From Spice to Survival: The Emerging Role of Curcumin in Cancer Immunotherapy
Simple Summary
Abstract
1. Introduction
2. Mechanisms of Action of Curcumin
2.1. Anti-Inflammatory Properties
2.2. Antioxidant Activity
2.3. Apoptosis Induction
2.3.1. Curcumin and Intrinsic Apoptosis
2.3.2. Curcumin and Extrinsic Apoptosis
2.4. Anti-Proliferative Effects of Curcumin
2.5. Inhibition of Angiogenesis and Metastasis
3. Curcumin and the Immune System
3.1. T Cells
3.2. Macrophages
3.3. Dendritic Cells
3.4. Natural Killer Cells
4. Curcumin in Combination with Immunotherapy
4.1. Curcumin and ICIs
4.2. Curcumin and Adoptive Cell Therapy
4.3. Curcumin and Cancer Vaccinations
5. Contextual Dualities
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society|Cancer Facts & Statistics. Available online: https://cancerstatisticscenter.cancer.org/?_gl=1 (accessed on 20 July 2025).
- Bai, R.; Cui, J. Development of Immunotherapy Strategies Targeting Tumor Microenvironment is Fiercely Ongoing. Front. Immunol. 2022, 13, 890166. [Google Scholar] [CrossRef]
- Decoding the Signs of Response to Cancer Immunotherapy. Available online: https://www.nature.com/articles/d42473-019-00064-0 (accessed on 20 July 2025).
- Mishra, A.P.; Swetanshu; Singh, P.; Yadav, S.; Nigam, M.; Seidel, V.; Rodrigues, C.F. Role of the Dietary Phytochemical Curcumin in Targeting Cancer Cell Signalling Pathways. Plants 2023, 12, 1782. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-ΚB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of Transcription Factors by Curcumin. Adv. Exp. Med. Biol. 2007, 595, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, N.; Samarghandian, S.; Hashemy, S.I. Immunomodulatory, Anti-Inflammatory, and Antioxidant Effects of Curcumin. J. Herbmed Pharmacol. 2018, 7, 211–219. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Kundu, J.; Na, H.-K. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Medica 2008, 74, 1526–1539. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL Death Receptor-Based Cancer Therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]
- Bhandarkar, S.S.; Arbiser, J.L. Curcumin as an Inhibitor of Angiogenesis. Adv. Exp. Med. Biol. 2007, 595, 185–195. [Google Scholar] [CrossRef]
- Katagiri, T.; Kameda, H.; Nakano, H.; Yamazaki, S. Regulation of T Cell Differentiation by the AP-1 Transcription Factor JunB. Immunol. Med. 2021, 44, 197–203. [Google Scholar] [CrossRef]
- Deng, J.; Golub, L.M.; Lee, H.-M.; Bhatt, H.-D.; Johnson, F.; Xu, T.; Gu, Y. A Novel Modified-Curcumin 2.24 Resolves Inflammation by Promoting M2 Macrophage Polarization. Sci. Rep. 2023, 13, 15513. [Google Scholar] [CrossRef]
- Rahimi, K.; Hassanzadeh, K.; Khanbabaei, H.; Haftcheshmeh, S.M.; Ahmadi, A.; Izadpanah, E.; Mohammadi, A.; Sahebkar, A. Curcumin: A Dietary Phytochemical for Targeting the Phenotype and Function of Dendritic Cells. Curr. Med. Chem. 2020, 27, 1549–1564. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, H. Improved Anti-Cancer Effect of Curcumin on Breast Cancer Cells by Increasing the Activity of Natural Killer Cells. J. Microbiol. Biotechnol. 2018, 28, 874–882. [Google Scholar] [CrossRef]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-ΚB Signaling Pathway by the Curcumin Analog, 3,5-Bis(2-Pyridinylmethylidene)-4-Piperidone (EF31): Anti-Inflammatory and Anti-Cancer Properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of Transcription Factor NF-ΚB is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Curcumin and Resveratrol Inhibit Nuclear Factor-KappaB-Mediated Cytokine Expression in Adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear Factor-ΚB—A Pivotal Transcription Factor in Chronic Inflammatory Diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by Curcumin as Revealed by Molecular Interaction Studies. Nat. Prod. Rep. 2011, 28, 1937. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Borra, S.; Gurumurthy, P.; Mahendra, J.; Jayamathi, K.; Cherian, C.; Chand, R. Antioxidant and Free Radical Scavenging Activity of Curcumin Determined by Using Different in Vitro and Ex Vivo Models. J. Med. Plants Res. 2013, 7, 2680–2690. [Google Scholar]
- Rahban, M.; Habibi-Rezaei, M.; Mazaheri, M.; Saso, L.; Moosavi-Movahedi, A.A. Anti-Viral Potential and Modulation of Nrf2 by Curcumin: Pharmacological Implications. Antioxidants 2020, 9, 1228. [Google Scholar] [CrossRef] [PubMed]
- Sarutipaiboon, I.; Settasatian, N.; Komanasin, N.; Kukongwiriyapan, U.; Sawanyawisuth, K.; Intharaphet, P.; Senthong, V.; Settasatian, C. Association of Genetic Variations in NRF2, NQO1, HMOX1, and MT with Severity of Coronary Artery Disease and Related Risk Factors. Cardiovasc. Toxicol. 2019, 20, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.H.; Rizzi, E.S.; Alves-Lopes, R.; Pinheiro, L.C.; Tostes, R.C.; Tanus-Santos, J.E. Antioxidant and Antihypertensive Responses to Oral Nitrite Involves Activation of the Nrf2 Pathway. Free Radic. Biol. Med. 2019, 141, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 Signaling in Regulation of Antioxidants and Phase 2 Enzymes in Cardiac Fibroblasts: Protection against Reactive Oxygen and Nitrogen Species-Induced Cell Injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef]
- Delgobo, M.; Gonçalves, R.M.; Delazeri, M.A.; Falchetti, M.; Zandoná, A.; Nascimento, R.; Almeida, K.; Fagundes, A.C.; Gelain, D.P.; Fracasso, J.I.; et al. Thioredoxin Reductase-1 Levels Are Associated with NRF2 Pathway Activation and Tumor Recurrence in Non-Small Cell Lung Cancer. Free Radic. Biol. Med. 2021, 177, 58–71. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Rahban, M.; Moosavi-Movahedi, Z.; Habibi-Rezaei, M.; Saso, L.; Moosavi-Movahedi, A.A. The Potential Role of Curcumin in Modulating the Master Antioxidant Pathway in Diabetic Hypoxia-Induced Complications. Molecules 2021, 26, 7658. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Zheng, S.; Wu, T. Curcumin Induces Apoptosis through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar] [CrossRef]
- Shankar, S.; Srivastava, R. Involvement of Bcl-2 Family Members, Phosphatidylinositol 3′-Kinase/AKT and Mitochondrial P53 in Curcumin (Diferulolylmethane)-Induced Apoptosis in Prostate Cancer. Int. J. Oncol. 2007, 30, 905–918. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, S. Curcumin Induces Autophagy, Apoptosis, and Cell Cycle Arrest in Human Pancreatic Cancer Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 5787218. [Google Scholar] [CrossRef]
- Wang, M.; Ruan, Y.; Chen, Q.; Li, S.; Wang, Q.; Cai, J. Curcumin Induced HepG2 Cell Apoptosis-Associated Mitochondrial Membrane Potential and Intracellular Free Ca2+ Concentration. Eur. J. Pharmacol. 2011, 650, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.A.; Somepalli, V.; Golakoti, T.; Kanugula, A.K.; Karnewar, S.; Rajendiran, K.; Vasagiri, N.; Prabhakar, S.; Kuppusamy, P.; Kotamraju, S.; et al. Mitochondrial-Targeted Curcuminoids: A Strategy to Enhance Bioavailability and Anticancer Efficacy of Curcumin. PLoS ONE 2014, 9, e89351. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kang, H.-J.; Moon, A. Inhibition of Invasion and Induction of Apoptosis by Curcumin in H-Ras-Transformed MCF10A Human Breast Epithelial Cells. Arch. Pharmacal Res. 2001, 24, 349–354. [Google Scholar] [CrossRef]
- Karmakar, S.; Banik, N.L.; Ray, S.K. Curcumin Suppressed Anti-Apoptotic Signals and Activated Cysteine Proteases for Apoptosis in Human Malignant Glioblastoma U87MG Cells. Neurochem. Res. 2007, 32, 2103–2113. [Google Scholar] [CrossRef]
- Zhu, L.; Han, M.B.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L.X. Curcumin Triggers Apoptosis via Upregulation of Bax/Bcl-2 Ratio and Caspase Activation in SW872 Human Adipocytes. Mol. Med. Rep. 2012, 12, 1151–1156. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and DATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Buchholz, T.A.; Garg, A.K.; Chakravarti, N.; Aggarwal, B.B.; Esteva, F.J.; Kuerer, H.M.; Singletary, S.E.; Hortobagyi, G.N.; Pusztai, L.; Cristofanilli, M.; et al. The Nuclear Transcription Factor ΚB/Bcl-2 Pathway Correlates with Pathologic Complete Response to Doxorubicin-Based Neoadjuvant Chemotherapy in Human Breast Cancer. Clin. Cancer Res. 2005, 11, 8398–8402. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, Z.; Fan, Y.; Guan, B.; Jia, J.; Gao, Y.; Wang, K.; Wu, K.; Wang, X.; Zheng, P.; et al. Curcumin Enhances Temsirolimus-Induced Apoptosis in Human Renal Carcinoma Cells through Upregulation of YAP/P53. Oncol. Lett. 2016, 12, 4999–5006. [Google Scholar] [CrossRef]
- Talib, W.H.; Al-hadid, S.A.; Ali, M.B.W.; AL-Yasari, I.H.; Ali, M.R.A. Role of Curcumin in Regulating P53 in Breast Cancer: An Overview of the Mechanism of Action. Breast Cancer Targets Ther. 2018, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.Y.; Kim, Y.H.; Choi, Y.S.; Kim, D.-G.; Herschorn, S.; Bae, J.-H.; Min, D.S.; Chang, J.H.; Jeong, Y.-J.; Lee, Y.H.; et al. Molecular Mechanisms of Curcumin-Induced Cytotoxicity: Induction of Apoptosis through Generation of Reactive Oxygen Species, Down-Regulation of Bcl-XL and IAP, the Release of Cytochrome c and Inhibition of Akt. Carcinogenesis 2003, 24, 1199–1208. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, J.; Huh, T.-L.; Lee, Y.S. Curcumin Induces Apoptosis in Human Colorectal Carcinoma (HCT-15) Cells by Regulating Expression of Prp4 and P53. Mol. Cells 2013, 35, 526–532. [Google Scholar] [CrossRef]
- Beevers, C.; Zhou, H.; Huang, S. Hitting the Golden TORget: Curcumin’s Effects on MTOR Signaling. Anti-Cancer Agents Med. Chem. 2013, 13, 988–994. [Google Scholar] [CrossRef]
- Khan, K.; Quispe, C.; Javed, Z.; Iqbal, M.J.; Sadia, H.; Raza, S.; Irshad, A.; Salehi, B.; Reiner, Ž.; Sharifi-Rad, J. Resveratrol, Curcumin, Paclitaxel and MiRNAs Mediated Regulation of PI3K/Akt/MTOR Pathway: Go Four Better to Treat Bladder Cancer. Cancer Cell Int. 2020, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Kyritsis, A.P.; Galani, V.; Lazari, D.; Sioka, C.; Voulgaris, S.; Alexiou, G.A. The Role of Curcumin in Cancer: A Focus on the PI3K/Akt Pathway. Cancers 2024, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Y.; Zhang, W.; Wang, Y.; Jao, Y.; Liu, J.; Zhang, M.; He, H. AMPK/MTOR/P70S6K Axis Prevents Apoptosis of Porphyromonas Gingivalis-Infected Gingival Epithelial Cells via BadSer136 Phosphorylation. Apoptosis 2023, 28, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.-N.; Gu, Y.; Guo, Q. Deltonin Enhances Gastric Carcinoma Cell Apoptosis and Chemosensitivity to Cisplatin via Inhibiting PI3K/AKT/MTOR and MAPK Signaling. World J. Gastrointest. Oncol. 2023, 15, 1739–1755. [Google Scholar] [CrossRef]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells Selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Yang, F.; Huang, J.; Lin, X.; Zhen, Z.; Chen, X. Apoptosis in Nasopharyngeal Carcinoma Cell Line NCE Induced by Curcumin and Its Molecular Mechanism. Chin. J. Otorhinolaryngol. Head Neck Surg. 2006, 41, 612–616. [Google Scholar]
- Jung, E.M. Curcumin Sensitizes Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Mediated Apoptosis through CHOP-Independent DR5 Upregulation. Carcinogenesis 2006, 27, 2008–2017. [Google Scholar] [CrossRef]
- Shankar, S.; Chen, Q.; Sarva, K.; Siddiqui, I.; Srivastava, R.K. Curcumin Enhances the Apoptosis-Inducing Potential of TRAIL in Prostate Cancer Cells: Molecular Mechanisms of Apoptosis, Migration and Angiogenesis. J. Mol. Signal. 2007, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Lin, M.C.; Weir, A.; Huang, B.; Xiong, L.; Chua, N.K.; Pang, J.; Santavanond, J.P.; Tixeira, R.; Doerflinger, M.; et al. Caspase-8-Driven Apoptotic and Pyroptotic Crosstalk Causes Cell Death and IL-1β Release in X-Linked Inhibitor of Apoptosis (XIAP) Deficiency. EMBO J. 2023, 42, e110468. [Google Scholar] [CrossRef]
- Luan, C.; Polinati, R.M.; Ferreira, C.; Nascimento, A.; Galinis, D.; Silva; Fialho, E. Curcumin and Melphalan Cotreatment Induces Cell Cycle Arrest and Apoptosis in MDA-MB-231 Breast Cancer Cells. Sci. Rep. 2023, 13, 56–63. [Google Scholar] [CrossRef]
- Cao, A.-L.; Tang, Q.-F.; Zhou, W.-C.; Qiu, Y.-Y.; Hu, S.-J.; Yin, P.-H. Ras/ERK Signaling Pathway is Involved in Curcumin-Induced Cell Cycle Arrest and Apoptosis in Human Gastric Carcinoma AGS Cells. J. Asian Nat. Prod. Res. 2014, 17, 56–63. [Google Scholar] [CrossRef]
- Sahu, R.P.; Batra, S.; Srivastava, S.K. Activation of ATM/Chk1 by Curcumin Causes Cell Cycle Arrest and Apoptosis in Human Pancreatic Cancer Cells. Br. J. Cancer 2009, 100, 1425–1433. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Li, B.; Zhang, K.; Wu, Y.; Xu, H.; Wang, J.; Zhang, J.; Fan, R.; Wei, J. Curcumin Promotes Cell Cycle Arrest and Inhibits Survival of Human Renal Cancer Cells by Negative Modulation of the PI3K/AKT Signaling Pathway. Cell Biochem. Biophys. 2015, 73, 681–686. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Queisser, N.; Wolfson, M.L.; Fraga, C.G.; Adamo, A.M.; Oteiza, P.I. Curcumin Induces Cell-Arrest and Apoptosis in Association with the Inhibition of Constitutively Active NF-ΚB and STAT3 Pathways in Hodgkin’s Lymphoma Cells. Int. J. Cancer 2008, 123, 56–65. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, M.K.; Kim, J.H. Curcumin Induces Cell Cycle Arrest and Apoptosis in Human Osteosarcoma (HOS) Cells. Anticancer Res. 2009, 29, 5039–5044. [Google Scholar] [PubMed]
- Shi, A.; Liu, L.; Li, S.; Qi, B. Natural Products Targeting the MAPK-Signaling Pathway in Cancer: Overview. J. Cancer Res. Clin. Oncol. 2024, 150, 1–20. [Google Scholar] [CrossRef]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in Cancer Metastasis and Translational Advances. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Deng, Q.; Liu, Y.; Zhao, P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/β-Catenin Signaling Pathway. Med. Sci. Monit. 2017, 23, 163–171. [Google Scholar] [CrossRef]
- Zhdanovskaya, N.; Lazzari, S.; Caprioglio, D.; Firrincieli, M.; Maioli, C.; Pace, E.; Imperio, D.; Talora, C.; Bellavia, D.; Checquolo, S.; et al. Identification of a Novel Curcumin Derivative Influencing Notch Pathway and DNA Damage as a Potential Therapeutic Agent in T-ALL. Cancers 2022, 14, 5772. [Google Scholar] [CrossRef]
- Elamin, M.H.; Shinwari, Z.; Hendrayani, S.-F.; Al-Hindi, H.; Al-Shail, E.; Khafaga, Y.; Al-kofide, A.; Aboussekhra, A. Curcumin Inhibits the Sonic Hedgehog Signaling Pathway and Triggers Apoptosis in Medulloblastoma Cells. Mol. Carcinog. 2009, 49, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin Suppresses the Colon Cancer Proliferation by Inhibiting Wnt/β-Catenin Pathways via MiR-130a. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-K.; Xia, J.; Chen, Z.; Zhang, S.; Ahmad, A.; Miele, L.; Sarkar, F.H.; Wang, Z. Inhibitory Effect of Curcumin on Oral Carcinoma CAL-27 Cells via Suppression of Notch-1 and NF-ΚB Signaling Pathways. J. Cell. Biochem. 2011, 112, 1055–1065. [Google Scholar] [CrossRef]
- Bae, Y.H.; Ryu, J.H.; Park, H.J.; Kim, K.R.; Wee, H.J.; Lee, O.H.; Jang, H.O.; Bae, M.K.; Kim, K.W.; Bae, S.K. Mutant P53-Notch1 Signaling Axis is Involved in Curcumin-Induced Apoptosis of Breast Cancer Cells. Korean J. Physiol. Pharmacol. 2013, 17, 291. [Google Scholar] [CrossRef]
- Subramaniam, D.; Ponnurangam, S.; Ramamoorthy, P.; Standing, D.; Battafarano, R.J.; Anant, S.; Sharma, P. Curcumin Induces Cell Death in Esophageal Cancer Cells through Modulating Notch Signaling. PLoS ONE 2012, 7, e30590. [Google Scholar] [CrossRef]
- Sun, X.D.; Liu, X.E.; Huang, D.S. Curcumin Reverses the Epithelial-Mesenchymal Transition of Pancreatic Cancer Cells by Inhibiting the Hedgehog Signaling Pathway. Oncol. Rep. 2013, 29, 2401–2407. [Google Scholar] [CrossRef]
- Niewiadomski, P.; Niedziółka, S.M.; Markiewicz, Ł.; Uśpieński, T.; Baran, B.; Chojnowska, K. Gli Proteins: Regulation in Development and Cancer. Cells 2019, 8, 147. [Google Scholar] [CrossRef]
- Meng, X.; Cai, J.; Liu, J.; Han, B.; Gao, F.; Gao, W.; Zhang, Y.; Zhang, J.; Zhao, Z. Curcumin Increases Efficiency of γ-Irradiation in Gliomas by Inhibiting Hedgehog Signaling Pathway. Cell Cycle 2017, 16, 1181–1192. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef]
- Ardizzone, A.; Bova, V.; Casili, G.; Repici, A.; Lanza, M.; Giuffrida, R.; Colarossi, C.; Mare, M.; Cuzzocrea, S.; Esposito, E.; et al. Role of Basic Fibroblast Growth Factor in Cancer: Biological Activity, Targeted Therapies, and Prognostic Value. Cells 2023, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Gabrielpillai, J.; Dietrich, J.; Zarbl, R.; Strieth, S.; Schröck, F.; Dietrich, D. Fibroblast Growth Factor (FGF), FGF Receptor (FGFR), and Cyclin D1 (CCND1) DNA Methylation in Head and Neck Squamous Cell Carcinomas is Associated with Transcriptional Activity, Gene Amplification, Human Papillomavirus (HPV) Status, and Sensitivity to Tyrosine Kinase Inhibitors. Clin. Epigenetics 2021, 13, 228. [Google Scholar] [CrossRef]
- Ronca, R.; Ghedini, G.C.; Maccarinelli, F.; Sacco, A.; Locatelli, S.L.; Foglio, E.; Taranto, S.; Grillo, E.; Matarazzo, S.; Castelli, R.; et al. FGF Trapping Inhibits Multiple Myeloma Growth through C-Myc Degradation–Induced Mitochondrial Oxidative Stress. Cancer Res. 2020, 80, 2340–2354. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S. Molecular Mechanisms of Curcumin Action: Gene Expression. BioFactors 2012, 39, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Sivak, J.; Ashton, P.; Russo, L.A.; Pham, B.Q.; Kasahara, N.; Raizman, M.B.; Fini, M. Elizabeth. Curcuminoids Inhibit the Angiogenic Response Stimulated by Fibroblast Growth Factor-2, Including Expression of Matrix Metalloproteinase Gelatinase B. J. Biol. Chem. 2000, 275, 10405–10412. [Google Scholar] [CrossRef]
- Pan, Z.; Zhuang, J.; Ji, C.; Cai, Z.; Liao, W.; Huang, Z. Curcumin Inhibits Hepatocellular Carcinoma Growth by Targeting VEGF Expression. Oncol. Lett. 2018, 15, 4821–4826. [Google Scholar] [CrossRef]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Effects of Curcumin on Tumor Angiogenesis and Biomarkers, COX-2 and VEGF, in Hepatocellular Carcinoma Cell-Implanted Nude Mice. Clin. Hemorheol. Microcirc. 2006, 34, 109–115. [Google Scholar]
- Goel, A.; Boland, C.; Chauhan, D.P. Specific Inhibition of Cyclooxygenase-2 (COX-2) Expression by Dietary Curcumin in HT-29 Human Colon Cancer Cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin Inhibits Cyclooxygenase-2 Transcription in Bile Acid- and Phorbol Ester-Treated Human Gastrointestinal Epithelial Cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef]
- Gately, S.; Li, W.W. Multiple Roles of COX-2 in Tumor Angiogenesis: A Target for Antiangiogenic Therapy. Semin. Oncol. 2004, 31, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Gururaj, A.E.; Belakavadi, M.; Venkatesh, D.A.; Marmé, D.; Salimath, B.P. Molecular Mechanisms of Anti-Angiogenic Effect of Curcumin. Biochem. Biophys. Res. Commun. 2002, 297, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.; Calaf, G.M. Curcumin and Epithelial-Mesenchymal Transition in Breast Cancer Cells Transformed by Low Doses of Radiation and Estrogen. Int. J. Oncol. 2016, 48, 2534–2542. [Google Scholar] [CrossRef]
- Pouliquen, D.; Boissard, A.; Henry, C.; Coqueret, O.; Guette, C. Curcuminoids as Modulators of EMT in Invasive Cancers: A Review of Molecular Targets with the Contribution of Malignant Mesothelioma Studies. Front. Pharmacol. 2022, 13, 934534. [Google Scholar] [CrossRef]
- Waldman, A.; Fritz, J.; Lenardo, M. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Eckert, R.L. Curcumin Suppresses AP1 Transcription Factor-Dependent Differentiation and Activates Apoptosis in Human Epidermal Keratinocytes. J. Biol. Chem. 2006, 282, 6707–6715. [Google Scholar] [CrossRef]
- Guilhem Lalle; Twardowski, J.; Grinberg-Bleyer, Y. NF-KB in Cancer Immunity: Friend or Foe? Cells 2021, 10, 355. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Jiang, B.; Guo, J. The Roles of Curcumin in Regulating the Tumor Immunosuppressive Microenvironment (Review). Oncol. Lett. 2020, 19, 3059–3070. [Google Scholar] [CrossRef]
- Kondělková, K.; Vokurková, D.; Krejsek, J.; Borská, L.; Fiala, Z.; Andrýs, C. Regulatory T Cells (Treg) and Their Roles in Immune System with Respect to Immunopathological Disorders. Acta Medica 2010, 53, 73–77. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Zou, J.Y.; Su, C.H.; Luo, H.H.; Lei, Y.Y.; Zeng, B.; Zhu, H.S.; Chen, Z.G. Curcumin Converts Foxp3+ Regulatory T Cells to T Helper 1 Cells in Patients with Lung Cancer. J. Cell. Biochem. 2018, 119, 1420–1428. [Google Scholar] [CrossRef]
- Kitz, A.; Dominguez-Villar, M. Molecular Mechanisms Underlying Th1-like Treg Generation and Function. Cell. Mol. Life Sci. 2017, 74, 4059–4075. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Chen, S.; Liang, H.; Ji, Y.; Kou, H.; Zhang, C.; Shang, G.; Shang, C.; Song, Z.; Yang, L.; Liu, L.; et al. Curcumin Modulates the Crosstalk between Macrophages and Bone Mesenchymal Stem Cells to Ameliorate Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 634650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin Modulates Macrophage Polarization through the Inhibition of the Toll-like Receptor 4 Expression and Its Signaling Pathways. Cell. Physiol. Biochem. 2015, 36, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Dong, G.; Guo, L.; Graves, D.T. The Function of Dendritic Cells in Modulating the Host Response. Mol. Oral Microbiol. 2018, 33, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Marciscano, A.E.; Anandasabapathy, N. The Role of Dendritic Cells in Cancer and Anti-Tumor Immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of Natural Killer Cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Portale, F.; Di Mitri, D. NK Cells in Cancer: Mechanisms of Dysfunction and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 9521. [Google Scholar] [CrossRef]
- Regis, S.; Dondero, A.; Caliendo, F.; Bottino, C.; Castriconi, R. NK Cell Function Regulation by TGF-β-Induced Epigenetic Mechanisms. Front. Immunol. 2020, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Jyothi, M.; Khar, A. Differential Modulation of Nitric Oxide Production by Curcumin in Host Macrophages and NK Cells. FEBS Lett. 2000, 483, 78–82. [Google Scholar] [CrossRef]
- Fiala, M. Curcumin and Omega-3 Fatty Acids Enhance NK Cell-Induced Apoptosis of Pancreatic Cancer Cells but Curcumin Inhibits Interferon-γ Production: Benefits of Omega-3 with Curcumin against Cancer. Molecules 2015, 20, 3020–3026. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, M.; Rapak, A. Effect of Natural Compounds on NK Cell Activation. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Day, D.; Nicholls, S.J.; Segelov, E. Immune Checkpoint Inhibitor Therapy in Oncology. JACC CardioOncology 2022, 4, 579–597. [Google Scholar] [CrossRef]
- Lim, S.-O.; Li, C.-W.; Xia, W.; Cha, J.-H.; Chan, L.-C.; Wu, Y.; Chang, S.-S.; Lin, W.-C.; Hsu, J.-M.; Hsu, Y.-H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef]
- Xiao, Z.; Su, Z.; Han, S.; Huang, J.; Lin, L.; Shuai, X. Dual PH-Sensitive Nanodrug Blocks PD-1 Immune Checkpoint and Uses T Cells to Deliver NF-ΚB Inhibitor for Antitumor Immunotherapy. Sci. Adv. 2020, 6, eaay7785. [Google Scholar] [CrossRef]
- Hoos, A. Development of Immuno-Oncology Drug—From CTLA4 to PD1 to the next Generations. Nat. Rev. Drug Discov. 2016, 15, 235–247. [Google Scholar] [CrossRef]
- Guo, L.; Li, H.; Fan, T.; Ma, Y.; Wang, L. Synergistic Efficacy of Curcumin and Anti-Programmed Cell Death-1 in Hepatocellular Carcinoma. Life Sci. 2021, 279, 119359. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced Anti-Tumor Effects of the PD-1 Blockade Combined with a Highly Absorptive Form of Curcumin Targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Roque, D.; Reader, J.; Lin, J. Combined Inhibition of IL-6 and IL-8 Pathways Suppresses Ovarian Cancer Cell Viability and Migration and Tumor Growth. Int. J. Oncol. 2022, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lu, Z.; Tang, L.; Wu, Z.; Wang, D.; Zheng, J.; Qiu, Q. Curcumin Inhibits Suppressive Capacity of Naturally Occurring CD4+CD25+ Regulatory T Cells in Mice in Vitro. Int. Immunopharmacol. 2012, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of Immune Checkpoints—PD-1, PD-L1, CTLA-4—New Opportunities for Cancer Patients and a New Challenge for Internists and General Practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, W.; Da, J.; Xu, M.; Wang, Y.; Zhou, J.; Wang, Z. Bisdemethoxycurcumin in Combination with α-PD-L1 Antibody Boosts Immune Response against Bladder Cancer. OncoTargets Ther. 2017, 10, 2675–2683. [Google Scholar] [CrossRef]
- Cancer Research Institute. Adoptive Cell Therapy. Available online: https://www.cancerresearch.org/immunotherapy-by-treatment-types/adoptive-cell-therapy (accessed on 20 July 2025).
- Chang, Y.-F.; Chuang, H.-Y.; Hsu, C.-H.; Liu, R.-S.; Gambhir, S.S.; Hwang, J.-J. Immunomodulation of Curcumin on Adoptive Therapy with T Cell Functional Imaging in Mice. Cancer Prev. Res. 2011, 5, 444–452. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-Cell Exhaustion in the Tumor Microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Limsakul, P.; Srifa, P.; Huang, Z.; Zhu, L.; Wu, Y.; Charupanit, K. Immunomodulatory Effects of Curcumin on CAR T-Cell Therapy. Antioxidants 2025, 14, 454. [Google Scholar] [CrossRef]
- Benmebarek, M.-R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef]
- Paul, S.; Sa, G. Curcumin as an Adjuvant to Cancer Immunotherapy. Front. Oncol. 2021, 11, 675923. [Google Scholar] [CrossRef]
- Yang, Y. Cancer Immunotherapy: Harnessing the Immune System to Battle Cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef]
- Lu, Y.; Miao, L.; Wang, Y.; Xu, Z.; Zhao, Y.; Shen, Y.; Xiang, G.; Huang, L. Curcumin Micelles Remodel Tumor Microenvironment and Enhance Vaccine Activity in an Advanced Melanoma Model. Mol. Ther. 2016, 24, 364–374. [Google Scholar] [CrossRef]
- Singh, M.; Ramos, I.; Asafu-Adjei, D.; Quispe-Tintaya, W.; Chandra, D.; Jahangir, A.; Zang, X.; Aggarwal, B.B.; Gravekamp, C. Curcumin Improves the Therapeutic Efficacy of L Isteria at-M Age-B Vaccine in Correlation with Improved T-Cell Responses in Blood of a Triple-Negative Breast Cancer Model 4T1. Cancer Med. 2013, 2, 571–582. [Google Scholar] [CrossRef]
- Jiang, G.-M.; Xie, W.-Y.; Wang, H.-S.; Du, J.; Wu, B.-P.; Xu, W.; Liu, H.-F.; Xiao, P.; Liu, Z.-G.; Li, H.-Y.; et al. Curcumin Combined with FAPαc Vaccine Elicits Effective Antitumor Response by Targeting Indolamine-2,3-Dioxygenase and Inhibiting EMT Induced by TNF-α in Melanoma. Oncotarget 2015, 6, 25932–25942. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Girisa, S.; Chetty, B.B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Volak, L.P.; Hanley, M.J.; Masse, G.; Hazarika, S.; Harmatz, J.S.; Badmaev, V.; Majeed, M.; Greenblatt, D.J.; Court, M.H. Effect of a Herbal Extract Containing Curcumin and Piperine on Midazolam, Flurbiprofen and Paracetamol (Acetaminophen) Pharmacokinetics in Healthy Volunteers. Br. J. Clin. Pharmacol. 2013, 75, 450–462. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Kothaplly, S.; Alukapally, S.; Nagula, N.; Maddela, R. Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans under Fasting Conditions. Adv. Ther. 2022, 39, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L. Safety of Anal Curcumin. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT06626230 (accessed on 21 July 2025).
- Curcumin Supplementation in Cervical Cancer. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT06080841 (accessed on 21 July 2025).
- Curcumin to Improve Inflammation and Symptoms in Patients with Clonal Cytopenia of Undetermined Significance, Low Risk Myelodysplastic Syndrome, and Myeloproliferative Neoplasms. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT06063486 (accessed on 21 July 2025).
- Curcumin VS Photo-bio-modulation Therapy of Oral Mucositis in Pediatric Patients Undergoing Anti-Cancer Non-invasive Treatment. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT06044142 (accessed on 21 July 2025).
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Kakhaki, R.D.; Saghafi, F. Efficacy of Curcumin for Amelioration of Radiotherapy-Induced Oral Mucositis: A Preliminary Randomized Controlled Clinical Trial. BMC Cancer 2023, 23, 1–9. [Google Scholar] [CrossRef]
- Role of Curcumin in Paclitaxel Induced PN. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT05966441?term=AREA%5BConditionSearch%5D(%22Neuropathy%22)%20AND%20AREA%5BInterventionSearch%5D(%22Antirheumatic%20Agents%22)&rank=10 (accessed on 21 July 2025).
- Concomitant Curcumin Palliative Radiotherapy in Advanced Cervical Cancer Trial (CuPRAC). Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT05947513 (accessed on 21 July 2025).
- Holdhoff, M.; Sahebjam, S.; Huang, P.; Kamson, D.O.; Iacoboni, M.; Dobson-Brown, T.; Schreck, K.C.; Kleinberg, L.; Redmond, K.J.; Croog, V.J.; et al. Liposomal Curcumin and Standard Radiation and Temozolomide for Newly Diagnosed High-Grade Gliomas: A Phase 1/2 Study. J. Clin. Oncol. 2025, 43 (Suppl. S16), TPS2095. [Google Scholar] [CrossRef]
- Clinical Study of Curcumin in Preventing Postoperative Adhesion of Bilateral Vocal Cords. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT05688488?a=1 (accessed on 21 July 2025).
- Thambamroong, T.; Seetalarom, K.; Saichaemchan, S.; Pumsutas, Y.; Prasongsook, N. Efficacy of Curcumin on Treating Cancer Anorexia-Cachexia Syndrome in Locally or Advanced Head and Neck Cancer: A Double-Blind, Placebo-Controlled Randomised Phase IIa Trial (CurChexia). J. Nutr. Metab. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Curcumin for the Treatment of Stage I-III Invasive Breast Cancer in Patients Undergoing Surgery. Cancer.gov. Available online: https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2020-00423&r=1 (accessed on 21 July 2025).
- Choi, Y.H.; Han, D.H.; Kim, S.; Kim, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Role of Curcumin in Prostate Cancer Patients with Intermittent Androgen Deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef]
- De Jaeghere, E.A.; Tuyaerts, S.; Van Nuffel, A.M.T.; Belmans, A.; Bogaerts, K.; Baiden-Amissah, R.; Lippens, L.; Vuylsteke, P.; Henry, S.; Trinh, X.B.; et al. Pembrolizumab, Radiotherapy, and an Immunomodulatory Five-Drug Cocktail in Pretreated Patients with Persistent, Recurrent, or Metastatic Cervical or Endometrial Carcinoma: Results of the Phase II PRIMMO Study. Cancer Immunol. Immunother. CII 2023, 72, 475–491. [Google Scholar] [CrossRef]
- Judith, P.J.; Maureen, B.; Mélanie, P.; Fabrice, K.; Isabelle, V.; Pascale, D.; Catherine, A.; Jean-Marc, N.; Hervé, C.; Valérie, D.; et al. Curcumin’s Effect in Advanced and Metastatic Breast Cancer Patients Treated with First or Second-Line Docetaxel: A Randomized Trial. Health Sci. Rep. 2024, 7, e70052. [Google Scholar] [CrossRef] [PubMed]
- Montalvan-Sanchez, E.E.; Hernandez-Marrero, J.; Norwood, D.A.; María González-Pons; Dominguez, R.L.; Rodriguez, L.M.; Richmond, E.; Limburg, P.J.; Cruz-Correa, M.; Morgan, D.R. Establishment of a Mesoamerican-Caribbean South-South Research Platform: Challenges in the Meriva (Curcuminoids) Gastric Cancer Chemoprevention Trial. Cancer Prev. Res. 2024, 17, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Prophylactic Topical Agents in Reducing Radiation-Induced Dermatitis in Patients With Non-inflammatory Breast Cancer (Curcumin-II). Clinicaltrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT02556632?term=AREA%5BConditionSearch%5D(Breast%20Cancer)%20AND%20AREA%5BBasicSearch%5D(curcumin)&rank=10 (accessed on 21 July 2025).
- Esfahani, K.; Boodaghians, L.; Kasymjanova, G.; Agulnik, J.S.; Pepe, C.; Sakr, L.; Small, D.I.; Jagoe, T.R.; Cohen, V. A Phase I Open Prospective Cohort Trial of Curcumin plus Tyrosine Kinase Inhibitors for EGFR-Mutant Advanced Non-Small Cell Lung Cancer. J. Clin. Oncol. 2019, 37 (Suppl. S15), e20611. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A Phase 1 Dose-Escalation Study on the Safety, Tolerability and Activity of Liposomal Curcumin (LipocurcTM) in Patients with Locally Advanced or Metastatic Cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef]
- Curcumin and Cholecalciferol in Treating Patients with Previously Untreated Stage 0-II Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT02100423?term=%22vitamin%20D%22%20AND%20%22CLL%22&viewType=Table&rank=2&tab=results (accessed on 21 July 2025).
- Tuyaerts, S.; Rombauts, K.; Everaert, T.; Van Nuffel, A.M.T.; Amant, F. A Phase 2 Study to Assess the Immunomodulatory Capacity of a Lecithin-Based Delivery System of Curcumin in Endometrial Cancer. Front. Nutr. 2019, 5, 138. [Google Scholar] [CrossRef]
- Radiosensitizing and Radioprotectve Effects of Curcumin in Prostate Cancer. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT01917890 (accessed on 21 July 2025).
- Phase II Study of Curcumin vs. Placebo for Chemotherapy-Treated Breast Cancer Patients Undergoing Radiotherapy. Clini-caltrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT01740323?term=AREA%5BBasicSearch%5D(curcumin%26pg%3D1)&rank=1 (accessed on 21 July 2025).
- Curcumin Biomarkers. Clinicaltrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT01333917?term=Curcumin&aggFilters=status:&viewType=Table&rank=8 (accessed on 21 July 2025).
- Curcumin Biomarker Trial in Head and Neck Cancer. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT01160302 (accessed on 21 July 2025).
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for Radiation Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Thirty Breast Cancer Patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef]
- Use of Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP). Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT00927485 (accessed on 21 July 2025).
- Phase III Trial of Gemcitabine, Curcumin and Celebrex in Patients With Metastatic Colon Cancer. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT00295035?id=%22NCT00295035%22OR%22NCT03026140%22OR%22NCT00005094%22OR%22NCT00159484%22OR%22NCT01729923%22&rank=3 (accessed on 21 July 2025).
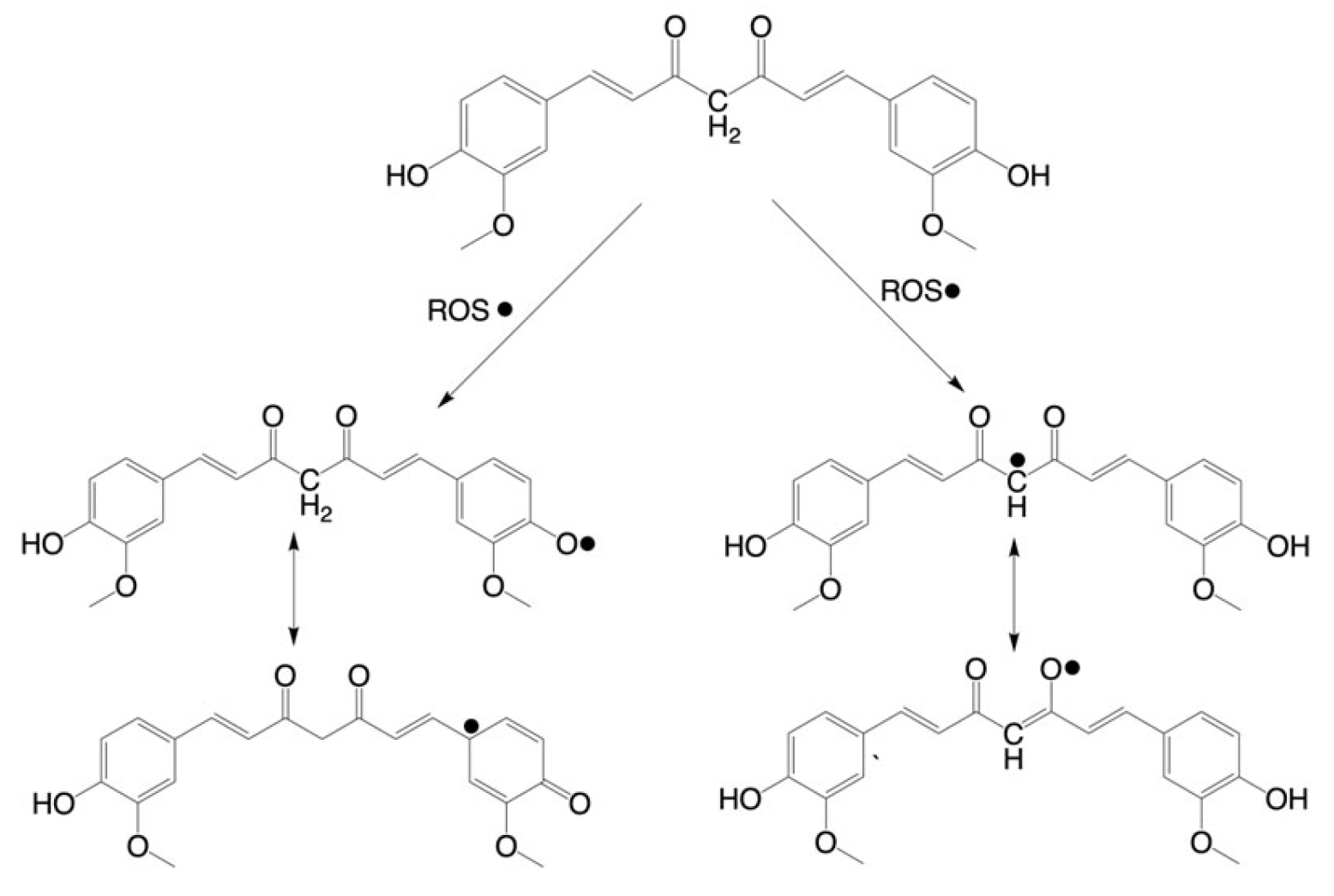
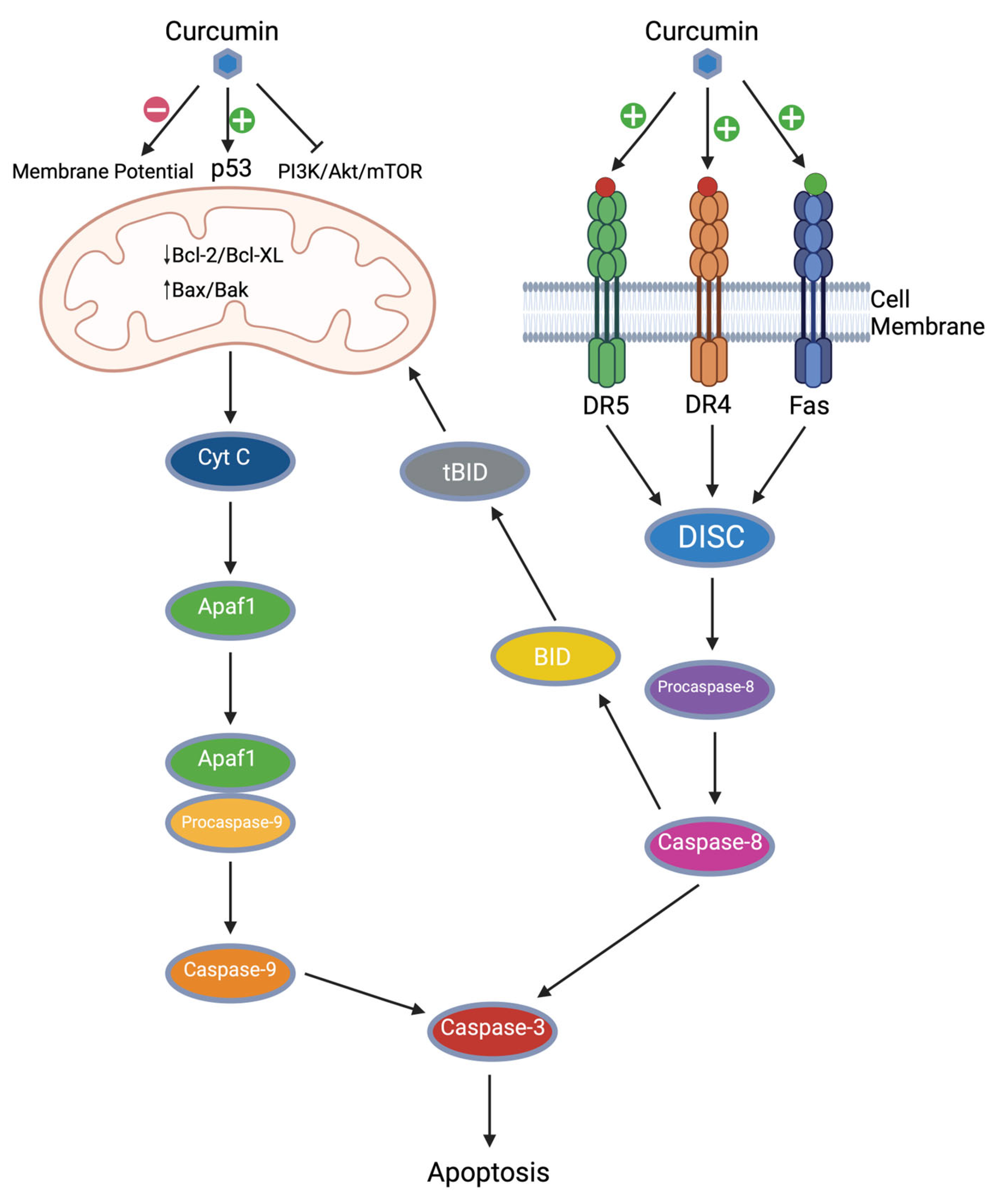

| Downstream Effect | Downstream Effect | Result | Reference |
|---|---|---|---|
| Anti-Inflammatory | ↓ TNF-⍺ | ↓ Inflammation | [8,19] |
| ↓IL-1 | ↓ Inflammation | [8,19] | |
| ↓ IL-6 | ↓ Inflammation | [8,19] | |
| ↓ IL-8 | ↓ Inflammation | [8] | |
| ↓ COX-2 | ↓ Inflammation | [8,19] | |
| ↓ iNOS | ↓ Inflammation | [20] | |
| Apoptosis | ↑ Bax | ↑ Apoptosis | [31,36,38,39,40] |
| ↑ Bak | ↑ Apoptosis | [31] | |
| ↑ PUMA | ↑ Apoptosis | [31] | |
| ↓ Bcl-2 | ↑ Apoptosis | [31,39,40] | |
| ↓ Bcl-XL | ↑ Apoptosis | [31,39,40] | |
| ↑ DR4 | ↑ Apoptosis | [50,52] | |
| ↑ DR5 | ↑ Apoptosis | [50,52] | |
| Proliferative | ↑ G2/M Arrest (via ATM/Chk1/p53) | ↓ Cancer Cell Proliferation | [7,47,60] |
| ↓ PI3K/Akt/STAT3 | ↓ Cancer Cell Proliferation | [47,59,78] | |
| Angiogenic | ↓ VEGF | ↓ Angiogenesis | [85] |
| ↓ FGF | ↓ Angiogenesis | [78] | |
| ↓ MMP-9 | ↓ Angiogenesis | [78] | |
| ↓ COX-2 | ↓ Angiogenesis | [84] | |
| ↓ PI3K/Akt/mTOR | ↓ Angiogenesis | [78] |
| Immune Cell Type | Subtype | Curcumin’s Effect | Mechanism | Reference |
|---|---|---|---|---|
| T Cells | Naïve T Cells | Inhibits activation and differentiation | Suppresses AP-1 and NF-κB signaling. Reduces IL-2 production and T-cell differentiation | [12] |
| Effector T Cells | Enhances resistance to apoptosis | Maintains NF-κB signaling homeostasis under oxidative stress | [12,91,92,94] | |
| Regulatory T cells | Converts Tregs to Th1 cells | Increases expression of IFN-γ, activating Jak/STAT1 cascade | [93,94,95,96] | |
| Macrophages | M1 Macrophages | Inhibits M1 polarization | Inhibits TLR-4-mediated signaling pathways | [99] |
| M2 macrophages | Inhibits M2 production | Inhibits IL-1β, TNF-⍺, CCR7, and iNOS preventing overproduction of M2 macrophages | [97,98] | |
| Dendritic Cells | Immature DCs | Inhibits maturation and antigen presentation | Blocks maturation markers, cytokines, and chemokines, including NF-κB, AP-1, and MAPK pathways | [14,100,101] |
| Mature DCs | Reduces immunostimulatory activity | Decreases expression of co-stimulatory molecules | [14,100,101] | |
| Natural Killer Cells | Enhances activity and cytotoxicity | Increases expression of CD56 and CD16, stimulates iNOS and nitric oxide production, and promotes IFN-γ | [15,104,105,106,107] |
| Trial Type | Trial Status | Cancer Focus | Intervention | Key Takeaways | Reference |
|---|---|---|---|---|---|
| NCT06626230 (Phase 1) | Ongoing | Safety | Curcumin via anal suppository | Objective: Trial testing for the best dose of curcumin in HIV patients with anal lesions. | [135] |
| NCT06080841 (Phase 1) | Ongoing | Cervical Cancer | Oral curcumin | Objective: Does curcumin supplementation increase the levels of p53 and apoptosis in cervical cancer patients, and what is the optimal dose of supplementation. | [136] |
| NCT06063486 (Phase 2) | Ongoing | Hematologic Cancers | Oral curcumin | Objective: Used to compare the change in inflammatory cytokine levels in patients treated with curcumin vs. placebo, as well as to measure symptomology in these groups. | [137] |
| NCT06044142 (Phase 1) | Ongoing | Pediatric Cancer | Curcumin vs. photo-biomodulation therapy | Objective: Trial will assess the impact of non-invasive photodynamic therapy with curcumin and photo-bio-modulation low-level laser treatment in managing mucositis induced by chemotherapy in pediatric patients. | [138] |
| NCT05982197 | Completed | Head and Neck Cancers | Topical curcumin against radiotherapy-induced oral mucositis | Outcome: Both curcumin mouthwash and nanocapsules were effective, safe, and well tolerated in treating radiation-induced oral mucositis, allowing for radiation to be better tolerated. | [139] |
| NCT05966441 (Phase 2) | Ongoing | Breast Cancer | Curcumin in combination with paclitaxel to prevent peripheral neuropathy | Objective: Trial testing for the neuroprotective efficacy of 2 g oral curcumin in breast cancer patients. Peripheral neuropathy is a major side effect of paclitaxel chemotherapy. | [140] |
| NCT05947513 | Ongoing | Cervical Cancer | Curcumin with palliative radiotherapy | Objective: Is adding curcumin to standard-of-care palliative radiotherapy of cervical cancer patients feasible and improve therapeutic responses while maintaining patient safety? To test, 250 mg curcumin capsules will be administered 4 times daily. | [141] |
| NCT05768919 (Phase I/II) | Ongoing | Gliomas | Liposomal curcumin with radiotherapy and temozolomide | Objectives: This study implements liposomal curcumin with standard radiation and adjuvant temozolomide in high-grade glioma patients to test dosage, safety, and feasibility. | [142] |
| NCT05688488 (Phase I/II) | Ongoing | Bilateral Vocal Cords | Curcumin use to prevent vocal cord adhesion in laryngeal papilloma surgery | Objectives: This study tests topical application of curcumin on bilateral vocal cords following vocal cord endoscopy to relieve vocal cord adhesion. | [143] |
| NCT04208334 (Phase IIa) | Completed | Head and Neck Cancer | Curcumin supplementation in Cancer anorexia–cachexia syndrome | Outcome: There was a significant benefit from curcumin in treating cancer anorexia–cachexia syndrome in patients with head and neck cancers by restoring metabolic processes. | [144] |
| NCT03980509 (Phase 1) | Ongoing | Breast Cancer | Curcumin window trial before surgery | Objective: Can curcumin reduce cancer load for stage I-III invasive breast cancer patients prior to undergoing surgery? | [145] |
| NCT03211104 | Completed | Prostate Cancer | Oral curcumin with intermittent androgen deprivation therapy | Outcome: Patients in the curcumin treatment group had significantly lower PSA progression compared to the placebo group. Additionally, there were significantly less adverse events in the curcumin group. However, curcumin did not reduce the amount of time patients could be off intermittent androgen deprivation. | [146] |
| NCT03192059 (Phase II) | Completed | Cervical Cancer | Immune modulatory 5-drug cocktail with pembrolizumab and radiation | Outcome: A 5-drug cocktail including cyclophosphamide, aspirin, lansoprazole, vitamin D, and curcumin started 2 weeks prior to radioimmunotherapy, resulted in significantly higher proportion of peripheral T cells in responders compared to nonresponders. Additionally, health-related quality of life was stable over time with acceptable toxicity. This trial failed to meet its primary objective, which was to measure objective response rate at week 26. | [147] |
| NCT03072992 (Phase II) | Completed | Breast Cancer | Curcumin with chemotherapy | Outcome: Curcumin in combination with docetaxel showed no difference in objective response rate, with a slight tendency toward longer progression-free survival at 12 months, which did not reach significance. | [148] |
| NCT02782949 (Phase II) | Ongoing | Gastric Cancer | Curcumin | Objective: Test whether patients treated with 500mg of curcumin for 180 days will have a protective effect against gastric cancer. | [149] |
| NCT02556632 (Phase II) | Completed | Breast Cancer | Topical curcumin in reducing radiation-induced dermatitis | Outcome: This study found that 2% topical curcumin significantly reduced redness and irritation of radiation-induced dermatitis in patients with non-inflammatory breast cancer, indicating curcumin made radiation therapy more tolerable. | [150] |
| NCT02321293 | Completed | Non-small cell lung cancer | Curcumin with tyrosine kinase inhibitors | Outcome: This study’s main goal was to examine feasibility and safety of curcumin treatment. | [151] |
| NCT02138955 (Phase 1) | Completed | General Cancer | Tolerance of IV liposomal curcumin (Lipocurc) | Outcome: The purpose of the study was to test dosage of IV curcumin. This trial found that 300 mg/m^2 liposomal curcumin was the maximal dose tolerated over 6h. The participants in the study included patients with metastatic tumors. | [152] |
| NCT02100423 (Phase II) | Completed | Chronic Lymphocytic Leukemia | Curcumin and cholecalciferol | Outcome: This study found that curcumin in combination with cholecalciferol was well tolerated and did not result in disease progression. Additionally, patients receiving daily oral treatment in cycles resulted in response-based continuation possible for up to 2 years. | [153] |
| NCT02017353 (Phase II) | Completed | Endometrial Cancer | Curcumin | Outcome: This study tested whether curcumin changed biomarkers in endometrial cancer; they found no difference in inflammatory biomarkers with a non-significant trend to improve quality of life. Finally, there was significant interpatient variability in biomarker levels. | [154] |
| NCT01917890 (Phase I) | Completed | Prostate Cancer | Curcumin and urosolic acid | Outcome: Unknown. | [155] |
| NCT01740323 (Phase II) | Completed | Breast Cancer | Curcumin (Meriva) | Outcome: This trial treated breast cancer patients undergoing radiation therapy. They found that 500 mg Meriva did not statistically affect markers of inflammation, but they found it may be beneficial for fatigue in women treated with neoadjuvant chemotherapy. | [156] |
| NCT01333917 (Phase 1) | Completed | Colorectal | Curcumin | Outcome: Participants received 4 g curcumin daily, followed by rectal biopsies to assess curcumin as a chemopreventative agent. No results have been published despite the clinical trial being marked as complete. | [157] |
| NCT01160302 (Phase I) | Completed | Head and Neck Cancer | Curcumin | Objective: Tested curcumin’s biomarker response in head and neck cancers. Despite data collection being complete, no data has been made available. | [158] |
| NCT01042938 (Phase II) | Completed | Breast Cancer | Curcumin | Outcome: This study tested whether curcumin could reduce radiation-induced dermatitis in breast cancer patients. They found that 2 g of oral curcumin three times a day resulted in reduced radiation dermatitis severity. | [159] |
| NCT00927485 | Completed | Familial Adenomatous Polyposis | Curcumin | Outcome: 4 g of oral curcumin for 30 days resulted in a significantly reduced number of aberrant crypt foci. | [160] |
| NCT00295035 (Phase III) | Ongoing | Colon Cancer | Curcumin with gemcitabine and celecoxib | Objective: The primary goal is to assess whether the combination therapy can increase the median time to tumor progression from 2.7 months to 4.0 months in metastatic colon cancer patients. | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, J.M.; Zhao, L.; Mayberry, T.G.; Cowan, B.C.; Wakefield, M.R.; Fang, Y. From Spice to Survival: The Emerging Role of Curcumin in Cancer Immunotherapy. Cancers 2025, 17, 2491. https://doi.org/10.3390/cancers17152491
Parker JM, Zhao L, Mayberry TG, Cowan BC, Wakefield MR, Fang Y. From Spice to Survival: The Emerging Role of Curcumin in Cancer Immunotherapy. Cancers. 2025; 17(15):2491. https://doi.org/10.3390/cancers17152491
Chicago/Turabian StyleParker, Jacob M., Lei Zhao, Trenton G. Mayberry, Braydon C. Cowan, Mark R. Wakefield, and Yujiang Fang. 2025. "From Spice to Survival: The Emerging Role of Curcumin in Cancer Immunotherapy" Cancers 17, no. 15: 2491. https://doi.org/10.3390/cancers17152491
APA StyleParker, J. M., Zhao, L., Mayberry, T. G., Cowan, B. C., Wakefield, M. R., & Fang, Y. (2025). From Spice to Survival: The Emerging Role of Curcumin in Cancer Immunotherapy. Cancers, 17(15), 2491. https://doi.org/10.3390/cancers17152491






