching, P.A.; Crown, J.; et al. Ribociclib plus endocrine therapy in early breast cancer. N. Engl. J. Med. 2024, 390, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Andre, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Mougalian, S.S.; Hernandez, M.; Lei, X.; Lynch, S.; Kuerer, H.M.; Symmans, W.F.; Theriault, R.L.; Fornage, B.D.; Hsu, L.; Buchholz, T.A.; et al. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016, 2, 508–516. [Google Scholar] [CrossRef] [PubMed]
- El Saghir, N.S.; Khalil, L.E.; El Dick, J.; Atwani, R.W.; Safi, N.; Charafeddine, M.; Al-Masri, A.; El Saghir, B.N.; Chaccour, M.; Tfayli, A.; et al. Improved survival of young patients with breast cancer 40 years and younger at diagnosis. JCO Glob. Oncol. 2023, 9, e2200354. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, R.L.; Maggi, N.; Castrezana, L.; Zehnpfennig, L.; Schwab, F.D.; Krol, J.; Oberhauser, I.; Weber, W.P.; Kurzeder, C.; Haug, M.D.; et al. The impact of neoadjuvant systemic treatment on postoperative complications in breast cancer surgery. Breast Cancer Res. Treat. 2023, 197, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.D.; Syrjala, K.L.; Andrykowski, M.A. Physical and psychological long-term and late effects of cancer. Cancer 2008, 112, 2577–2592. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Kehlet, H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J. Pain 2011, 12, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Janda, M.; Cornish, B.; Battistutta, D.; Newman, B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J. Clin. Oncol. 2008, 26, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; O’Shaughnessy, J.; Boyle, F.; Toi, M.; Broom, R.; Blancas, I.; Gumus, M.; Yamashita, T.; Im, Y.H.; Rastogi, P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Safety and patient-reported outcomes from the monarchE study. Ann. Oncol. 2022, 33, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, Z.; Garg, R.; Listo, M.; Zavitsanos, P.; Wazer, D.E.; Huber, K.E. Late complications of radiation therapy for breast cancer: Evolution in techniques and risk over time. Gland. Surg. 2018, 7, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Maass, S.W.; Roorda, C.; Berendsen, A.J.; Verhaak, P.F.; de Bock, G.H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Bustos, A.; de Pedro, C.G.; Romero-Elias, M.; Ramos, J.; Osorio, P.; Cantos, B.; Maximiano, C.; Mendez, M.; Fiuza-Luces, C.; Mendez-Otero, M.; et al. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support. Care Cancer 2021, 29, 6523–6534. [Google Scholar] [CrossRef] [PubMed]
- Zeng, E.; He, W.; Smedby, K.E.; Czene, K. Adjuvant hormone therapy-related hot flashes predict treatment discontinuation and worse breast cancer prognosis. J. Natl. Compr. Cancer Netw. 2022, 20, 683–689 e682. [Google Scholar] [CrossRef] [PubMed]
- Digkas, E.; Smith, D.R.; Wennstig, A.K.; Matikas, A.; Tegnelius, E.; Valachis, A. Incidence and risk factors of hypothyroidism after treatment for early breast cancer: A population-based cohort study. Breast Cancer Res. Treat. 2024, 204, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, Y.H.; Jeon, M.J. Risk factors for negative impacts on sexual activity and function in younger breast cancer survivors. Psychooncology 2015, 24, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Izci, F.; Ilgun, A.S.; Findikli, E.; Ozmen, V. Psychiatric symptoms and psychosocial problems in patients with breast cancer. J. Breast Health 2016, 12, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-term effects of breast cancer surgery, treatment, and survivor care. J. Midwifery Womens Health 2019, 64, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Harbeck, N.; Barrios, C.H.; Bergh, J.; Cortes, J.; El Saghir, N.; Francis, P.A.; Hudis, C.A.; Ohno, S.; Partridge, A.H.; et al. Research needs in breast cancer. Ann. Oncol. 2017, 28, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Y.; Soto-Rubio, A.; Ramos-Campos, M.; Escriche-Saura, A.; Martinez, M.; Gavila, J. Impact of hormone therapy side effects on health-related quality of life, distress, and well-being of breast cancer survivors. Sci. Rep. 2022, 12, 18673. [Google Scholar] [CrossRef] [PubMed]
- Hamood, R.; Hamood, H.; Merhasin, I.; Keinan-Boker, L. Chronic pain and other symptoms among breast cancer survivors: Prevalence, predictors, and effects on quality of life. Breast Cancer Res. Treat. 2018, 167, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Wiskemann, J.; Steindorf, K. Quality of life, problems, and needs of disease-free breast cancer survivors 5 years after diagnosis. Qual. Life Res. 2018, 27, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Maass, S.; Brandenbarg, D.; Boerman, L.M.; Verhaak, P.F.M.; de Bock, G.H.; Berendsen, A.J. Fatigue among long-term breast cancer survivors: A controlled cross-sectional study. Cancers 2021, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Zambetti, M.; Moliterni, A.; Materazzo, C.; Stefanelli, M.; Cipriani, S.; Valagussa, P.; Bonadonna, G.; Gianni, L. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J. Clin. Oncol. 2001, 19, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bonneterre, J.; Roche, H.; Kerbrat, P.; Fumoleau, P.; Goudier, M.J.; Fargeot, P.; Montcuquet, P.; Clavere, P.; Barats, J.C.; Monnier, A.; et al. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group. J. Clin. Oncol. 2004, 22, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A.; Hussey, M.A.; Moinpour, C.M.; Unger, J.M.; Hutchins, L.F.; Dakhil, S.R.; Giguere, J.K.; Goodwin, J.W.; Martino, S.; Albain, K.S. Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on Southwest Oncology Group protocol s8897. J. Clin. Oncol. 2008, 26, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, L.; Bruce, J.; Scott, N.W.; Smith, W.C.; Chambers, W.A. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br. J. Cancer 2005, 92, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Levenhagen, K.; Ryans, K.; Perdomo, M.; Gilchrist, L. Interventions for breast cancer-related lymphedema: Clinical practice guideline from the Academy of Oncologic Physical Therapy of APTA. Phys. Ther. 2020, 100, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Torgbenu, E.; Luckett, T.; Buhagiar, M.A.; Chang, S.; Phillips, J.L. Prevalence and incidence of cancer related lymphedema in low and middle-income countries: A systematic review and meta-analysis. BMC Cancer 2020, 20, 604. [Google Scholar] [CrossRef] [PubMed]
- Classen, J.; Nitzsche, S.; Wallwiener, D.; Kristen, P.; Souchon, R.; Bamberg, M.; Brucker, S. Fibrotic changes after postmastectomy radiotherapy and reconstructive surgery in breast cancer. A retrospective analysis in 109 patients. Strahlenther. Onkol. 2010, 186, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Filion, K.B.; Al-Qurashi, S.; Torabi, N.; Bouganim, N.; Suissa, S.; Azoulay, L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 2017, 28, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Eckhoff, L.; Knoop, A.; Jensen, M.B.; Ewertz, M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur. J. Cancer 2015, 51, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Hashimoto, K.; Shimizu, C.; Hirakawa, A.; Harano, K.; Yunokawa, M.; Yonemori, K.; Katsumata, N.; Tamura, K.; Ando, M.; et al. Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int. J. Clin. Oncol. 2013, 18, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Onzi, G.R.; D’Agustini, N.; Garcia, S.C.; Guterres, S.S.; Pohlmann, P.R.; Rosa, D.D.; Pohlmann, A.R. Chemobrain in breast cancer: Mechanisms, clinical manifestations, and potential interventions. Drug Saf. 2022, 45, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Ferguson, D.W.; Gill, J.; Paul, J.; Symonds, P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Luz, P.; Carvalho, A.N.; Oliveira, A.; Menezes, M.; Dinis, R.; Gosalbez, B. Prevalence of fear of death among young breast cancer patients during adjuvant endocrine therapy: Results from a portuguese cohort. Acta Medica Port. 2021, 34, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Carmen, A.; Anne, O.; Monika, S.; Daniel, E.; Johannes, G.; Verena, M.; Michael, H.; Christine, B. Does the toxicity of endocrine therapy persist into long-term survivorship?: Patient-reported outcome results from a follow-up study beyond a 10-year-survival. Breast Cancer Res. Treat. 2022, 198, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Raggio, G.A.; Butryn, M.L.; Arigo, D.; Mikorski, R.; Palmer, S.C. Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol. Health 2014, 29, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Blondeaux, E.; Bruzzone, M.; Perachino, M.; Anderson, R.A.; de Azambuja, E.; Poorvu, P.D.; Kim, H.J.; Villarreal-Garza, C.; Pistilli, B.; et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2021, 39, 3293–3305. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L. Osteoporosis: A long-term and late-effect of breast cancer treatments. Cancers 2020, 12, 3094. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.J.; Asher, A.; Smith, S.R. A targeted approach to post-mastectomy pain and persistent pain following breast cancer treatment. Cancers 2021, 13, 5191. [Google Scholar] [CrossRef] [PubMed]
- Gartner, R.; Jensen, M.B.; Nielsen, J.; Ewertz, M.; Kroman, N.; Kehlet, H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009, 302, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Rosas, J.C.; Fink, H.; Behrens, S.; Chang-Claude, J.; Seibold, P. Longitudinal changes of health-related quality of life over 10 years in breast cancer patients treated with radiotherapy following breast-conserving surgery. Qual. Life Res. 2023, 32, 2639–2652. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Piroth, M.D.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther. Onkol. 2019, 195, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Puckett, L.L.; Saba, S.G.; Henry, S.; Rosen, S.; Rooney, E.; Filosa, S.L.; Gilbo, P.; Pappas, K.; Laxer, A.; Eacobacci, K.; et al. Cardiotoxicity screening of long-term, breast cancer survivors—The CAROLE (Cardiac-Related Oncologic Late Effects) study. Cancer Med. 2021, 10, 5051–5061. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Basal, C.; Seluzicki, C.; Li, S.Q.; Seidman, A.D.; Mao, J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016, 159, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Hardy-Leger, I.; Licaj, I.; Pistilli, B.; Rigal, O.; Le Fel, J.; Levy, C.; Capel, A.; Coutant, C.; Meyer, J.; et al. Cognitive impairment in patients with breast cancer before surgery: Results from a CANTO cohort subgroup. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Caruso, R.; Riba, M.B.; Lloyd-Williams, M.; Kissane, D.; Rodin, G.; McFarland, D.; Campos-Ródenas, R.; Zachariae, R.; Santini, D.; et al. Anxiety and depression in adult cancer patients: ESMO Clinical Practice Guideline. ESMO Open 2023, 8, 101155. [Google Scholar] [CrossRef] [PubMed]
- Vickberg, S.M. The Concerns About Recurrence Scale (CARS): A systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann. Behav. Med. 2003, 25, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, H.J.G.; Gielissen, M.F.M.; Schmits, I.C.; Verhagen, C.; Rovers, M.M.; Knoop, H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12,327 breast cancer survivors. Ann. Oncol. 2016, 27, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Seav, S.M.; Dominick, S.A.; Stepanyuk, B.; Gorman, J.R.; Chingos, D.T.; Ehren, J.L.; Krychman, M.L.; Su, H.I. Management of sexual dysfunction in breast cancer survivors: A systematic review. Women’s Midlife Health 2015, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Poorvu, P.D.; Gelber, S.I.; Zheng, Y.; Ruddy, K.J.; Tamimi, R.M.; Peppercorn, J.; Schapira, L.; Borges, V.F.; Come, S.E.; Lambertini, M.; et al. Pregnancy after breast cancer: Results from a prospective cohort of young women with breast cancer. Cancer 2021, 127, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.L. De-escalation of breast cancer surgery. Surgery 2023, 174, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Morgan, J.; Wyld, L. A qualitative study exploring the views of global healthcare professionals towards de-escalation of axillary surgery in early breast cancer. Eur. J. Surg. Oncol. 2025, 51, 110079. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, L.; Blankenship, S.A.; Chatterjee, A.; Geha, R.; Nocera, N.; Czerniecki, B.J.; Tchou, J.; Fisher, C.S. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: A systematic literature review. Ann. Surg. Oncol. 2016, 23, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Caffo, O.; Amichetti, M.; Ferro, A.; Lucenti, A.; Valduga, F.; Galligioni, E. Pain and quality of life after surgery for breast cancer. Breast Cancer Res. Treat. 2003, 80, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Shamsesfandabadi, P.; Shams Esfand Abadi, M.; Yin, Y.; Carpenter, D.J.; Peluso, C.; Hilton, C.; Coopey, S.B.; Gomez, J.; Beriwal, S.; Champ, C.E. Resistance training and lymphedema in breast cancer survivors. JAMA Netw. Open 2025, 8, e2514765. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.D.; Rush, S.C.; Kostroff, K.; Derisi, D.; Farber, L.A.; Maurer, V.E.; Bosworth, J.L. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Batenburg, M.C.T.; Bartels, M.; Maarse, W.; Witkamp, A.; Verkooijen, H.M.; van den Bongard, H.J.G.D. Factors associated with late local radiation toxicity after post-operative breast irradiation. Breast J. 2022, 2022, 6745954. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, A.; Mousavi, E.; Nikoubin-Boroujeni, M.; Tabasi, S.; Arjmandi, N.; Ghelishli, N.; Arab-Bafrani, Z. Cardiac toxicity under concurrent administration of trastuzumab (anti-HER2 therapy) and radiotherapy: Systematic review and meta-analysis. Health Sci. Rep. 2025, 8, e70966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, Y.; Hao, M. Cardiotoxicity induced by chemotherapy and immunotherapy in cancer treatment: A bibliometric analysis. Discov. Oncol. 2025, 16, 376. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Trapani, D.; Nicolò, E.; Corti, C.; Valenza, C.; Criscitiello, C.; Curigliano, G.; Carey, L.A. Cardiotoxicity of agents used in patients with breast cancer. JCO Oncol. Pract. 2024, 20, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Strongman, H.; Gadd, S.; Matthews, A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; Dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019, 394, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Lin, Z.; Yang, M.; Li, C. Cardiac toxicity from adjuvant targeting treatment for breast cancer post-surgery. Front. Oncol. 2022, 12, 706861. [Google Scholar] [CrossRef] [PubMed]
- Mata Caballero, R.; Serrano Antolin, J.M.; Jimenez Hernandez, R.M.; Talavera Calle, P.; Curcio Ruigomez, A.; Del Castillo Arrojo, S.; Graupner Abad, C.; Cristobal Varela, C.; Alonso Martin, J.J. Incidence of long-term cardiotoxicity and evolution of the systolic function in patients with breast cancer treated with anthracyclines. Cardiol. J. 2022, 29, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, N.I.; Jager, A.; Liesting, C.; Kofflard, M.J.M.; Brugts, J.J.; Kitzen, J.; Boersma, E.; Levin, M.D. Cardiac monitoring in HER2-positive patients on trastuzumab treatment: A review and implications for clinical practice. Breast 2020, 52, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Dent, S.F.; Moore, H.; Raval, P.; Alder, L.; Guha, A. How to manage and monitor cardiac dysfunction in patients with metastatic HER2-positive breast cancer. JACC CardioOncol. 2022, 4, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, P.; Wang, Q. Evolving therapeutics and ensuing cardiotoxicities in triple-negative breast cancer. Cancer Treat. Rev. 2024, 130, 102819. [Google Scholar] [CrossRef] [PubMed]
- Sund, M.; Garcia-Argibay, M.; Garmo, H.; Ahlgren, J.; Wennstig, A.K.; Fredriksson, I.; Lindman, H.; Valachis, A. Aromatase inhibitors use and risk for cardiovascular disease in breast cancer patients: A population-based cohort study. Breast 2021, 59, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Jung, E.A.; Kim, Z.; Kim, B.Y. Risk of cardiovascular events and lipid profile change in patients with breast cancer taking aromatase inhibitor: A systematic review and meta-analysis. Curr. Oncol. 2023, 30, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.M.; Ramchand, S.K.; Yeo, B.; Grossmann, M. Cardiometabolic effects of endocrine treatment of estrogen receptor-positive early breast cancer. J. Endocr. Soc. 2019, 3, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Cheang, I.; Gue, Y.; Wu, M.Z.; Huang, J.Y.; Ren, Q.W.; Chen, Z.; Tse, Y.K.; Li, H.L.; Chan, Y.H.; Tse, H.F.; et al. Cardiovascular risks associated with adjuvant endocrine therapy in women with breast cancer: A population-based cohort study. BMC Cancer 2025, 25, 1063. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.F.; Sestak, I.; Howell, A.; Bonanni, B.; Bundred, N.; Levy, C.; von Minckwitz, G.; Eiermann, W.; Neven, P.; Stierer, M.; et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): A double-blind, randomised controlled trial. Lancet 2016, 387, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Cuppone, F.; Bria, E.; Verma, S.; Pritchard, K.I.; Gandhi, S.; Carlini, P.; Milella, M.; Nistico, C.; Terzoli, E.; Cognetti, F.; et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer 2008, 112, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Filion, K.B.; Bouganim, N.; Suissa, S.; Azoulay, L. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: A population-based cohort study. Circulation 2020, 141, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Lakritz, S.; Schreiber, A.R.; Kuna, E.M.; Bradley, C.J.; Kondapalli, L.; Diamond, J.R. Major cardiovascular adverse events in older adults with early-stage triple-negative breast cancer treated with adjuvant taxane + anthracycline versus taxane-based chemotherapy regimens: A SEER-medicare study. Eur. J. Cancer 2024, 196, 113426. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.; Pahl, R.; Huber, K.; Eilf, K.; Dunst, J. Cardiac toxicity after radiotherapy for breast cancer: Myths and facts. Breast Care 2015, 10, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Schwab, L.; Visovsky, C. Psychological distress and quality of life in breast cancer survivors with taxane-induced peripheral neuropathy: A scoping review. Front. Oncol. 2022, 12, 1005083. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotte, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Di Leone, A.; Terribile, D.; Magno, S.; Sanchez, A.M.; Scardina, L.; Mason, E.J.; D’Archi, S.; Maggiore, C.; Rossi, C.; Di Micco, A.; et al. Neoadjuvant chemotherapy in breast cancer: An advanced personalized multidisciplinary prehabilitation model (APMP-M) to optimize outcomes. J. Pers. Med. 2021, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; George, R.P.; O’Malley, L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 2135. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J. Chemotherapy associated central nervous system damage. Adv. Exp. Med. Biol. 2010, 678, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Arrillaga-Romany, I.C.; Dietrich, J. Imaging findings in cancer therapy-associated neurotoxicity. Semin. Neurol. 2012, 32, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.A. How chemotherapy damages the central nervous system. J. Biol. 2008, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Le, N.K.; Gabrick, K.S.; Chouairi, F.; Mets, E.J.; Avraham, T.; Alperovich, M. Impact of socioeconomic status on psychological functioning in survivorship following breast cancer and reconstruction. Breast J. 2020, 26, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Bickel, E.A.; Fleer, J.; Ranchor, A.V.; Schroevers, M.J. Are cancer patients with high depressive symptom levels able to manage these symptoms without professional care? The role of coping and social support. Psychooncology 2022, 31, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, A.; Havas, J.; Martin, E.; Pistilli, B.; Menvielle, G.; Dumas, A.; Charles, C.; Everhard, S.; Martin, A.-L.; Coutant, C.; et al. Assessing the risk of severe post-treatment (tx) cancer-related fatigue (CRF) among breast cancer survivors (BCS) in the CANcer TOxicity (CANTO) cohort. J. Clin. Oncol. 2021, 39, 12022. [Google Scholar] [CrossRef]
- Roila, F.; Fumi, G.; Fatigoni, S. Management of fatigue following breast cancer treatment. Breast Cancer Manag. 2016, 5, 79–87. [Google Scholar] [CrossRef]
- Haidinger, R.; Bauerfeind, I. Long-term side effects of adjuvant therapy in primary breast cancer patients: Results of a web-based survey. Breast Care 2019, 14, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Campbell, K.L.; Hayes, S.C. Weight management and its role in breast cancer rehabilitation. Cancer 2012, 118, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Chen, W.Y.; Rosner, B.; Holmes, M.D. Weight, weight gain, and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.B.; Trentham-Dietz, A.; Egan, K.M.; Titus-Ernstoff, L.; Holmes, M.D.; Bersch, A.J.; Holick, C.N.; Hampton, J.M.; Stampfer, M.J.; Willett, W.C.; et al. Body mass index before and after breast cancer diagnosis: Associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Vance, V.; Mourtzakis, M.; McCargar, L.; Hanning, R. Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obes. Rev. 2011, 12, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Heudel, P.E.; Van Praagh-Doreau, I.; Duvert, B.; Cauvin, I.; Hardy-Bessard, A.C.; Jacquin, J.P.; Stefani, L.; Vincent, L.; Dramais, D.; Guastalla, J.P.; et al. Does a homeopathic medicine reduce hot flushes induced by adjuvant endocrine therapy in localized breast cancer patients? A multicenter randomized placebo-controlled phase III trial. Support. Care Cancer 2019, 27, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Ayers, B.; Smith, M.; Hellier, J.; Mann, E.; Hunter, M.S. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): A randomized controlled trial. Menopause 2012, 19, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Carmody, J.F.; Crawford, S.; Salmoirago-Blotcher, E.; Leung, K.; Churchill, L.; Olendzki, N. Mindfulness training for coping with hot flashes: Results of a randomized trial. Menopause 2011, 18, 611–620. [Google Scholar] [CrossRef] [PubMed]
- McCurry, S.M.; Guthrie, K.A.; Morin, C.M.; Woods, N.F.; Landis, C.A.; Ensrud, K.E.; Larson, J.C.; Joffe, H.; Cohen, L.S.; Hunt, J.R.; et al. Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: A MsFLASH randomized clinical trial. JAMA Intern. Med. 2016, 176, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Christiaens, M.R.; Enzlin, P.; Neven, P.; Amant, F. Sexual functioning in women after mastectomy versus breast conserving therapy for early-stage breast cancer: A prospective controlled study. Breast 2014, 23, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Niman, S.M.; Ruggeri, M.; Peccatori, F.A.; Azim, H.A., Jr.; Colleoni, M.; Saura, C.; Shimizu, C.; Saetersdal, A.B.; Kroep, J.R.; et al. Who are the women who enrolled in the POSITIVE trial: A global study to support young hormone receptor positive breast cancer survivors desiring pregnancy. Breast 2021, 59, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.; Lee, Y.C.; Tew, W.P. Managing adverse effects associated with poly (ADP-ribose) polymerase inhibitors in ovarian cancer: A synthesis of clinical trial and real-world data. Soc. Clin. Oncol. Educ. Book 2023, 43, e390876. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Solmunde, E.; Falstie-Jensen, A.M.; Lorenzen, E.L.; Ewertz, M.; Reinertsen, K.V.; Dekkers, O.M.; Cronin-Fenton, D.P. Breast cancer, breast cancer-directed radiation therapy and risk of hypothyroidism: A systematic review and meta-analysis. Breast 2023, 68, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wen, J.; Yang, A.; Wang, Y.; Li, N.; Yu, P.; Wei, W.; Tang, J. The influence of hormone therapy on secondary diabetes mellitus in breast cancer: A meta-analysis. Clin. Breast Cancer 2022, 22, e48–e58. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Marhold, M.; Udovica, S.; Halstead, A.; Hirdler, M.; Ferner, M.; Wimmer, K.; Bago-Horvath, Z.; Exner, R.; Fitzal, F.; Strasser-Weippl, K.; et al. Emergence of immune-related adverse events correlates with pathological complete response in patients receiving pembrolizumab for early triple-negative breast cancer. Oncoimmunology 2023, 12, 2275846. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Giobbie-Hurder, A. Benefits and adverse effects of endocrine therapy. Ann. Oncol. 2010, 21, vii107–vii111. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Banks, L.M.; Girgis, S.I.; Vrdoljak, E.; Fox, J.; Cawthorn, S.J.; Patel, A.; Bliss, J.M.; Coombes, R.C.; Kilburn, L.S. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res. Treat. 2010, 124, 153–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F.; on behalf of the ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Fleischer, J.B.; Johnson, M.K.; Brown, I.N.; Joe, A.K.; Hershman, D.L.; McMahon, D.J.; Silverberg, S.J. Prevention of bone loss after withdrawal of tamoxifen. Endocr. Pract. 2008, 14, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Rabaglio, M.; Sun, Z.; Price, K.N.; Castiglione-Gertsch, M.; Hawle, H.; Thurlimann, B.; Mouridsen, H.; Campone, M.; Forbes, J.F.; Paridaens, R.J.; et al. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann. Oncol. 2009, 20, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Barchiesi, G.; Resuli, B.; Verrico, M.; Speranza, I.; Cristofani, L.; Pediconi, F.; Tomao, F.; Botticelli, A.; Santini, D. Sarcopenia in breast cancer patients: A systematic review and meta-analysis. Cancers 2024, 16, 596. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, Z.; Ruan, G.; Tu, C.; Ding, W. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: A literature-based meta-analysis of randomized trials. Breast Cancer Res. Treat. 2020, 179, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Godet, I.; Gilkes, D.M. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr. Cancer Sci. Ther. 2017, 4, 10.15761. [Google Scholar] [CrossRef] [PubMed]
- Blondeaux, E.; Sonnenblick, A.; Agostinetto, E.; Bas, R.; Kim, H.J.; Franzoi, M.A.; Bernstein-Molho, R.; Linn, S.; Kwong, A.; Pogoda, K.; et al. Association between risk-reducing surgeries and survival in young BRCA carriers with breast cancer: An international cohort study. Lancet Oncol. 2025, 26, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.M.; Garber, J.E. BRCA1/2 testing: Therapeutic implications for breast cancer management. Br. J. Cancer 2018, 119, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Huszno, J.; Budryk, M.; Kolosza, Z.; Nowara, E. The influence of BRCA1/BRCA2 mutations on toxicity related to chemotherapy and radiotherapy in early breast cancer patients. Oncology 2013, 85, 278–282. [Google Scholar] [CrossRef] [PubMed]
- van Barele, M.; Akdeniz, D.; Heemskerk-Gerritsen, B.A.M.; Genepso; Andrieu, N.; Nogues, C.; Hebon; van Asperen, C.J.; Wevers, M.; Ausems, M.; et al. Contralateral breast cancer risk in patients with breast cancer and a germline-BRCA1/2 pathogenic variant undergoing radiation. J. Natl. Cancer Inst. 2023, 115, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, J.; Mobus, V.; Schneeweiss, A.; Rhiem, K.; Tesch, H.; Blohmer, J.U.; Lubbe, K.; Untch, M.; Salat, C.; Huober, J.; et al. Germline BRCA1/2 mutations and severe haematological toxicities in patients with breast cancer treated with neoadjuvant chemotherapy. Eur. J. Cancer 2021, 145, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, S.; Zhou, J.Z.; Bassett, R.; Gutierrez Barrera, A.M.; Layman, R.M.; Valero, V.; Arun, B. Clinical outcome and toxicity from taxanes in breast cancer patients with BRCA1 and BRCA2 pathogenic germline mutations. Breast J. 2020, 26, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Drooger, J.C.; Heemskerk-Gerritsen, B.A.M.; Smallenbroek, N.; Epskamp, C.; Seynaeve, C.M.; Jager, A. Toxicity of (neo)adjuvant chemotherapy for BRCA1- and BRCA2-associated breast cancer. Breast Cancer Res. Treat. 2016, 156, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Shanley, S.; McReynolds, K.; Ardern-Jones, A.; Ahern, R.; Fernando, I.; Yarnold, J.; Evans, G.; Eccles, D.; Hodgson, S.; Ashley, S.; et al. Late toxicity is not increased in BRCA1/BRCA2 mutation carriers undergoing breast radiotherapy in the United Kingdom. Clin. Cancer Res. 2006, 12, 7025–7032. [Google Scholar] [CrossRef] [PubMed]
- Pierce, L.J.; Strawderman, M.; Narod, S.A.; Oliviotto, I.; Eisen, A.; Dawson, L.; Gaffney, D.; Solin, L.J.; Nixon, A.; Garber, J.; et al. Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J. Clin. Oncol. 2000, 18, 3360–3369. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, D.H.; Noh, J.M.; Huh, S.J.; Park, W.; Nam, S.J.; Lee, J.E. Acute skin toxicity in Korean breast cancer patients carrying BRCA mutations. Int. J. Radiat. Biol. 2014, 90, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Vliek, S.; Hilbers, F.S.; van Werkhoven, E.; Mandjes, I.; Kessels, R.; Kleiterp, S.; Lips, E.H.; Mulder, L.; Kayembe, M.T.; Loo, C.E.; et al. High-dose alkylating chemotherapy in BRCA-altered triple-negative breast cancer: The randomized phase III NeoTN trial. NPJ Breast Cancer 2023, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Vuilleumier, A.; Viassolo, V.; Ayme, A.; De Talhouet, S.; Combes, J.D.; Peron, J.; Bodmer, A.; Giraud, S.; Buisson, A.; et al. BRCA1/BRCA2 germline mutations and chemotherapy-related hematological toxicity in breast cancer patients. Breast Cancer Res. Treat. 2019, 174, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Fradley, M.; Sun, W.; Kim, J.; Zhao, X.; Pal, T.; Ismail-Khan, R. An exploratory study to determine whether BRCA1 and BRCA2 mutation carriers have higher risk of cardiac toxicity. Genes 2017, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Barac, A.; Lynce, F.; Smith, K.L.; Mete, M.; Shara, N.M.; Asch, F.M.; Nardacci, M.P.; Wray, L.; Herbolsheimer, P.; Nunes, R.A.; et al. Cardiac function in BRCA1/2 mutation carriers with history of breast cancer treated with anthracyclines. Breast Cancer Res. Treat. 2016, 155, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Pearson, E.J.; Nair, A.; Daoud, Y.; Blum, J.L. The incidence of cardiomyopathy in BRCA1 and BRCA2 mutation carriers after anthracycline-based adjuvant chemotherapy. Breast Cancer Res. Treat. 2017, 162, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Demissei, B.G.; Lv, W.; Wilcox, N.S.; Sheline, K.; Smith, A.M.; Sturgeon, K.M.; McDermott-Roe, C.; Musunuru, K.; Lefebvre, B.; Domchek, S.M.; et al. BRCA1/2 mutations and cardiovascular function in breast cancer survivors. Front. Cardiovasc. Med. 2022, 9, 833171. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Goldrat, O.; Toss, A.; Azim, H.A., Jr.; Peccatori, F.A.; Ignatiadis, M.; Del Mastro, L.; Demeestere, I. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat. Rev. 2017, 59, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Finch, A.; Lubinski, J.; Byrski, T.; Ghadirian, P.; Kim-Sing, C.; Lynch, H.T.; Ainsworth, P.J.; Neuhausen, S.L.; Greenblatt, E.; et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2013, 31, 3914–3919. [Google Scholar] [CrossRef] [PubMed]
- Ringwald, J.; Wochnowski, C.; Bosse, K.; Giel, K.E.; Schaffeler, N.; Zipfel, S.; Teufel, M. Psychological distress, anxiety, and depression of cancer-affected BRCA1/2 mutation carriers: A systematic review. J. Genet. Couns. 2016, 25, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Cecco, S.; Puligheddu, S.; Fusaroli, M.; Gerratana, L.; Yan, M.; Zamagni, C.; De Ponti, F.; Raschi, E. Emerging toxicities of antibody-drug conjugates for breast cancer: Clinical prioritization of adverse events from the FDA Adverse Event Reporting System. Target. Oncol. 2024, 19, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Xu, W.; Dai, M.; Li, S.; Xin, W.; Tong, Y.; He, C.; Mi, X.; Zhan, Z.; Fang, L. Hematological toxicity of cyclin-dependent kinase 4/6 inhibitors in patients with breast cancer: A network meta-analysis and pharmacovigilance study. Expert Opin. Drug Saf. 2024, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pang, D.; Yang, H.; Li, W.; Wang, S.; Cui, S.; Liao, N.; Wang, Y.; Wang, C.; Chang, Y.C.; et al. Neoadjuvant-adjuvant pertuzumab in HER2-positive early breast cancer: Final analysis of the randomized phase III PEONY trial. Nat. Commun. 2024, 15, 2153. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Bardia, A.; Quiroga, V.; Park, Y.H.; Blancas, I.; Alonso-Romero, J.L.; Vasiliev, A.; Adamchuk, H.; Salgado, M.; Yardley, D.A.; et al. Neoadjuvant palbociclib plus either giredestrant or anastrozole in oestrogen receptor-positive, HER2-negative, early breast cancer (coopERA Breast Cancer): An open-label, randomised, controlled, phase 2 study. Lancet Oncol. 2023, 24, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Connors, C.; Valente, S.A.; ElSherif, A.; Escobar, P.; Chichura, A.; Kopicky, L.; Roesch, E.; Ritner, J.; McIntire, P.; Wu, Y.; et al. Real-world outcomes with the KEYNOTE-522 regimen in early-stage triple-negative breast cancer. Ann. Surg. Oncol. 2025, 32, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Segura, T.; Medrano, I.H.; Collazo, S.; Maté, C.; Sguera, C.; Del Rio-Bermudez, C.; Casero, H.; Salcedo, I.; García-García, J.; Alcahut-Rodríguez, C.; et al. Symptoms timeline and outcomes in amyotrophic lateral sclerosis using artificial intelligence. Sci. Rep. 2023, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Panero, J.L.; Esteban Mur, R.; Jarque, I.; Romero-Gómez, M.; Group, S.R.; García Labrador, L.; González Calvo, J. Chronic liver disease-associated severe thrombocytopenia in Spain: Results from a retrospective study using machine learning and natural language processing. Gastroenterol. Hepatol. 2024, 47, 236–245. [Google Scholar] [CrossRef] [PubMed]
- González-Juanatey, C.; Anguita-Sánchez, M.; Barrios, V.; Núñez-Gil, I.; Gómez-Doblas, J.J.; García-Moll, X.; Lafuente-Gormaz, C.; Rollán-Gómez, M.J.; Peral-Disdier, V.; Martínez-Dolz, L.; et al. Impact of advanced age on the incidence of major adverse cardiovascular events in patients with type 2 diabetes mellitus and stable coronary artery disease in a real-world setting in spain. J. Clin. Med. 2023, 12, 5218. [Google Scholar] [CrossRef] [PubMed]
- Abrisqueta-Costa, P.; García-Marco, J.A.; Gutiérrez, A.; Hernández-Rivas, J.; Andreu-Lapiedra, R.; Arguello-Tomas, M.; Leiva-Farré, C.; López-Roda, M.D.; Callejo-Mellén, Á.; Álvarez-García, E.; et al. Real-world evidence on adverse events and healthcare resource utilization in patients with chronic lymphocytic leukaemia in Spain using natural language processing: The SRealCLL study. Cancers 2024, 16, 4004. [Google Scholar] [CrossRef] [PubMed]
| Long-Term Adverse Events | Time Span |
|---|---|
| Chest wall and breast adverse events | |
| PMPS | 7–12 years after BC diagnosis [47] |
| Lymphedema | 10 years after BC diagnosis [48] After BC primary treatment [49] |
| Skin and soft tissue affection | After BC primary treatment [50] |
| Cardiologic | |
| Heart failure | >6 months after BC diagnosis [51], 5–8 years after BC diagnosis [45], 8 years after BC diagnosis [45], 10–13 years after BC diagnosis [46], and 11 years after BC diagnosis [44] |
| Arrhythmia, acute ischemic heart disease, ischemic stroke, or transient ischemic attack | >6 months after BC diagnosis [51] |
| Neurotoxicity | |
| CIPN | >3 weeks after BC treatment [52], after the first administration of chemotherapy [53] |
| Cognitive dysfunction | During chemotherapy treatment, after cessation of treatment, >6 months post-treatment cessation, >1 year post-treatment cessation, >3 years post-treatment cessation [54] |
| Psychological alterations | |
| Anxiety | Different timepoints ranging from 1.8 to 21 years after BC diagnosis [55] |
| Depression | Different timepoints ranging from 1.8 to 21 years after BC diagnosis [55] |
| Fear of death | >1 year after BC diagnosis [56] |
| Women’s health | |
| Fatigue | After BC primary treatment, >5 years after BC diagnosis [43] |
| Hormonal alterations | After BC primary treatment [40], >8 years after BC diagnosis [57] |
| Sexual disorders | >3 years after BC diagnosis [58] |
| Reduced fertility | >2 years after BC diagnosis [59] |
| GI symptoms | |
| Diarrhea | After BC primary treatment [30] |
| Endocrine symptoms | |
| Hypothyroidism | >3 years after BC diagnosis [35] |
| Osteomuscular adverse events | |
| Osteoporosis | After BC primary treatment [60] |
| Type of Adverse Event | Prevalence | Risk Factors | Management |
|---|---|---|---|
| Chest wall and breast | |||
| PMPS [61,62] | 28.2–65% | Postoperative pain, younger age, high BMI, axillary radiation, and axillary lymph node dissection | Analgesics, surgical interventions, acupuncture, or hypnosis |
| Lymphedema [28,48,49] | 27–40% | ALND, mastectomy, adjuvant therapies, high BMI | Physiotherapy |
| Skin and soft tissue affections [31,63] | Up to 43% | Radiotherapy | Physiotherapy, anti-inflammatory drugs |
| Cardiologic | |||
| Cardiac toxicity [64,65,66] | 1–51.5% | Age, history of heart disease, maximum cumulative dose of anthracyclines, endocrine therapy, radiation to the left breast | Prevention: use of alternative chemotherapeutic agents, cardioprotective agents Treatment: same guidelines for heart failure for other causes |
| Neurologic | |||
| CIPN [29,67] | 23–80% | Age, taxane treatment, baseline neuropathy, smoking, diabetes | Duloxetine (level I evidence), venlafaxine, pregabalin, amitriptyline, and tramadol. In selected patients, acupuncture can also be an option |
| Cognitive dysfunction [54,68] | 28–33% | Age, chemotherapy, endocrine therapy | Cognitive rehabilitation, physical exercise, and low evidence for pharmacological treatment |
| Psychological alterations | |||
| Depression [32,69] | 9.4–66.1% | Younger age at diagnosis, history of psychological disorder, substance abuse, poor social support, and lower socioeconomic status. | Psychological/psychiatric support and cognitive–behavioral therapy |
| Anxiety [32,70] | 17.9–33.3% | Younger age, physical symptoms, chemotherapy, poor social and cognitive functioning, and communication problems with healthcare providers | Psychological/psychiatric support and cognitive–behavioral therapy |
| Fear of death [56,71] | 71% | Uncertain future, young age, breast-conserving surgery | Psychological/psychiatric support and cognitive–behavioral therapy |
| Women’s health | |||
| Fatigue [33,72] | 30–50% | Relation with long-term adverse events such as cardiac, menopause, or psychological | Lifestyle modifications, such as regular exercise, adequate sleep, stress reduction techniques, and treatment of other comorbidities or late adverse events |
| Hormonal alterations [34,40] | 33–48.7% | Endocrine therapy, chemotherapy | Gabapentin or SSRIs/SNRIs for hot flashes. Physical exercise, cognitive-behavioral therapy, and mindfulness |
| Sexual disorders [58,73] | 90% | Body image alterations, endocrine therapy, and psychological impairment, such as depression or anxiety | The treatment of associated factors (vaginal dryness, dyspareunia, depression, or anxiety, etc.) Sexual counseling |
| Reduced fertility [59,74] | 60% | Gonadotoxic chemotherapy | Oncofertility counseling |
| GI symptoms | |||
| Diarrhea [30] | 29.4–83% | Treatment with abemaciclib or immunotherapy | Dose reduction or interruption according to severity. Loperamide for abemaciclib toxicity and corticosteroids for immunotherapy according to severity |
| Nausea [30] | 23.0–77% | Treatment with abemaciclib, olaparib, or ribociclib | Integration of strategies to prevent or lessen its impact |
| Vomiting [30] | 40% | Treatment with olaparib | Integration of strategies to prevent or lessen its impact |
| Endocrine symptoms | |||
| Hypothyroidism [35] | 5–6% | Radiotherapy treatment | Hormonal supplementation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obispo, B.; Bailleux, C.; Cantos, B.; Zamora, P.; Jhawar, S.R.; Varghese, J.; Cabal-Hierro, L.; Luz, P.; Berrocal-Almanza, L.; Xu, X. Long-Term Adverse Events Following Early Breast Cancer Treatment with a Focus on the BRCA-Mutated Population. Cancers 2025, 17, 2506. https://doi.org/10.3390/cancers17152506
Obispo B, Bailleux C, Cantos B, Zamora P, Jhawar SR, Varghese J, Cabal-Hierro L, Luz P, Berrocal-Almanza L, Xu X. Long-Term Adverse Events Following Early Breast Cancer Treatment with a Focus on the BRCA-Mutated Population. Cancers. 2025; 17(15):2506. https://doi.org/10.3390/cancers17152506
Chicago/Turabian StyleObispo, Berta, Caroline Bailleux, Blanca Cantos, Pilar Zamora, Sachin R. Jhawar, Jajini Varghese, Lucia Cabal-Hierro, Paulo Luz, Luis Berrocal-Almanza, and Xiaoqing Xu. 2025. "Long-Term Adverse Events Following Early Breast Cancer Treatment with a Focus on the BRCA-Mutated Population" Cancers 17, no. 15: 2506. https://doi.org/10.3390/cancers17152506
APA StyleObispo, B., Bailleux, C., Cantos, B., Zamora, P., Jhawar, S. R., Varghese, J., Cabal-Hierro, L., Luz, P., Berrocal-Almanza, L., & Xu, X. (2025). Long-Term Adverse Events Following Early Breast Cancer Treatment with a Focus on the BRCA-Mutated Population. Cancers, 17(15), 2506. https://doi.org/10.3390/cancers17152506






