Principles of Endoscopic Surveillance of Extrapapillary Duodenal Lesions in Familial Adenomatous Polyposis: A 14-Year Single-Center Observation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Surveillance Endpoints
- Detection of invasive cancer and subsequent qualification for surgical resection.
- Excision of high-risk lesions, such as polyps with diameter >10 mm, foci of high-grade dysplasia, and LSTs.
- Evaluation of polyposis severity using the Spigelman score.
2.2. Diagnostic Criteria for Polyposis
2.3. Statistical Analysis
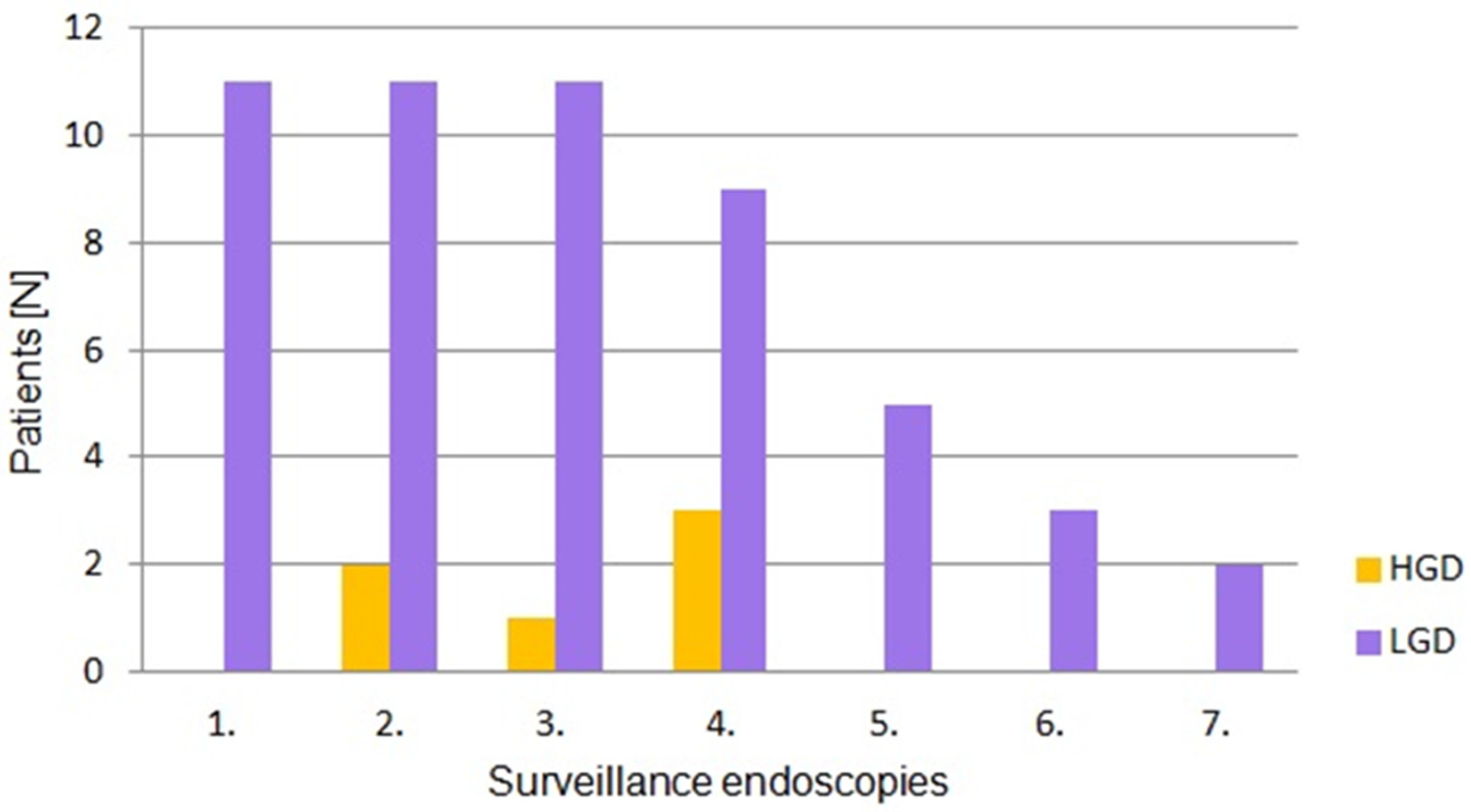
3. Results
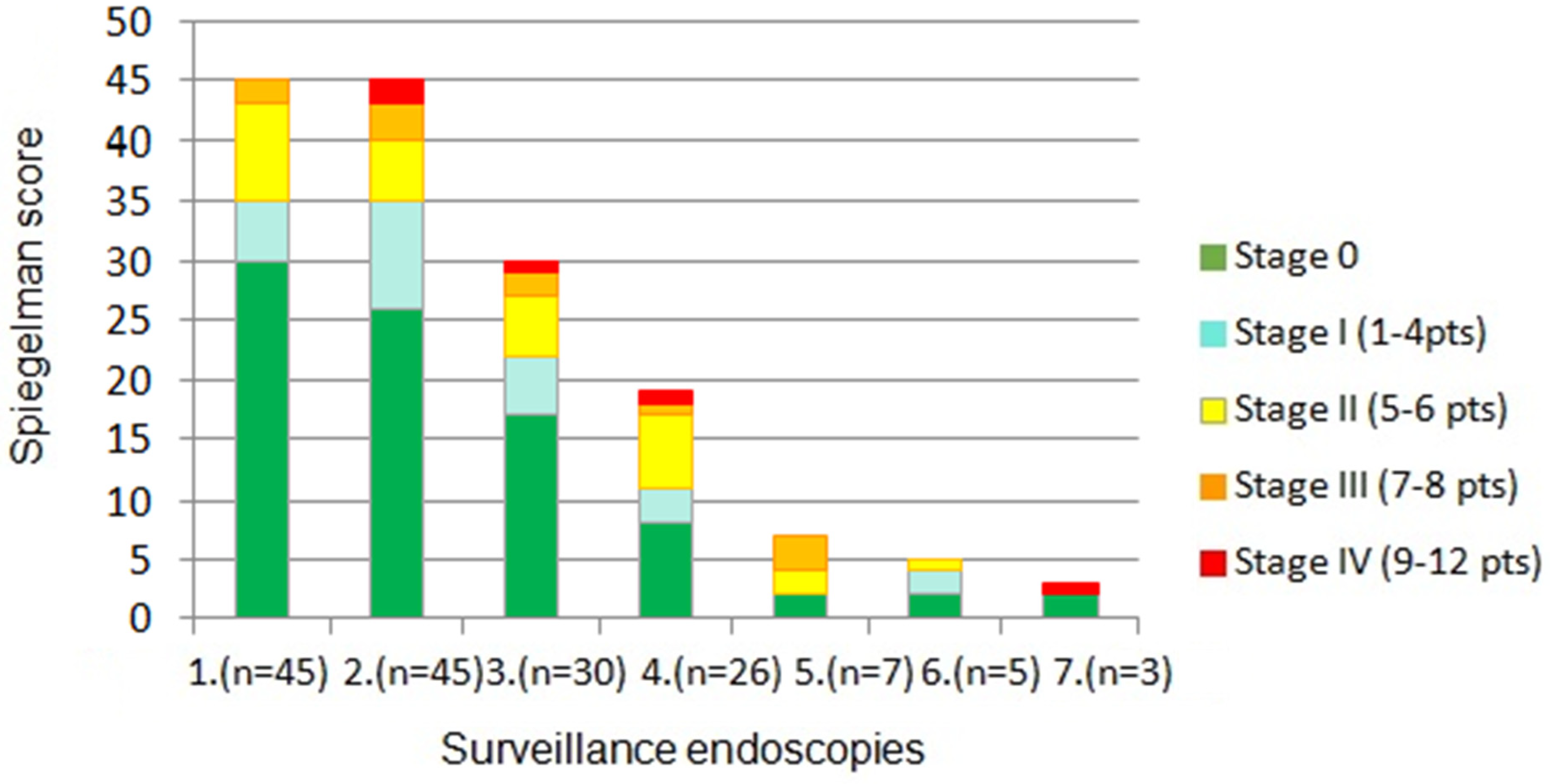
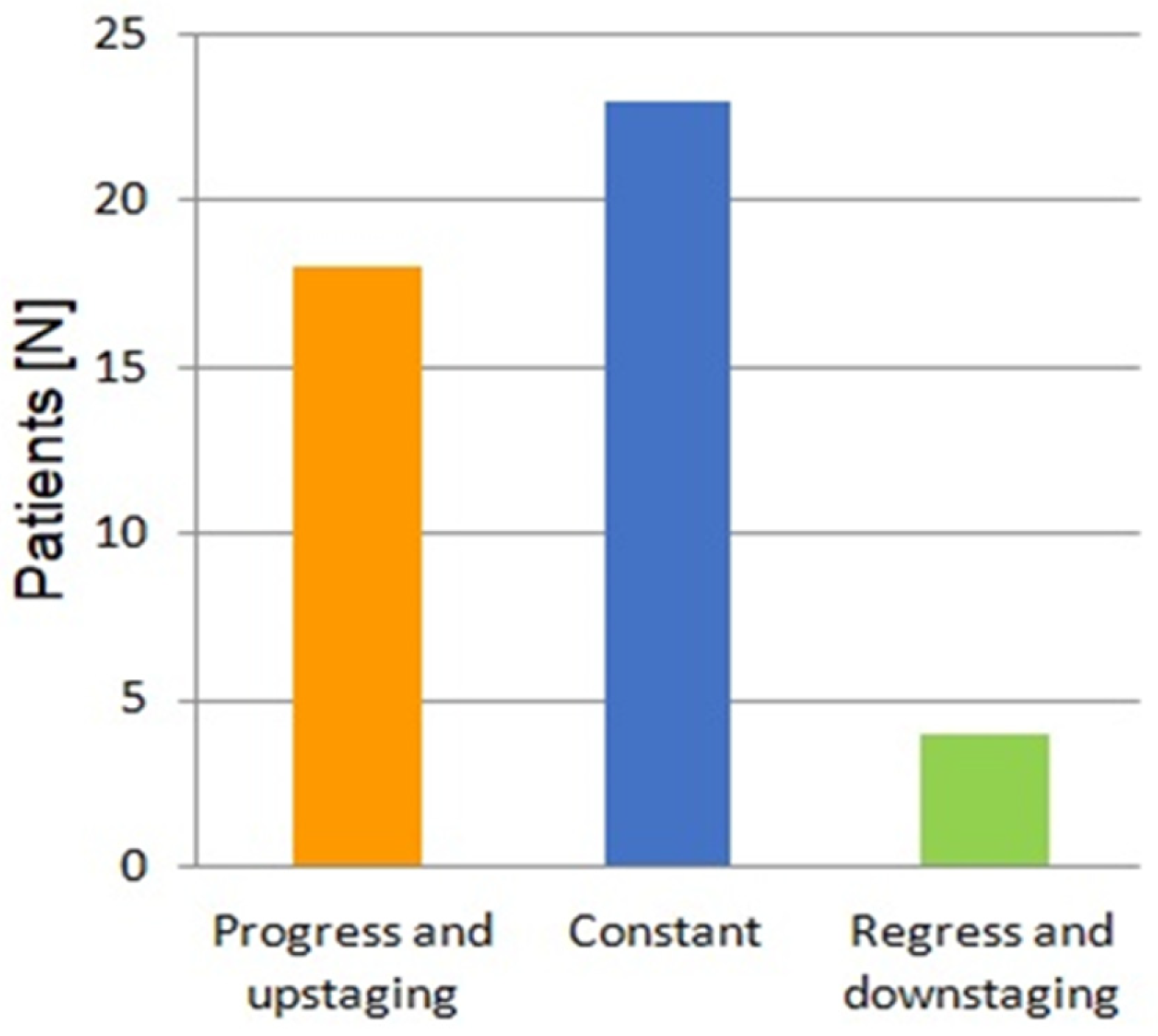
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FAP | Familial Adenomatous Polyposis |
| HGD | High-Grade Dysplasia |
| APC | Adenomatous Polyposis Coli |
| NCCN | National Comprehensive Cancer Network |
| GI | Gastrointestinal |
| SS | Spigelman Score |
| LSTs | Laterally Spreading Tumors |
| JNET | Japan NBI Expert Team |
| LGD | Low-Grade Dysplasia |
| SM | Submucosal Invasive Cancer |
| EMR | Endoscopic Mucosal Resection |
| TDE | Transduodenal Excision |
| PSD | Pancreas-Sparing Duodenectomy |
| PD | Pancreaticoduodenectomy |
| ESGE | European Society of Gastrointestinal Endoscopy |
| BSG | British Society of Gastroenterology |
| ASGE | American Society of Gastrointestinal Endoscopy |
| EHTG | European Hereditary Tumor Group |
| ESCP | European Society of Coloproctology |
| ICC | Intraclass Correlation Coefficient |
| AFI | Autofluorescence Imaging |
| CSP | Cold Snare Polypectomy |
| HSP | Hot Snare Polypectomy |
| UEMR | Underwater Endoscopic Mucosal Resection |
| EMR-C | Cap-Assisted Endoscopic Mucosal Resection |
| ESD | Endoscopic Submucosal Dissection |
References
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and Characterization of the Familial Adenomatous Polyposis Coli Gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Paszkowski, J.; Samborski, P.; Kucharski, M.; Cwaliński, J.; Banasiewicz, T.; Pławski, A. Endoscopic Surveillance and Treatment of Upper GI Tract Lesions in Patients with Familial Adenomatous Polyposis-A New Perspective on an Old Disease. Genes 2022, 13, 2329. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Gupta, S.; Burke, C.A.; Axell, L.; Chen, L.M.; Chung, D.C.; Clayback, K.M.; Dallas, S.; Felder, S.; Gbolahan, O.; et al. NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 1122–1132. [Google Scholar]
- McConnell, R.B. Familial Polyposis Coli. Family Studies, Histopathology, Differential Diagnosis, and Results of Treatment. J. Med. Genet. 1976, 13, 415. [Google Scholar] [CrossRef]
- Hopper, A.D. Role of Endoscopy in Patients with Familial Adenomatous Polyposis. Frontline Gastroenterol. 2022, 13, E72–E79. [Google Scholar] [CrossRef]
- Ma, M.X.; Bourke, M.J. Management of Duodenal Polyps. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 389–399. [Google Scholar] [CrossRef]
- Campos, F.G.; Martinez, C.A.R.; Lopez, L.A.B.; Kanno, D.T.; Nahas, S.C.; Cecconello, I. Advanced Duodenal Neoplasia and Carcinoma in Familial Adenomatous Polyposis: Outcomes of Surgical Management. J. Gastrointest. Oncol. 2017, 8, 877–884. [Google Scholar] [CrossRef]
- Chini, P.; Draganov, P.V. Diagnosis and Management of Ampullary Adenoma: The Expanding Role of Endoscopy. World J. Gastrointest. Endosc. 2011, 3, 241. [Google Scholar] [CrossRef]
- Zare, B.; Monahan, K.J. Guidelines for Familial Adenomatous Polyposis (FAP): Challenges in Defining Clinical Management for a Rare Disease. Fam. Cancer 2025, 24, 35. [Google Scholar] [CrossRef]
- Roos, V.H.; Bastiaansen, B.A.J.; Dekker, E. Challenges and Pitfalls of Investigating Duodenal Cancer in Patients with Familial Adenomatous Polyposis. Gastrointest. Endosc. 2019, 89, 355–356. [Google Scholar] [CrossRef]
- Bertoni, G.; Sassatelli, R.; Nigrisoli, E.; Pennazio, M.; Tansini, P.; Arrigoni, A.; Ponz De Leon, M.; Rossini, F.P.; Bedogni, G. High Prevalence of Adenomas and Microadenomas of the Duodenal Papilla and Periampullary Region in Patients with Familial Adenomatous Polyposis. Eur. J. Gastroenterol. Hepatol. 1996, 8, 1201–1206. [Google Scholar] [CrossRef]
- Aelvoet, A.S.; Buttitta, F.; Ricciardiello, L.; Dekker, E. Management of Familial Adenomatous Polyposis and MUTYH-Associated Polyposis; New Insights. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101793. [Google Scholar] [CrossRef]
- Skipworth, J.R.A.; Morkane, C.; Raptis, D.A.; Vyas, S.; Olde Damink, S.W.; Imber, C.J.; Pereira, S.P.; Malago, M.; West, N.; Phillips, R.K.S.; et al. Pancreaticoduodenectomy for Advanced Duodenal and Ampullary Adenomatosis in Familial Adenomatous Polyposis. HPB 2011, 13, 342. [Google Scholar] [CrossRef]
- Groves, C.J.; Saunders, B.P.; Spigelman, A.D.; Phillips, R.K.S. Duodenal Cancer in Patients with Familial Adenomatous Polyposis (FAP): Results of a 10 Year Prospective Study. Gut 2002, 50, 636–641. [Google Scholar] [CrossRef]
- Van Leerdam, M.E.; Roos, V.H.; Van Hooft, J.E.; Dekker, E.; Jover, R.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Pellisé, M.; Saurin, J.C.; et al. Endoscopic Management of Polyposis Syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 877–895. [Google Scholar] [CrossRef]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the Management of Hereditary Colorectal Cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef]
- Yang, J.; Gurudu, S.R.; Koptiuch, C.; Agrawal, D.; Buxbaum, J.L.; Fehmi, S.M.A.; Fishman, D.S.; Khashab, M.A.; Jamil, L.H.; Jue, T.L.; et al. American Society for Gastrointestinal Endoscopy Guideline on the Role of Endoscopy in Familial Adenomatous Polyposis Syndromes. Gastrointest. Endosc. 2020, 91, 963–982.e2. [Google Scholar] [CrossRef]
- Zaffaroni, G.; Mannucci, A.; Koskenvuo, L.; de Lacy, B.; Maffioli, A.; Bisseling, T.; Half, E.; Cavestro, G.M.; Valle, L.; Ryan, N.; et al. Updated European Guidelines for Clinical Management of Familial Adenomatous Polyposis (FAP), MUTYH-Associated Polyposis (MAP), Gastric Adenocarcinoma, Proximal Polyposis of the Stomach (GAPPS) and Other Rare Adenomatous Polyposis Syndromes: A Joint EHTG-ESCP Revision. Br. J. Surg. 2024, 111, znae070. [Google Scholar] [CrossRef]
- Vasen, H.F.A.; Möslein, G.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Blanco, I.; Bülow, S.; Burn, J.; Capella, G.; et al. Guidelines for the Clinical Management of Familial Adenomatous Polyposis (FAP). Gut 2008, 57, 704–713. [Google Scholar] [CrossRef]
- Half, E.; Bercovich, D.; Rozen, P. Familial Adenomatous Polyposis. Orphanet J. Rare Dis. 2009, 4, 1–23. [Google Scholar] [CrossRef]
- Aelvoet, A.S.; Pellisé, M.; Bastiaansen, B.A.J.; van Leerdam, M.E.; Jover, R.; Balaguer, F.; Kaminski, M.F.; Karstensen, J.G.; Saurin, J.-C.; Hompes, R.; et al. Personalized Endoscopic Surveillance and Intervention Protocols for Patients with Familial Adenomatous Polyposis: The European FAP Consortium Strategy. Endosc. Int. Open 2023, 11, E386–E393. [Google Scholar] [CrossRef]
- Ghorbanoghli, Z.; Bastiaansen, B.A.J.; Langers, A.M.J.; Nagengast, F.M.; Poley, J.W.; Hardwick, J.C.H.; Koornstra, J.J.; Sanduleanu, S.; De Vos Tot Nederveen Cappel, W.H.; Witteman, B.J.M.; et al. Extracolonic Cancer Risk in Dutch Patients with APC (Adenomatous Polyposis Coli)-Associated Polyposis. J. Med. Genet. 2018, 55, 11–14. [Google Scholar] [CrossRef]
- Vasen, H.F.A.; Bülow, S.; Myrhøj, T.; Mathus-Vliegen, L.; Griffioen, G.; Buskens, E.; Taal, B.G.; Nagengast, F.; Slors, J.F.M.; De Ruiter, P. Decision Analysis in the Management of Duodenal Adenomatosis in Familial Adenomatous Polyposis. Gut 1997, 40, 716–719. [Google Scholar] [CrossRef]
- Bülow, S.; Christensen, I.J.; Højen, H.; Björk, J.; Elmberg, M.; Järvinen, H.; Lepistö, A.; Nieuwenhuis, M.; Vasen, H. Duodenal Surveillance Improves the Prognosis after Duodenal Cancer in Familial Adenomatous Polyposis. Colorectal Dis. 2012, 14, 947–952. [Google Scholar] [CrossRef]
- Spigelman, A.D.; Talbot, I.C.; Williams, C.B.; Domizio, P.; Phillips, R.K.S. Upper Gastrointestinal Cancer in Patients with Familial Adenomatous Polyposis. Lancet 1989, 2, 783–785. [Google Scholar] [CrossRef]
- Mannucci, A.; Puzzono, M.; Goel, A.; Möslein, G.; Balafas, S.; Di Serio, M.S.; Cavestro, G.M. The Spigelman Staging System and the Risk of Duodenal and Papillary Cancer in Familial Adenomatous Polyposis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2024, 119, 617–624. [Google Scholar] [CrossRef]
- Dekker, E.; Boparai, K.S.; Poley, J.W.; Mathus-Vliegen, E.M.H.; Offerhaus, G.J.A.; Kuipers, E.J.; Fockens, P.; Dees, J. High Resolution Endoscopy and the Additional Value of Chromoendoscopy in the Evaluation of Duodenal Adenomatosis in Patients with Familial Adenomatous Polyposis. Endoscopy 2009, 41, 666–669. [Google Scholar] [CrossRef]
- Thiruvengadam, S.S.; Lopez, R.; O’Malley, M.; LaGuardia, L.; Church, J.M.; Kalady, M.; Walsh, R.M.; Burke, C.A. Spigelman Stage IV Duodenal Polyposis Does Not Precede Most Duodenal Cancer Cases in Patients with Familial Adenomatous Polyposis. Gastrointest. Endosc. 2019, 89, 345–354.e2. [Google Scholar] [CrossRef]
- Sourrouille, I.; Lefèvre, J.H.; Shields, C.; Colas, C.; Bellanger, J.; Desaint, B.; Paye, F.; Tiret, E.; Parc, Y. Surveillance of Duodenal Polyposis in Familial Adenomatous Polyposis: Should the Spigelman Score Be Modified? Dis. Colon. Rectum 2017, 60, 1137–1146. [Google Scholar] [CrossRef]
- Sano, Y.; Kobayashi, M.; Kozu, T. Development and clinical application of a narrow band imaging (NBI) system with builtin narrow-band RGB filters. Stom. Intestig. 2001, 36, 1283–1287. [Google Scholar]
- Machida, H.; Sano, Y.; Hamamoto, Y.; Muto, M.; Kozu, T.; Tajiri, H.; Yoshida, S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: A pilot study. Endoscopy 2004, 36, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Kashida, H.; Kudo, S.E.; Misawa, M.; Ikehara, N.; Hamatani, S. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig. Endosc. 2010, 22, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Yamada, M.; Takamaru, H.; Sakamoto, T.; Matsuda, T.; Sekine, S.; Igarashi, Y.; Saito, Y. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United Eur. Gastroenterol. J. 2019, 7, 914–923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanbiervliet, G.; Moss, A.; Arvanitakis, M.; Arnelo, U.; Beyna, T.; Busch, O.; Deprez, P.H.; Kunovsky, L.; Larghi, A.; Manes, G.; et al. Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Tajika, M.; Tanaka, T.; Oonishi, S.; Yamada, K.; Kamiya, T.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Haba, S.; Kuraishi, Y.; et al. Endoscopic Management of Adenomas in the Ileal Pouch and the Rectal Remnant after Surgical Treatment in Familial Adenomatous Polyposis. J. Clin. Med. 2022, 11, 3562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubio, C.A. Serrated Adenoma of the Duodenum. J. Clin. Pathol. 2004, 57, 1219–1221. [Google Scholar] [CrossRef]
- Burke, C.A.; Beck, G.J.; Church, J.M.; Van Stolk, R.U. The Natural History of Untreated Duodenal and Ampullary Adenomas in Patients with Familial Adenomatous Polyposis Followed in an Endoscopic Surveillance Program. Gastrointest. Endosc. 1999, 49, 358–364. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiao, Y.; Yang, S.; Zhou, A.; Zhao, G.; Guo, S.; Li, P.; Zhang, S. Endoscopic Diagnosis and Treatment of Superficial Non-Ampullary Duodenal Epithelial Tumors: A Review. J. Transl. Int. Med. 2023, 11, 206. [Google Scholar] [CrossRef]
- Bourke, M.J.; Lo, S.K.; Buerlein, R.C.D.; Das, K.K. AGA Clinical Practice Update on Nonampullary Duodenal Lesions: Expert Review. Gastroenterology 2025, 168, 169–175. [Google Scholar] [CrossRef]
- Jaganmohan, S.; Lynch, P.M.; Raju, R.P.; Ross, W.A.; Lee, J.E.; Raju, G.S.; Bhutani, M.S.; Fleming, J.B.; Lee, J.H. Endoscopic Management of Duodenal Adenomas in Familial Adenomatous Polyposis--a Single-Center Experience. Dig. Dis. Sci. 2012, 57, 732–737. [Google Scholar] [CrossRef]
- Aelvoet, A.S.; Karstensen, J.G.; Bastiaansen, B.A.J.; van Leerdam, M.E.; Balaguer, F.; Kaminski, M.; Hompes, R.; Bossuyt, P.M.M.; Ricciardiello, L.; Latchford, A.; et al. Cold Snare Polypectomy for Duodenal Adenomas in Familial Adenomatous Polyposis: A Prospective International Cohort Study. Endosc. Int. Open 2023, 11, E1056–E1062. [Google Scholar] [CrossRef]
- Maruoka, D.; Matsumura, T.; Kasamatsu, S.; Ishigami, H.; Taida, T.; Okimoto, K.; Nakagawa, T.; Katsuno, T.; Arai, M. Cold Polypectomy for Duodenal Adenomas: A Prospective Clinical Trial. Endoscopy 2017, 49, 776–783. [Google Scholar] [CrossRef]
- Shinozaki, S.; Kobayashi, Y.; Hayashi, Y.; Sakamoto, H.; Lefor, A.K.; Yamamoto, H. Efficacy and Safety of Cold versus Hot Snare Polypectomy for Resecting Small Colorectal Polyps: Systematic Review and Meta-Analysis. Dig. Endosc. 2018, 30, 592–599. [Google Scholar] [CrossRef]
- Kuroki, K.; Sanomura, Y.; Oka, S.; Yorita, N.; Kurihara, M.; Mizumoto, T.; Yoshifuku, Y.; Arihiro, K.; Tanaka, S.; Chayama, K. Clinical Outcomes of Endoscopic Resection for Superficial Non-Ampullary Duodenal Tumors. Endosc. Int. Open 2020, 8, E354–E359. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.; Lourdusamy, V.; Zhu, X.; Hawes, R.; Varadarajulu, S. Efficacy and Safety of Endoscopic Mucosal Resection of Non-Ampullary Duodenal Polyps: A Systematic Review. Endosc. Int. Open 2016, 4, E699–E708. [Google Scholar] [CrossRef]
- Probst, A.; Freund, S.; Neuhaus, L.; Ebigbo, A.; Braun, G.; Goelder, S.; Weber, T.; Märkl, B.; Anthuber, M.; Messmann, H. Complication Risk despite Preventive Endoscopic Measures in Patients Undergoing Endoscopic Mucosal Resection of Large Duodenal Adenomas. Endoscopy 2020, 52, 847–855. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Uedo, N.; Takeuchi, Y.; Ishihara, R.; Okada, H.; Iishi, H. Current Status of Endoscopic Resection for Superficial Nonampullary Duodenal Epithelial Tumors. Digestion 2018, 97, 45–51. [Google Scholar] [CrossRef]
- Conio, M.; De Ceglie, A.; Filiberti, R.; Fisher, D.A.; Siersema, P.D. Cap-Assisted EMR of Large, Sporadic, Nonampullary Duodenal Polyps. Gastrointest. Endosc. 2012, 76, 1160–1169. [Google Scholar] [CrossRef]
- Yahagi, N.; Kato, M.; Ochiai, Y.; Maehata, T.; Sasaki, M.; Kiguchi, Y.; Akimoto, T.; Nakayama, A.; Fujimoto, A.; Goto, O.; et al. Outcomes of Endoscopic Resection for Superficial Duodenal Epithelial Neoplasia. Gastrointest. Endosc. 2018, 88, 676–682. [Google Scholar] [CrossRef]
- Hopper, A.D.; Bourke, M.J.; Williams, S.J.; Swan, M.P. Giant Laterally Spreading Tumors of the Papilla: Endoscopic Features, Resection Technique, and Outcome (with Videos). Gastrointest. Endosc. 2010, 71, 967–975. [Google Scholar] [CrossRef]
- Fanning, S.B.; Bourke, M.J.; Williams, S.J.; Chung, A.; Kariyawasam, V.C. Giant Laterally Spreading Tumors of the Duodenum: Endoscopic Resection Outcomes, Limitations, and Caveats. Gastrointest. Endosc. 2012, 75, 805–812. [Google Scholar] [CrossRef]
- ei Kudo, S.; Lambert, R.; Allen, J.I.; Fujii, H.; Fujii, T.; Kashida, H.; Matsuda, T.; Mori, M.; Saito, H.; Shimoda, T.; et al. Nonpolypoid Neoplastic Lesions of the Colorectal Mucosa. Gastrointest. Endosc. 2008, 68, S3–S47. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Wang, G.; Li, S.; Chen, T.; He, L.; Xu, A.; Xu, M. Endoscopic Submucosal Dissection for Large Duodenal Laterally Spreading Lesions Is Feasible: A Multi-Center Retrospective Study. Surg. Laparosc. Endosc. Percutan Technol. 2023, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, M.; Bilder, H.; Duarte, B.; Lasa, J.; Zubiaurre, I. Underwater Endoscopic Mucosal Resection of Recurrent Duodenal Lateral Spreading Tumor. Endoscopy 2021, 53, E98–E99. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chai, N.; Linghu, E.; Zhai, Y.; Li, Z.; Du, C.; Li, L. Clinical Outcomes of Endoscopic Resection for Non-Ampullary Duodenal Laterally Spreading Tumors. Surg. Endosc. 2019, 33, 4048–4056. [Google Scholar] [CrossRef]
- Grega, T.; Kmochova, K.; Hejcmanova, K.; Ngo, O.; Brodyuk, N.; Majek, O.; Bures, J.; Urbanek, P.; Zavoral, M. Impact of narrow band imaging in prediction of histology of advanced colorectal neoplasia. Sci. Rep. 2025, 15, 1414. [Google Scholar] [CrossRef]

| Spigelman Stages | Surveillance Interval | |||
|---|---|---|---|---|
| ESGE, 2019r. [15] | BSG, 2020r. [16] | ASGE, 2020r. [17] | Joint EHTG-ESCP Revision; 2024 r. [18] | |
| 0 | 5 years | 5 years | 5 years | 3–5 years |
| I | 5 years | 5 years | 5 years | 2–3 years or 2 years * |
| II | 3 years | 3 years | 3 years | 2 years or 1 year * |
| III | 1 year | 1 year | 6–12 months | 1 year or 6–12 months * |
| IV | 6 months | 6–12 months | 3–6 months | 6–12 months |
| Advantages | Disadvantages |
|---|---|
| Guideline integration | Underestimation of malignancy risk |
| Simplicity and accessibility | Equal weighting of criteria |
| High reliability | Exclusion of papillary pathology |
| Association with cancer risk | Advancement in diagnostics not yet integrated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cwaliński, J.; Kot, G.; Grochowska, W.; Budzyńska, K.; Cwalińska, A.; Paszkowski, J. Principles of Endoscopic Surveillance of Extrapapillary Duodenal Lesions in Familial Adenomatous Polyposis: A 14-Year Single-Center Observation. Cancers 2025, 17, 2490. https://doi.org/10.3390/cancers17152490
Cwaliński J, Kot G, Grochowska W, Budzyńska K, Cwalińska A, Paszkowski J. Principles of Endoscopic Surveillance of Extrapapillary Duodenal Lesions in Familial Adenomatous Polyposis: A 14-Year Single-Center Observation. Cancers. 2025; 17(15):2490. https://doi.org/10.3390/cancers17152490
Chicago/Turabian StyleCwaliński, Jarosław, Gabriela Kot, Wiktoria Grochowska, Katarzyna Budzyńska, Agnieszka Cwalińska, and Jacek Paszkowski. 2025. "Principles of Endoscopic Surveillance of Extrapapillary Duodenal Lesions in Familial Adenomatous Polyposis: A 14-Year Single-Center Observation" Cancers 17, no. 15: 2490. https://doi.org/10.3390/cancers17152490
APA StyleCwaliński, J., Kot, G., Grochowska, W., Budzyńska, K., Cwalińska, A., & Paszkowski, J. (2025). Principles of Endoscopic Surveillance of Extrapapillary Duodenal Lesions in Familial Adenomatous Polyposis: A 14-Year Single-Center Observation. Cancers, 17(15), 2490. https://doi.org/10.3390/cancers17152490







