Cognitive Impairment in Prostate Cancer Patients Receiving Androgen Deprivation Therapy: A Scoping Review
Simple Summary
Abstract
1. Introduction
1.1. Prevalence and Treatment of Prostate Cancer
1.2. Androgen Deprivation Therapy and Side Effects
1.3. Cardiovascular and Metabolic Effects
1.4. Bone Density and Fracture Risk
1.5. Muscle Mass and Body Composition
1.6. Sexual Dysfunction
1.7. Cognitive Dysfunction
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Study Population and Setting
2.4. Data Extraction
3. Results
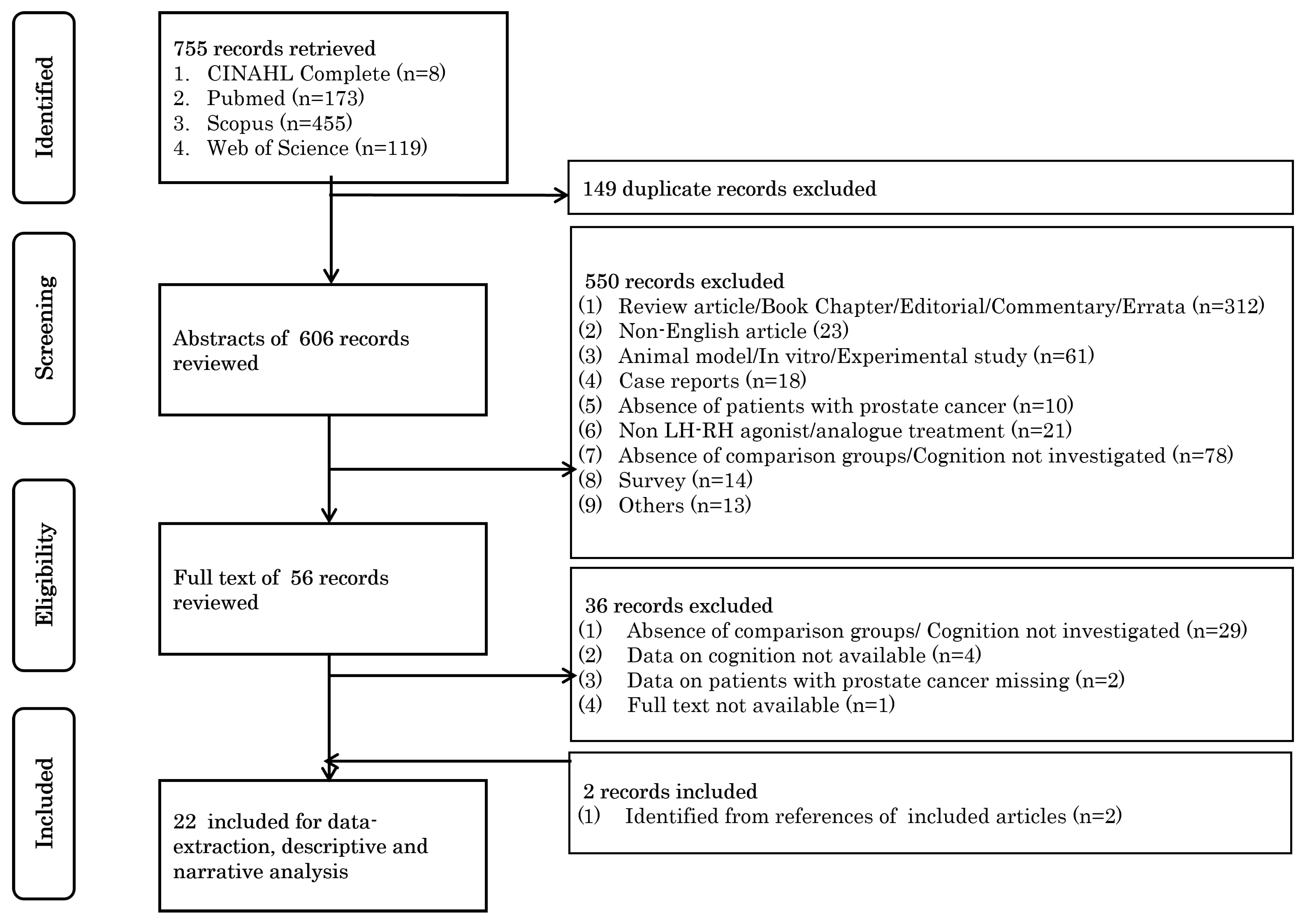
3.1. Study Selection
3.2. General Characteristics of the Studies
3.3. Study Design
3.4. Inclusion and Exclusion Criteria
3.5. Conceptual and Operational Definitions of Cognitive Performance
3.6. General Cognitive Function
3.7. Memory (Verbal and Visual)
3.8. Attention and Processing Speed
3.9. Executive Function
3.10. Visuospatial Abilities
3.11. Contents and the Effect of ADT Intervention
3.11.1. ADT Intervention
3.11.2. Effect of ADT Intervention
3.12. Memory Domains (Working, Verbal, and Visual Memory)
3.13. Attention and Processing Speed
3.14. Executive Function
3.15. Global Cognitive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Cancer Research Fund. Prostate Cancer Statistics. Available online: https://www.wcrf.org/dietandcancer/prostate-cancer-statistics/ (accessed on 2 August 2023).
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. Seer Cancer Statistics Review 1975–2012; National Cancer Institute: Bathedsa, MD, USA, 2014. [Google Scholar]
- Cowan, B.A.; Olivier, K.; Tombal, B.; Wefel, J.S. Treatment-Related Cognitive Impairment in Patients with Prostate Cancer: Patients’ Real-World Insights for Optimizing Outcomes. Adv. Ther. 2024, 41, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Oefelein, M.G.; Feng, A.; Scolieri, M.J.; Ricchiutti, D.; Resnick, M.I. Reassessment of the Definition of Castrate Levels of Testosterone: Implications for Clinical Decision Making. Urology 2000, 56, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; de Reijke, T.M.; Van Tienhoven, G.; Van den Bergh, A.C.; Oddens, J.; Poortmans, P.M.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of Androgen Suppression in the Treatment of Prostate Cancer. N. Engl. J. Med. 2009, 360, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Orihuela, E.; Goodwin, J.S. Increasing Use of Gonadotropin-Releasing Hormone Agonists for the Treatment of Localized Prostate Carcinoma. Cancer 2005, 103, 1615–1624. [Google Scholar] [CrossRef]
- Wilding, G. The Importance of Steroid Hormones in Prostate Cancer. Cancer Surv. 1992, 14, 113–130. [Google Scholar]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-Targeted Therapy in Men with Prostate Cancer: Evolving Practice and Future Considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Gründker, C.; Emons, G. The Role of Gonadotropin-Releasing Hormone in Cancer Cell Proliferation and Metastasis. Front. Endocrinol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Filicori, M. Pulsatile Gonadotropin-Releasing Hormone: Clinical Applications of a Physiologic Paradigm. FS Rep. 2023, 4, 20–26. [Google Scholar] [CrossRef]
- Cooke, B.A.; Sullivan, M.H. The Mechanisms of Lhrh Agonist Action in Gonadal Tissues. Mol. Cell Endocrinol. 1985, 41, 115–122. [Google Scholar] [CrossRef]
- Nguyen, C.; Lairson, D.R.; Swartz, M.D.; Du, X.L. Risks of Major Long-Term Side Effects Associated with Androgen-Deprivation Therapy in Men with Prostate Cancer. Pharmacotherapy 2018, 38, 999–1009. [Google Scholar] [CrossRef]
- Bosco, C.; Bosnyak, Z.; Malmberg, A.; Adolfsson, J.; Keating, N.L.; Van Hemelrijck, M. Quantifying Observational Evidence for Risk of Fatal and Nonfatal Cardiovascular Disease Following Androgen Deprivation Therapy for Prostate Cancer: A Meta-Analysis. Eur. Urol. 2015, 68, 386–396. [Google Scholar] [CrossRef]
- Goldray, D.; Weisman, Y.; Jaccard, N.; Merdler, C.; Chen, J.; Matzkin, H. Decreased Bone Density in Elderly Men Treated with the Gonadotropin-Releasing Hormone Agonist Decapeptyl (D-Trp6-Gnrh). J. Clin. Endocrinol. Metab. 1993, 76, 288–290. [Google Scholar] [CrossRef]
- Hu, J.R.; Duncan, M.S.; Morgans, A.K.; Brown, J.D.; Meijers, W.C.; Freiberg, M.S.; Salem, J.E.; Beckman, J.A.; Moslehi, J.J. Cardiovascular Effects of Androgen Deprivation Therapy in Prostate Cancer: Contemporary Meta-Analyses. Arter. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e55–e64. [Google Scholar] [CrossRef]
- Basaria, S.; Muller, D.C.; Carducci, M.A.; Egan, J.; Dobs, A.S. Hyperglycemia and Insulin Resistance in Men with Prostate Carcinoma Who Receive Androgen-Deprivation Therapy. Cancer 2006, 106, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Holmes-Walker, D.J.; Woo, H.; Gurney, H.; Do, V.T.; Chipps, D.R. Maintaining Bone Health in Patients with Prostate Cancer. Med. J. Aust. 2006, 184, 176–179. [Google Scholar] [CrossRef]
- Matsushima, H.; Taguchi, T.; Kodama, S.; Okubo, N.; Saito, K.; Jabłońska, K.; Fukumoto, S.; Matsumoto, T. Androgen Deprivation Therapy-Related Fracture Risk in Prostate Cancer: An Insurance Claims Database Study in Japan. J. Bone Min. Miner. Metab. 2024, 42, 223–232. [Google Scholar] [CrossRef]
- Clay, C.A.; Perera, S.; Wagner, J.M.; Miller, M.E.; Nelson, J.B.; Greenspan, S.L. Physical Function in Men with Prostate Cancer on Androgen Deprivation Therapy. Phys. Ther. 2007, 87, 1325–1333. [Google Scholar] [CrossRef]
- Basaria, S.; Lieb, J., 2nd; Tang, A.M.; DeWeese, T.; Carducci, M.; Eisenberger, M.; Dobs, A.S. Long-Term Effects of Androgen Deprivation Therapy in Prostate Cancer Patients. Clin. Endocrinol. 2002, 56, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Potosky, A.L.; Knopf, K.; Clegg, L.X.; Albertsen, P.C.; Stanford, J.L.; Hamilton, A.S.; Gilliland, F.D.; Eley, J.W.; Stephenson, R.A.; Hoffman, R.M. Quality-of-Life Outcomes after Primary Androgen Deprivation Therapy: Results from the Prostate Cancer Outcomes Study. J. Clin. Oncol. 2001, 19, 3750–3757. [Google Scholar] [CrossRef]
- Shim, M.; Bang, W.J.; Oh, C.Y.; Lee, Y.S.; Cho, J.S. Androgen Deprivation Therapy and Risk of Cognitive Dysfunction in Men with Prostate Cancer: Is There a Possible Link? Prostate Int. 2022, 10, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Adhya, D.; Annuario, E.; Lancaster, M.A.; Price, J.; Baron-Cohen, S.; Srivastava, D.P. Understanding the Role of Steroids in Typical and Atypical Brain Development: Advantages of Using a “Brain in a Dish” Approach. J. Neuroendocr. 2018, 30, e12547. [Google Scholar] [CrossRef]
- Leranth, C.; Petnehazy, O.; MacLusky, N.J. Gonadal Hormones Affect Spine Synaptic Density in the Ca1 Hippocampal Subfield of Male Rats. J. Neurosci. 2003, 23, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Dart, D.A.; Bevan, C.L.; Uysal-Onganer, P.; Jiang, W.G. Analysis of Androgen Receptor Expression and Activity in the Mouse Brain. Sci. Rep. 2024, 14, 11115. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, T.; Lin, S.; Zhang, B.; Li, X. Dihydrotestosterone Reduces Neuroinflammation in Spinal Cord Injury through Nf-Κb and Mapk Pathway. Cell Mol. Biol. (Noisy-Le-Grand) 2024, 70, 213–218. [Google Scholar] [CrossRef]
- Westlye, L.T.; Kaufmann, T.; Alnæs, D.; Hullstein, I.R.; Bjørnebekk, A. Brain Connectivity Aberrations in Anabolic-Androgenic Steroid Users. Neuroimage Clin. 2017, 13, 62–69. [Google Scholar] [CrossRef]
- Song, J. Amygdala Activity and Amygdala-Hippocampus Connectivity: Metabolic Diseases, Dementia, and Neuropsychiatric Issues. Biomed. Pharmacother. 2023, 162, 114647. [Google Scholar] [CrossRef] [PubMed]
- Moffat, S.D.; Zonderman, A.B.; Metter, E.J.; Kawas, C.; Blackman, M.R.; Harman, S.M.; Resnick, S.M. Free Testosterone and Risk for Alzheimer Disease in Older Men. Neurology 2004, 62, 188–193. [Google Scholar] [CrossRef]
- Hsu, B.; Cumming, R.G.; Waite, L.M.; Blyth, F.M.; Naganathan, V.; Le Couteur, D.G.; Seibel, M.J.; Handelsman, D.J. Longitudinal Relationships between Reproductive Hormones and Cognitive Decline in Older Men: The Concord Health and Ageing in Men Project. J. Clin. Endocrinol. Metab. 2015, 100, 2223–2230. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, H.; Li, S.; Zhang, D. Low Serum Testosterone Concentrations Are Associated with Poor Cognitive Performance in Older Men but Not Women. Front. Aging Neurosci. 2021, 13, 712237. [Google Scholar] [CrossRef]
- Morgans, A.K.; Renzulli, J., 2nd; Olivier, K.; Shore, N.D. Risk of Cognitive Effects in Comorbid Patients with Prostate Cancer Treated with Androgen Receptor Inhibitors. Clin. Genitourin. Cancer 2021, 19, 467.e1–467.e11. [Google Scholar] [CrossRef]
- Cai, Z.; Li, H. An Updated Review: Androgens and Cognitive Impairment in Older Men. Front. Endocrinol. 2020, 11, 586909. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.M.; Matsumoto, A.M.; Amory, J.K.; Johnson, M.; Craft, S.; Peskind, E.R.; Raskind, M.A. Characterization of Verbal and Spatial Memory Changes from Moderate to Supraphysiological Increases in Serum Testosterone in Healthy Older Men. Psychoneuroendocrinology 2007, 32, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.; Pu, S.J. A Pilot Study on the Effects of Testosterone in Hypogonadal Aging Male Patients with Alzheimer’s Disease. Aging Male 2003, 6, 13–17. [Google Scholar] [CrossRef]
- Lu, P.H.; Masterman, D.A.; Mulnard, R.; Cotman, C.; Miller, B.; Yaffe, K.; Reback, E.; Porter, V.; Swerdloff, R.; Cummings, J.L. Effects of Testosterone on Cognition and Mood in Male Patients with Mild Alzheimer Disease and Healthy Elderly Men. Arch. Neurol. 2006, 63, 177–185. [Google Scholar] [CrossRef]
- Green, H.J.; Pakenham, K.I.; Headley, B.C.; Yaxley, J.; Nicol, D.L.; Mactaggart, P.N.; Swanson, C.; Watson, R.B.; Gardiner, R.A. Altered Cognitive Function in Men Treated for Prostate Cancer with Luteinizing Hormone-Releasing Hormone Analogues and Cyproterone Acetate: A Randomized Controlled Trial. BJU Int. 2002, 90, 427–432. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Timilshina, N.; Duff-Canning, S.; Breunis, H.; Tannock, I.F.; Naglie, G.; Fleshner, N.E.; Krahn, M.D.; Warde, P.; Marzouk, S.; et al. Effects of Long-Term Androgen Deprivation Therapy on Cognitive Function over 36 Months in Men with Prostate Cancer. Cancer 2017, 123, 237–244. [Google Scholar] [CrossRef]
- Gunlusoy, B.; Ceylan, Y.; Koskderelioglu, A.; Gedizlioglu, M.; Degirmenci, T.; Ortan, P.; Kozacioglu, Z. Cognitive Effects of Androgen Deprivation Therapy in Men with Advanced Prostate Cancer. Urology 2017, 103, 167–172. [Google Scholar] [CrossRef]
- McGinty, H.L.; Phillips, K.M.; Jim, H.S.; Cessna, J.M.; Asvat, Y.; Cases, M.G.; Small, B.J.; Jacobsen, P.B. Cognitive Functioning in Men Receiving Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-Analysis. Support. Care Cancer 2014, 22, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. Prisma Extension for Scoping Reviews (Prisma-Scr): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated Methodological Guidance for the Conduct of Scoping Reviews. JBI Evid. Implement. 2021, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Alibhai, S.M.; Breunis, H.; Timilshina, N.; Johnston, C.; Tomlinson, G.; Tannock, I.; Krahn, M.; Fleshner, N.E.; Warde, P.; Canning, S.D.; et al. Impact of Androgen-Deprivation Therapy on Physical Function and Quality of Life in Men with Nonmetastatic Prostate Cancer. J. Clin. Oncol. 2010, 28, 5038–5045. [Google Scholar] [CrossRef]
- Gilbert, D.C.; Duong, T.; Kynaston, H.G.; Alhasso, A.A.; Cafferty, F.H.; Rosen, S.D.; Kanaga-Sundaram, S.; Dixit, S.; Laniado, M.; Madaan, S.; et al. Quality-of-Life Outcomes from the Prostate Adenocarcinoma: Transcutaneous Hormones (Patch) Trial Evaluating Luteinising Hormone-Releasing Hormone Agonists Versus Transdermal Oestradiol for Androgen Suppression in Advanced Prostate Cancer. BJU Int. 2017, 119, 667–675. [Google Scholar] [CrossRef]
- Green, H.J.; Pakenham, K.I.; Headley, B.C.; Yaxley, J.; Nicol, D.L.; Mactaggart, P.N.; Swanson, C.E.; Watson, R.B.; Gardiner, R.A. Quality of Life Compared During Pharmacological Treatments and Clinical Monitoring for Non-Localized Prostate Cancer: A Randomized Controlled Trial. BJU Int. 2004, 93, 975–979. [Google Scholar] [CrossRef]
- Herr, H.W.; O’Sullivan, M. Quality of Life of Asymptomatic Men with Nonmetastatic Prostate Cancer on Androgen Deprivation Therapy. J. Urol. 2000, 163, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, V.A.; Bloomfield, D.J.; Shilling, V.M.; Edginton, T.L. Does Neoadjuvant Hormone Therapy for Early Prostate Cancer Affect Cognition? Results from a Pilot Study. BJU Int. 2005, 96, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Jim, H.S.; Small, B.J.; Patterson, S.; Salup, R.; Jacobsen, P.B. Cognitive Impairment in Men Treated with Luteinizing Hormone-Releasing Hormone Agonists for Prostate Cancer: A Controlled Comparison. Support. Care Cancer 2010, 18, 21–27. [Google Scholar] [CrossRef]
- Joly, F.; Alibhai, S.M.; Galica, J.; Park, A.; Yi, Q.L.; Wagner, L.; Tannock, I.F. Impact of Androgen Deprivation Therapy on Physical and Cognitive Function, as Well as Quality of Life of Patients with Nonmetastatic Prostate Cancer. J. Urol. 2006, 176 Pt 1, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Karunasinghe, N.; Zhu, Y.; Han, D.Y.; Lange, K.; Zhu, S.; Wang, A.; Ellett, S.; Masters, J.; Goudie, M.; Keogh, J.; et al. Quality of Life Effects of Androgen Deprivation Therapy in a Prostate Cancer Cohort in New Zealand: Can We Minimize Effects Using a Stratification Based on the Aldo-Keto Reductase Family 1, Member C3 Rs12529 Gene Polymorphism? BMC Urol. 2016, 16, 48. [Google Scholar] [CrossRef]
- Lebret, T.; Culine, S.; Davin, J.L.; Hennequin, C.; Mignard, J.P.; Moreau, J.L.; Rossi, D.; Zerbib, M.; Mahmoudi, A.; Latorzeff, I. Quality of Life of 1276 Elderly Patients with Prostate Cancer, Starting Treatment with a Gonadotropin-Releasing Hormone Agonist: Results of a French Observational Study. Aging Male 2014, 17, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Tabernero, Á.J.L.; Álvarez Ossorio, J.L.; Ciria, J.P.; Domínguez-Escrig, J.L.; Vázquez, F.; Angulo, J.; López, F.J.; de La Iglesia, R.; Romero, J. Cognitive Function in Patients with Prostate Cancer Receiving Luteinizing Hormone-Releasing Hormone Analogues: A Prospective, Observational, Multicenter Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 590–594. [Google Scholar] [CrossRef]
- Okamoto, K.; Sekine, Y.; Nomura, M.; Koike, H.; Matsui, H.; Shibata, Y.; Ito, K.; Suzuki, K. Effects of a Luteinizing Hormone-Releasing Hormone Agonist on Cognitive, Sexual, and Hormonal Functions in Patients with Prostate Cancer: Relationship with Testicular and Adrenal Androgen Levels. Basic. Clin. Androl. 2015, 25, 3. [Google Scholar] [CrossRef]
- Sánchez-Martínez, V.; Buigues, C.; Navarro-Martínez, R.; García-Villodre, L.; Jeghalef, N.; Serrano-Carrascosa, M.; Rubio-Briones, J.; Cauli, O. Analysis of Brain Functions in Men with Prostate Cancer under Androgen Deprivation Therapy: A One-Year Longitudinal Study. Life 2021, 11, 227. [Google Scholar] [CrossRef]
- Wiechno, P.J.; Sadowska, M.; Kalinowski, T.; Michalski, W.; Demkow, T. Does Pharmacological Castration as Adjuvant Therapy for Prostate Cancer after Radiotherapy Affect Anxiety and Depression Levels, Cognitive Functions and Quality of Life? Psychooncology 2013, 22, 346–351. [Google Scholar] [CrossRef]
- Ihrig, A.; Pernt, P.M.; Zschäbitz, S.; Huber, J.; Friederich, H.C.; Bugaj, T.J.; Maatouk, I. Neurocognitive Effects of Androgen Deprivation Therapy and New Hormonal Agents in a Sample of Patients with Metastatic Prostate Cancer. Int. Urol. Nephrol. 2023, 55, 2733–2739. [Google Scholar] [CrossRef]
- Shah, S.I.A.; Minhas, U.M.; Nasir, N. Impact of the Duration of Androgen Deprivation Therapy for Prostate Cancer on Cognition: A Study Using Mini-Mental State Examination. Pak. J. Med. Health Sci. 2018, 12, 3. [Google Scholar]
- Cherrier, M.M.; Rose, A.L.; Higano, C. The Effects of Combined Androgen Blockade on Cognitive Function During the First Cycle of Intermittent Androgen Suppression in Patients with Prostate Cancer. J. Urol. 2003, 170, 1808–1811. [Google Scholar] [CrossRef]
- Chao, H.H.; Uchio, E.; Zhang, S.; Hu, S.; Bednarski, S.R.; Luo, X.; Rose, M.; Concato, J.; Li, C.S. Effects of Androgen Deprivation on Brain Function in Prostate Cancer Patients - a Prospective Observational Cohort Analysis. BMC Cancer 2012, 12, 371. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Akashi, Y.; Kiba, K.; Hirayama, A.; Uemura, H. Impact of Androgen Deprivation Therapy on Cognitive Function in Men with Prostate Cancer. BJUI Compass 2024, 5, 356–358. [Google Scholar] [CrossRef]
- Tan, W.W.; Heckman, M.G.; Vishnu, P.; Crook, J.E.; Younkin, L.H.; Covil, E.G.; Ferman, T.J.; Graff-Radford, N.R.; Younkin, S.G.; Smallridge, R.C.; et al. Effect of Leuprolide on Serum Amyloid-Β Peptide Levels and Memory in Patients with Prostate Cancer with Biochemical Recurrence. Urology 2013, 81, 150–154. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force Recommendations to Harmonise Studies of Cognitive Function in Patients with Cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J. Candidate Mechanisms for Chemotherapy-Induced Cognitive Changes. Nat. Rev. Cancer 2007, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
| Component | Details |
|---|---|
| Population (P) | Men with a confirmed histological diagnosis of adenocarcinoma of the prostate, including metastatic or non-metastatic, localized, or advanced prostate cancer. |
| Concept (C) | Cognitive change. |
| Context (C) | Treatment with LH-RH analogues or agonists. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreira, J.V.; Barreira, P.; Falcão, G.; Garcez, D.; Silva, P.; Santos, G.; Fontes-Sousa, M.; Leão Mendes, J.; Reis, F.; Santos, C.F.; et al. Cognitive Impairment in Prostate Cancer Patients Receiving Androgen Deprivation Therapy: A Scoping Review. Cancers 2025, 17, 2501. https://doi.org/10.3390/cancers17152501
Barreira JV, Barreira P, Falcão G, Garcez D, Silva P, Santos G, Fontes-Sousa M, Leão Mendes J, Reis F, Santos CF, et al. Cognitive Impairment in Prostate Cancer Patients Receiving Androgen Deprivation Therapy: A Scoping Review. Cancers. 2025; 17(15):2501. https://doi.org/10.3390/cancers17152501
Chicago/Turabian StyleBarreira, João Vasco, Pedro Barreira, Gil Falcão, Daniela Garcez, Pedro Silva, Gustavo Santos, Mário Fontes-Sousa, José Leão Mendes, Filipa Reis, Carla F. Santos, and et al. 2025. "Cognitive Impairment in Prostate Cancer Patients Receiving Androgen Deprivation Therapy: A Scoping Review" Cancers 17, no. 15: 2501. https://doi.org/10.3390/cancers17152501
APA StyleBarreira, J. V., Barreira, P., Falcão, G., Garcez, D., Silva, P., Santos, G., Fontes-Sousa, M., Leão Mendes, J., Reis, F., Santos, C. F., Ribeiro, F., & Capelas, M. L. (2025). Cognitive Impairment in Prostate Cancer Patients Receiving Androgen Deprivation Therapy: A Scoping Review. Cancers, 17(15), 2501. https://doi.org/10.3390/cancers17152501







