Drug Repurposing of New Treatments for Neuroendocrine Tumors
Simple Summary
Abstract
1. Introduction
2. Techniques for Drug Repurposing
3. Drug Repurposing for Neuroendocrine Tumors (NETs)
3.1. Significance and a Few Successful Examples
3.2. Drugs with Clinical Significance and a Potential Role in the Field of NETs
3.3. Drugs with Preliminary Clinical Data in the Field of NETs
3.4. Drugs with a Preclinical Rationale Without Clinically Proven Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.L. Drug repurposing for cancer therapy. Signal Transduct. Target Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Benevento, E.; Liccardi, A.; Cannavale, G.; Minotta, R.; DI Iasi, G.; Colao, A. Recent advances and future challenges in the diagnosis of neuroendocrine neoplasms. Minerva Endocrinol. 2024, 49, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Sedlack, A.J.H.; Varghese, D.G.; Naimian, A.; Yazdian Anari, P.; Bodei, L.; Hallet, J.; Riechelmann, R.P.; Halfdanarson, T.; Capdevilla, J.; Del Rivero, J. Update in the management of gastroenteropancreatic neuroendocrine tumors. Cancer 2024, 130, 3090–3105. [Google Scholar] [CrossRef]
- Fazio, N.; La Salvia, A. Precision medicine in gastroenteropancreatic neuroendocrine neoplasms: Where are we in 2023? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101794. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Fanciulli, G.; Malandrino, P.; Ramundo, V.; Faggiano, A.; Colao, A.; NIKE Group. Predictive factors of response to mTOR inhibitors in neuroendocrine tumours. Endocr. Relat. Cancer 2016, 23, R173–R183. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Applications Covered by Section 505(b)(2). Available online: https://www.fda.gov/media/72419/download (accessed on 23 June 2025).
- FDA. Breakthrough Therapy. Available online: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy (accessed on 23 June 2025).
- EMA. (EMA) Has the Type II Variation. Available online: https://www.ema.europa.eu/en/glossary-terms/type-ii-variation (accessed on 23 June 2025).
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorg. Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2018, 175, 168–180. [Google Scholar] [CrossRef]
- March-Vila, E.; Pinzi, L.; Sturm, N.; Tinivella, A.; Engkvist, O.; Chen, H.; Rastelli, G. On the Integration of In Silico Drug Design Methods for Drug Repurposing. Front. Pharmacol. 2017, 8, 298. [Google Scholar] [CrossRef]
- Park, K. A review of computational drug repurposing. Transl. Clin. Pharmacol. 2019, 27, 59–63. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- EMA Afinitor (Everolimus). Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/afinitor-epar-product-information_en.pdf (accessed on 23 June 2025).
- Liu, E.; Marincola, P.; Oberg, K. Everolimus in the treatment of patients with advanced pancreatic neuroendocrine tumors: Latest findings and interpretations. Ther. Adv. Gastroenterol. 2013, 6, 412–419. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, nonfunctional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.S.; Scarpa, A.; Lawlor, R.T.; Sadanandam, A.; Tafuto, S.; Krogh, M.; Teule, A.; Garcia-Carbonero, R.; Klumpen, H.J.; Cremer, B.; et al. Search for biomarkers to personalize treatment with streptozotocin plus 5-fluorouracil or everolimus in patients with advanced pancreatic neuroendocrine tumors: The randomized phase III SEQTOR trial (GETNE-1206). Ann. Oncol. 2024, 35, S754. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Everolimus in treatment of neuroendocrine tumors: Efficacy, side-effects, resistance and factors affecting its place in the treatment sequence. Expert Opin. Pharmacother. 2018, 19, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Medici, B.; Caffari, E.; Maculan, Y.; Benatti, S.; Piacentini, F.; Dominici, M.; Gelsomino, F. Everolimus in the Treatment of Neuroendocrine Tumors: Lights and Shadows. Biomedicines 2025, 13, 455. [Google Scholar] [CrossRef]
- Chomanicova, N.; Gazova, A.; Adamickova, A.; Valaskova, S.; Kyselovic, J. The role of AMPK/mTOR signaling pathway in anticancer activity of metformin. Physiol. Res. 2021, 70, 501–508. [Google Scholar] [CrossRef]
- Cigrovski Berkovic, M.; Coppola, A.; Sesa, V.; Mrzljak, A.; Lai, Q. Metformin and pancreatic neuroendocrine tumors: A systematic review and meta-analysis. World J. Gastroenterol. 2024, 30, 759–769. [Google Scholar] [CrossRef]
- Pusceddu, S.; Vernieri, C.; Di Maio, M.; Marconcini, R.; Spada, F.; Massironi, S.; Ibrahim, T.; Brizzi, M.P.; Campana, D.; Faggiano, A.; et al. Metformin Use Is Associated with Longer Progression-Free Survival of Patients with Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology 2018, 155, 479–489.e7. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The potential use of simvastatin for cancer treatment: A review. Biomed. Pharmacother. 2021, 141, 111858. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; La Salvia, A.; Liccardi, A.; Cozzolino, A.; Di Sarno, A.; Russo, F.; Colao, A.; Faggiano, A.; NIKE group. Dyslipidemia, lipid-lowering agents and neuroendocrine neoplasms: New horizons. Endocrine 2024, 85, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; Russo, F.; Zamponi, V.; Sesti, F.; Puliani, G.; Modica, R.; Malandrino, P.; Ferraù, F.; Rinzivillo, M.; Di Muzio, M.; et al. Impact of dyslipidemia and lipid-lowering therapy with statins in patients with neuroendocrine tumors. J. Neuroendocrinol. 2025, 37, e13485. [Google Scholar] [CrossRef]
- Jahchan, N.S.; Dudley, J.T.; Mazur, P.K.; Flores, N.; Yang, D.; Palmerton, A.; Zmoos, A.F.; Vaka, D.; Tran, K.Q.; Zhou, M.; et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013, 3, 1364–1377. [Google Scholar] [CrossRef]
- Wang, J.; Byers, L.A. Teaching an old dog new tricks: Drug repositioning in small cell lung cancer. Cancer Discov. 2013, 3, 1333–1335. [Google Scholar] [CrossRef][Green Version]
- Riess, J.W.; Jahchan, N.S.; Das, M.; Zach Koontz, M.; Kunz, P.L.; Wakelee, H.A.; Schatzberg, A.; Sage, J.; Neal, J.W. A phase IIa study repositioning desipramine in small cell lung and other high-grade neuroendocrine tumors. Cancer Treat Res. Commun. 2020, 23, 100174. [Google Scholar] [CrossRef]
- Eleutherakis-Papaiakovou, V.; Bamias, A.; Dimopoulos, M.A. Thalidomide in cancer medicine. Ann. Oncol. 2004, 15, 1151–1160. [Google Scholar] [CrossRef]
- D’Amato, R.J.; Loughran, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar] [CrossRef]
- EMA. Thalidomide. Available online: https://www.ema.europa.eu/en/documents/product-information/thalidomide-bms-epar-product-information_en.pdf (accessed on 23 June 2025).
- Durán, I.; Salazar, R.; Casanovas, O.; Arrazubi, V.; Vilar, E.; Siu, L.L.; Yao, J.; Tabernero, J. New drug development in digestive neuroendocrine tumors. Ann. Oncol. 2007, 18, 1307–1313. [Google Scholar] [CrossRef]
- Koumarianou, A.; Kaltsas, G.; Kulke, M.H.; Oberg, K.; Strosberg, J.R.; Spada, F.; Galdy, S.; Barberis, M.; Fumagalli, C.; Berruti, A.; et al. Temozolomide in Advanced Neuroendocrine Neoplasms: Pharmacological and Clinical Aspects. Neuroendocrinology 2015, 101, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Song, L.; Liu, W.; Zhou, Y.; Miao, Y.; Fang, W.; Tan, H.; Shi, S.; Jiang, H.; Xu, J.; et al. S-1/temozolomide versus S-1/temozolomide plus thalidomide in advanced pancreatic and non-pancreatic neuroendocrine tumours (STEM): A randomised, open-label, multicentre phase 2 trial. EClinicalMedicine 2022, 54, 101667. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Borrego, M.C.; L-López, F.; Gálvez-Moreno, M.A.; Fuentes-Fayos, A.C.; Venegas-Moreno, E.; Herrera-Martínez, A.D.; Blanco-Acevedo, C.; Solivera, J.; Landsman, T.; Gahete, M.D.; et al. A New Generation Somatostatin-Dopamine Analogue Exerts Potent Antitumoral Actions on Pituitary Neuroendocrine Tumor Cells. Neuroendocrinology 2020, 110, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Feng, Y.; Liu, Y.; Zhang, Y.; Liu, L.; Zhang, B.; Zhang, T.; Su, Z.; Xue, L.; Wu, Z.B. Disulfiram mediated anti-tumour effect in pituitary neuroendocrine tumours by inducing cuproptosis. Int. Immunopharmacol. 2024, 134, 112159. [Google Scholar] [CrossRef]
- Liyun, C.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Bian, C.; Zheng, Z.; Su, J.; Chang, S.; Yu, H.; Bao, J.; Xin, Y.; Jiang, X. Copper homeostasis and cuproptosis in tumor pathogenesis and therapeutic strategies. Front. Pharmacol. 2023, 14, 1271613. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, Y. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. ecancermedicalscience 2017, 11, 781. [Google Scholar] [CrossRef]
- Nakano, K.; Masui, T.; Yogo, A.; Uchida, Y.; Sato, A.; Kasai, Y.; Nagai, K.; Anazawa, T.; Kawaguchi, Y.; Uemoto, S. Chloroquine induces apoptosis in pancreatic neuroendocrine neoplasms via endoplasmic reticulum stress. Endocr. Relat. Cancer 2020, 27, 431–439. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Tang, T.; Zhou, M.; Ye, F. Research progress on nonsteroidal anti-inflammatory drugs in the treatment of pituitary neuroendocrine tumors. Front. Pharmacol. 2024, 15, 1407387. [Google Scholar] [CrossRef]
- Sulsenti, R.; Frossi, B.; Bongiovanni, L.; Cancila, V.; Ostano, P.; Fischetti, I.; Enriquez, C.; Guana, F.; Chiorino, G.; Tripodo, C.; et al. Repurposing of the Antiepileptic Drug Levetiracetam to Restrain Neuroendocrine Prostate Cancer and Inhibit Mast Cell Support to Adenocarcinoma. Front. Immunol. 2021, 12, 622001. [Google Scholar] [CrossRef]
- Elhasasna, H.; Khan, R.; Bhanumathy, K.K.; Vizeacoumar, F.S.; Walke, P.; Bautista, M.; Dahiya, D.K.; Maranda, V.; Patel, H.; Balagopal, A.; et al. A Drug Repurposing Screen Identifies Fludarabine Phosphate as a Potential Therapeutic Agent for N-MYC Overexpressing Neuroendocrine Prostate Cancers. Cells 2022, 11, 2246. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, B.; Chen, L.; Li, A.; Shen, K.; Su, R.; Zhang, W.; Zhu, Y.; Wang, Q.; Xue, W. Repurposing ketotifen as a therapeutic strategy for neuroendocrine prostate cancer by targeting the IL-6/STAT3 pathway. Cell. Oncol. 2023, 46, 1445–1456. [Google Scholar] [CrossRef]
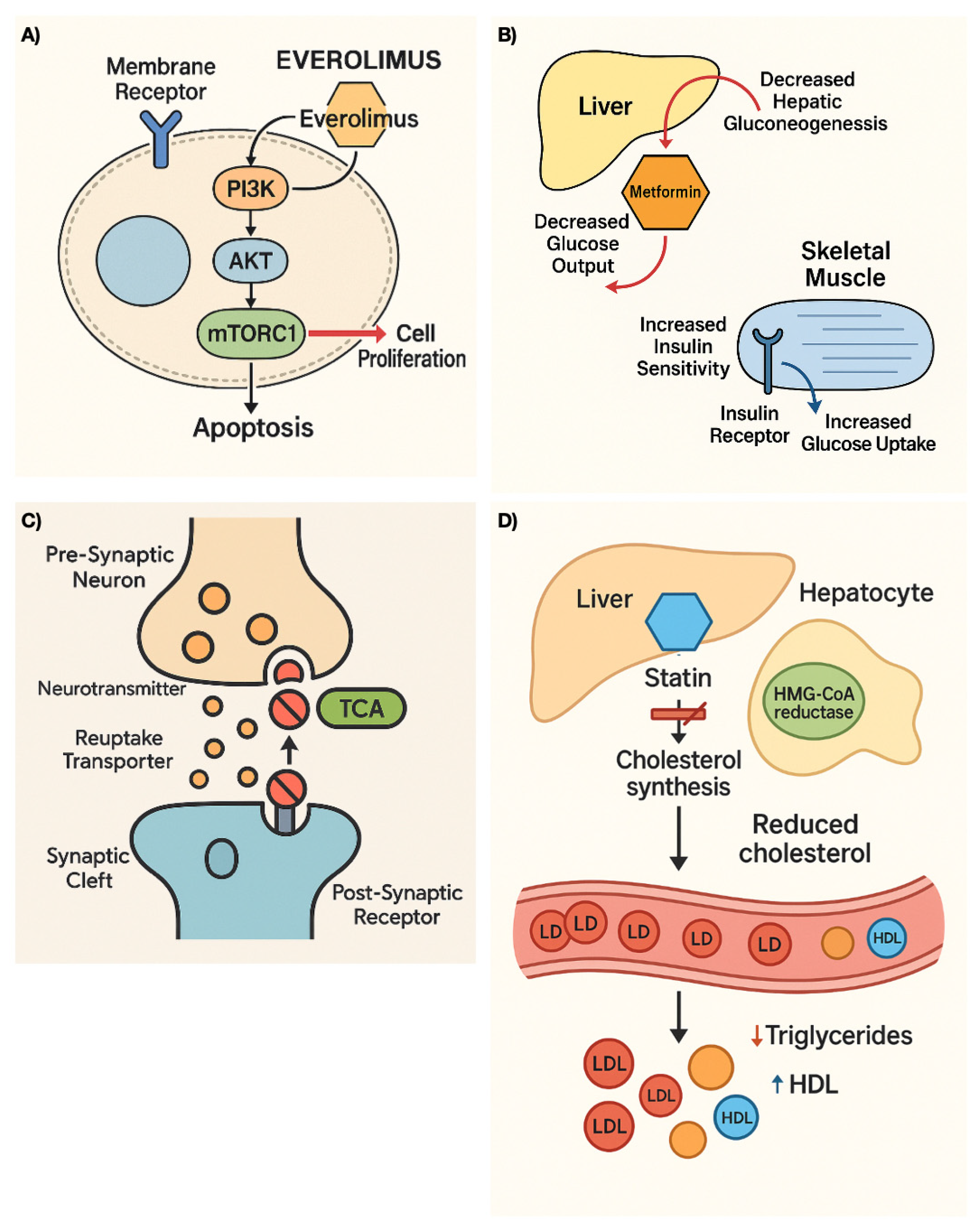
| Active Substance | Mechanism of Action | Clinical Indication (Level of Evidence) | Repurposing Approach |
|---|---|---|---|
| Drug repurposed for NETs | |||
| Everolimus | mTOR inhibitor | pNETs extra pNETs (Phase III randomized controlled studies) | Experimental |
| Drugs with clinical significance and potential role in the field of NETs | |||
| Metformin | Inhibits mitochondrial oxidative phosphorylation, leading to activation of AMPK and subsequent downregulation of the mTOR pathway. | pNET (Systematic review, Retrospective studies) | Experimental |
| Statins | Inhibit the enzyme HMG-CoA reductase in the liver, decreasing hematic cholesterol levels; reduce proteins involved in tumor proliferation and angiogenesis; induce cell apoptosis. | pNETs PitNETs SCLC Lung NETs Small Bowel NETs Pheochromocytoma Merkel cell carcinoma (Observational retrospective studies) | Experimental |
| Drugs with preliminary clinical data in the field of NETs | |||
| Tricyclic Antidepressants | Inhibits serotonin and epinephrine reuptake, and antagonize cholinergic, histaminic, and adrenergic receptors. Activate stress pathways and in-duce cell death. | SCLC pNETs (Preclinical models, Phase II study) | Computational Experimental |
| Thalidomide | Inhibits angiogenesis by interrupting processes mediated by bFGF and/or VEGF. Inhibits TNF-α synthesis and blocks the activation of nuclear factor (NF)-κB. | pNETs (Phase II studies) | Experimental |
| Drugs with preclinical rationale without clinically proven implications | |||
| Dopastatins | Enhanced efficacy in suppressing GH hypersecretion. | PitNETs (Preclinical studies) | Experimental |
| Disulfiram | Inhibits the enzyme aldehyde dehydrogenase (ALDH). Induces cuproptosis leading to proteotoxic stress and cell death. | PitNETs (Preclinical studies) | Experimental |
| Chloroquine Hydroxychloroquine | Inhibit autophagy by disrupting lysosomal acidification and can suppress cancer cell proliferation by interfering with the CXCL12/CXCR4 signaling pathway. | pNETs (Preclinical studies) | Experimental |
| Celecoxib | Inhibits cyclooxygenase-2 (COX-2), an enzyme involved in the production of prostaglandins, which are mediators of pain and inflammation. | PitNETs (In vitro studies) | Experimental (target mechanism-based) |
| Levetiracetam | Modulation of neurotransmitter release through binding to the synaptic vesicle glycoprotein 2A. | NEPC (In vitro experiments, preclinical studies) | Computational Experimental |
| Fludarabine Phosphate | Inhibits DNA synthesis, primarily by interfering with the activity of several enzymes involved in DNA replication. | NEPC (In vitro experiments) | Computational |
| Ketotifen | Suppresses neuroendocrine differentiation, reduces cell viability, and reverses lineage switch via targeting the IL-6/STAT3 pathway. | NEPC (In vitro experiments) | Computational |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellino, S.; Lucente, D.; La Salvia, A. Drug Repurposing of New Treatments for Neuroendocrine Tumors. Cancers 2025, 17, 2488. https://doi.org/10.3390/cancers17152488
Bellino S, Lucente D, La Salvia A. Drug Repurposing of New Treatments for Neuroendocrine Tumors. Cancers. 2025; 17(15):2488. https://doi.org/10.3390/cancers17152488
Chicago/Turabian StyleBellino, Stefania, Daniela Lucente, and Anna La Salvia. 2025. "Drug Repurposing of New Treatments for Neuroendocrine Tumors" Cancers 17, no. 15: 2488. https://doi.org/10.3390/cancers17152488
APA StyleBellino, S., Lucente, D., & La Salvia, A. (2025). Drug Repurposing of New Treatments for Neuroendocrine Tumors. Cancers, 17(15), 2488. https://doi.org/10.3390/cancers17152488







