Beyond Treatment Decisions: The Predictive Value of Comprehensive Geriatric Assessment in Older Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.2.1. G8 Questionnaire
2.2.2. CGA
- Activities of Daily Living—ADL for the assessment of functional capacity [16].
- Instrumental Activity of Daily Life—IADL for the assessment of instrumental capacity [17].
- Geriatric Depression Scale—short form—GDS to assess the emotional status [18].
- Exton Smith scale—ESS to determine the risk of developing pressure sores [21].
- The Mini Nutritional Assessment (MNA) to determine nutritional status [22].
- The Cumulative Illness Rating Scale (CIRS) for the assessment of comorbidities [23].
2.2.3. Balducci Evaluation
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Sample at the Baseline
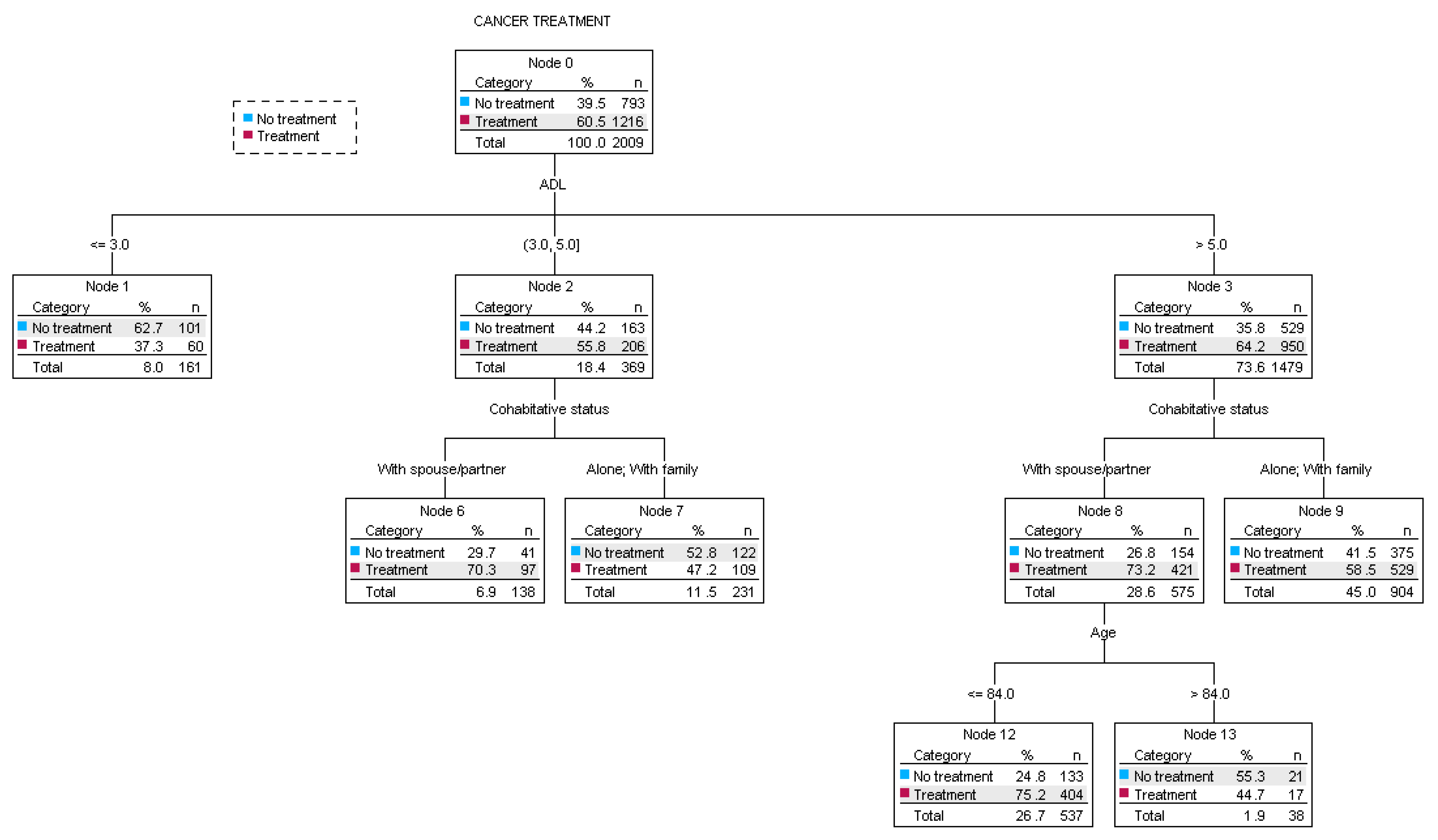
3.2. CGA Parameters and Clinical Decision Making
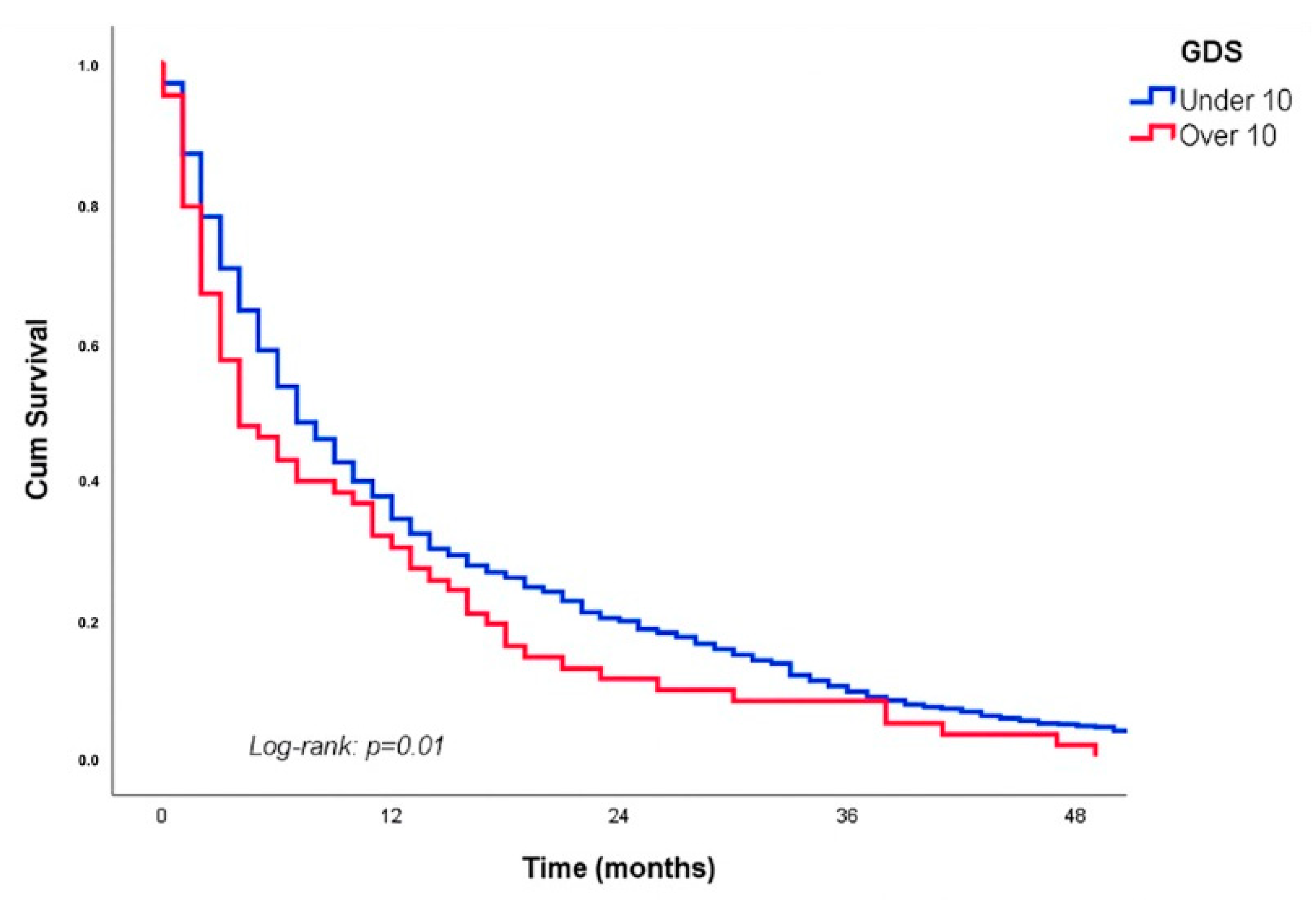
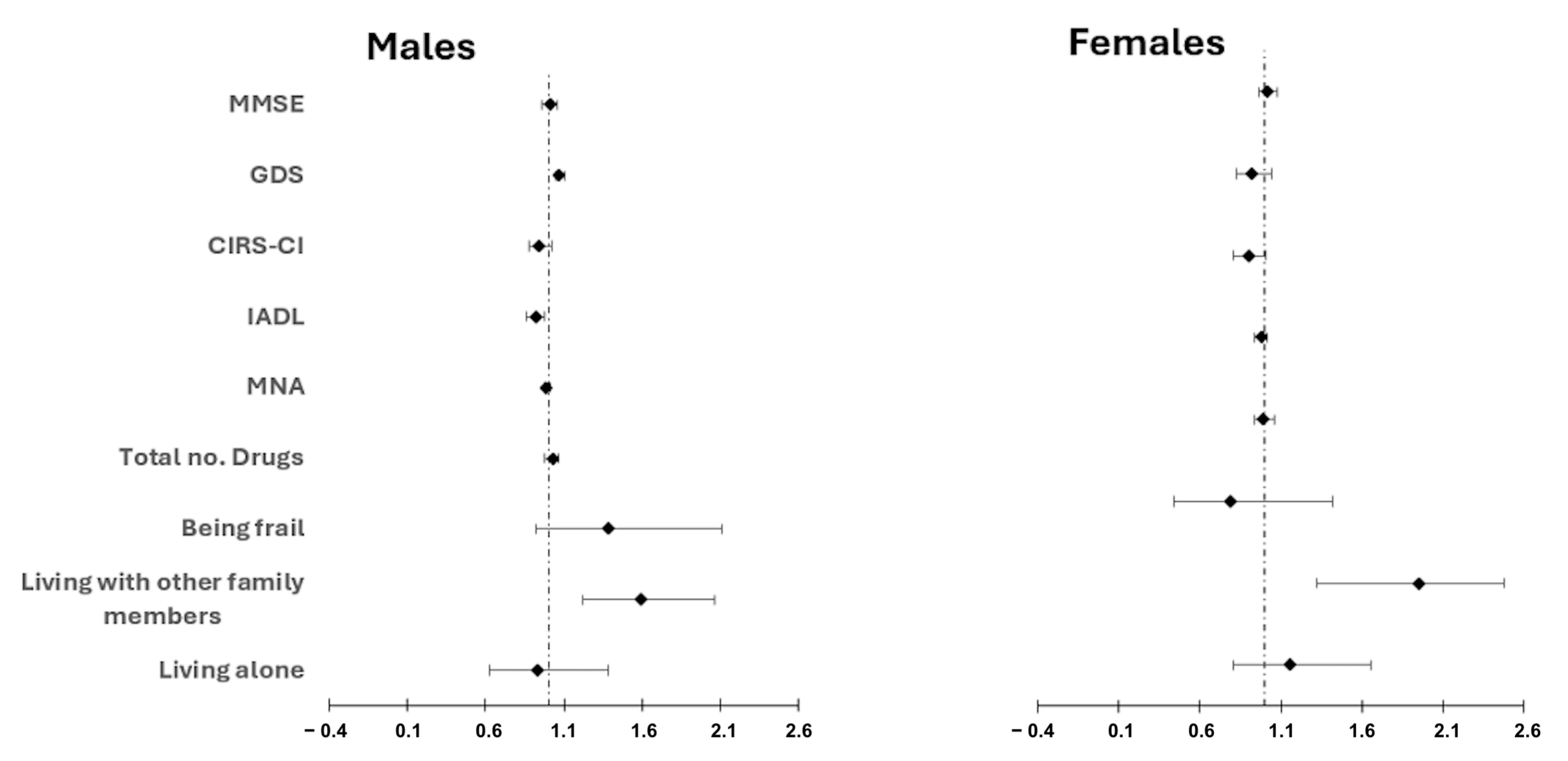
3.3. Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ries, L.A.G.; Melbert, D.; Krapcho, M.; Mariotto, A.; Miller, B.A.; Feuer, E.J.; Clegg, L.; Horner, M.J.; Howlader, N.; Eisner, M.P.; et al. SEER Cancer Statistics Review 1975–2004—Previous Version—SEER Cancer Statistics [Internet]; National Cancer Institute: Bethesda, MD, USA, 2007; pp. 112–121.
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef] [PubMed]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Goede, V. Frailty and Cancer: Current Perspectives on Assessment and Monitoring. Clin. Interv. Aging 2023, 18, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M.; Aapro, M.; Bernabei, R.; Cohen, H.J.; Droz, J.P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; Sørbye, L.; et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. Hematol. 2005, 55, 241–252. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Karnofsky, D.A.; Burchenal, J.H. Present status of clinical cancer chemotherapy. Am. J. Med. 1950, 8, 767–788. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Chiusole, B.; Tortorelli, I.; Galiano, A.; Murtas, F.; Ahcene-Djaballah, S.; Tierno, G.; Bergo, E.; Banzato, A.; Gatti, M.; Di Maggio, A.; et al. Management and Outcomes of Older Patients (Age ≥ 70 Years) with Advanced Soft Tissue Sarcoma and Role of Geriatric Assessment and Oncological Multidimensional Prognostic Index (Onco-MPI) in a Real-World Setting. Cancers 2023, 15, 1043. [Google Scholar] [CrossRef]
- Fusco, D.; Ferrini, A.; Pasqualetti, G.; Giannotti, C.; Cesari, M.; Laudisio, A.; Ballestrero, A.; Scabini, S.; Odetti, P.R.; Colloca, G.F.; et al. Comprehensive geriatric assessment in older adultswith cancer: Recommendations by the Italian Society of Geriatrics and Gerontology (SIGG). Eur. J. Clin. Investig. 2021, 51, e13347. [Google Scholar] [CrossRef]
- Mohile, S.G.; Mohamed, M.R.; Xu, H.; Culakova, E.; Loh, K.P.; Magnuson, A.; Flannery, M.A.; Obrecth, S.; Gilmore, N.; Ramsdale, E.; et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet 2021, 398, 1894–1904. [Google Scholar] [CrossRef]
- Dale, W.; Klepin, H.D.; Williams, G.R.; Alibhai, S.M.H.; Bergerot, C.; Brintzenhofeszoc, K.; Hopkins, J.O.; Jhawer, M.P.; Katheria, V.; Poh Loh, K.; et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline update. J. Clin. Oncol. 2023, 41, 4293–4312. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.L.; Kim, H.; Soto-Perez-de-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Chien, L.C.; Charles, K.; et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults with Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef]
- Akinoso-Imran, A.Q.; O’Rorke, M.; Kee, F.; Jordao, H.; Walls, G.; Bannon, F.J. Surgical under-treatment of older adult patients with cancer: A systematic review and meta-analysis. J. Geriatr. Oncol. 2022, 13, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Pottel, L.; Lycke, M.; Boterberg, T.; Pottel, H.; Goethals, L.; Duprez, F.; Rottey, S.; Lievens, Y.; Van Den Noortgate, N.; Geldhof, K.; et al. G-8 indicates overall and quality-adjusted survival in older head and neck cancer patients treated with curative radiochemotherapy. BMC Cancer 2015, 15, 875. [Google Scholar] [CrossRef] [PubMed]
- Katz, S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. J. Aging Ment. Health 1986, 5, 165–173. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Bliss, M.R.; McLaren, R.; Exton-Smith, A.N. Mattresses for preventing pressure sores in geriatric patients. Mon. Bull. Minist. Health Public Health Lab. Serv. 1966, 25, 238–268. [Google Scholar]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative Illness Rating Scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Balducci, L.; Extermann, M. Management of cancer in the older person: A practical approach. Oncologist 2000, 5, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Lu, Y. Decision tree methods: Applications for classification and prediction. Shanghai Arch. Psychiatry 2015, 27, 130–135. [Google Scholar] [PubMed]
- Cohen, H.J.; Feussner, J.R.; Weinberger, M.; Carnes, M.; Hamdy, R.C.; Hsieh, F.; Phibbs, C.; Courtney, D.; Lyles, K.W.; May, C.; et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N. Engl. J. Med. 2002, 346, 905–912. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health Consensus Development Conference Statement: Geriatric assessment methods for clinical decision-making. J. Am. Geriatr. Soc. 1988, 36, 342–347. [CrossRef]
- Zuccarino, S.; Monacelli, F.; Antognoli, R.; Nencioni, A.; Monzani, F.; Ferrè, F.; Seghieri, C.; Incalzi, R.A. Exploring Cost-Effectiveness of the Comprehensive Geriatric Assessment in Geriatric Oncology: A Narrative Review. Cancers 2022, 14, 3235. [Google Scholar] [CrossRef]
- Nadaraja, S.; Matzen, L.E.; Jørgensen, T.L.; Dysager, L.; Knudsen, A.Ø.; Jeppesen, S.S.; Moller, S.; Herrstedt, J. The impact of comprehensive geriatric assessment for optimal treatment of older patients with cancer: A randomized parallel-group clinical trial. J. Geriatr. Oncol. 2020, 11, 488–495. [Google Scholar] [CrossRef]
- Feng, M.A.; McMillan, D.T.; Crowell, K.; Muss, H.; Nielsen, M.E.; Smith, A.B. Geriatric assessment in surgical oncology: A systematic review. J. Surg. Res. 2015, 193, 265–272. [Google Scholar] [CrossRef]
- Van Walree, I.C.; Scheepers, E.; van Huis-Tanja, L.; Emmelot-Vonk, M.H.; Bellera, C.; Soubeyran, P.; Hamaker, M.E. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J. Geriatr. Oncol. 2019, 10, 847–858. [Google Scholar] [CrossRef]
- Pierantoni, F.; Basso, U.; Maruzzo, M.; Lamberti, E.; Bimbatti, D.; Tierno, G.; Bergo, E.; Brunello, A.; Zagonel, V. Comprehensive geriatric assessment is an independent prognostic factor in older patients with metastatic renal cell cancer treated with first-line Sunitinib or Pazopanib: A single center experience. J. Geriatr. Oncol. 2021, 12, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Brunello, A.; Fontana, A.; Zafferri, V.; Panza, F.; Fiduccia, P.; Basso, U.; Copetti, M.; Lonardi, S.; Roma, A.; Falci, C.; et al. Development of an oncological-multidimensional prognostic index (Onco-MPI) for mortality prediction in older cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Procaccio, L.; Bergamo, F.; Gatti, M.; Chiusole, B.; Tierno, G.; Bergo, E.; Daniel, F.; Nappo, F.; Maddalena, G.; Rasola, C.; et al. The oncological multidimensional prognostic index is a promising decision-making tool: A real-world analysis in older patients with metastatic colorectal cancer. Eur. J. Cancer 2022, 177, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Spar, M.J.; Nicosia, F.M.; Steinman, M.A.; Brown, R.T. Current approaches to measuring functional status among older adults in VA primary care clinics. Fed. Pract. 2017, 34, 26–31. [Google Scholar]
- Ceolin, C.; Bano, G.; Biz, C.; Dianin, M.; Bedogni, M.; Guarnaccia, A.; Berizzi, A.; Ruggieri, P.; Coin, A.; Sergi, G. Functional autonomy and 12-month mortality in older adults with proximal femoral fractures in an orthogeriatric setting: Risk factors and gender differences. Aging Clin. Exp. Res. 2023, 35, 1063–1071. [Google Scholar] [CrossRef]
- Brauner, D.J.; Muir, J.C.; Sachs, G.A. Treating nondementia illnesses in patients with dementia. JAMA 2000, 283, 3230–3235. [Google Scholar] [CrossRef]
- Couderc, A.L.; Boulahssass, R.; Nouguerède, E.; Gobin, N.; Guérin, O.; Villani, P.; Barlesi, F.; Paillaud, E. Functional status in a geriatric oncology setting: A review. J. Geriatr. Oncol. 2019, 10, 884–894. [Google Scholar] [CrossRef]
- Mackey, D.C.; Cauley, J.A.; Barrett-Connor, E.; Schousboe, J.T.; Cawthon, P.M.; Cummings, S.R. Life-space mobility and mortality in older men: A prospective cohort study. J. Am. Geriatr. Soc. 2014, 62, 1288–1296. [Google Scholar] [CrossRef]
- Kanesvaran, R.; Li, H.; Koo, K.N.; Poon, D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3620–3627. [Google Scholar] [CrossRef]
- Lund, R.; Due, P.; Modvig, J.; Holstein, B.E.; Damsgaard, M.T.; Andersen, P.K. Cohabitation and marital status as predictors of mortality--an eight year follow-up study. Soc. Sci. Med. 2002, 55, 673–679. [Google Scholar] [CrossRef]
- Jeong, H.; Yim, H.W.; Nam, B.W. Independent predictors of depressive symptoms and social isolation on 2-year all-cause mortality among the Korean elderly in a population-based cohort study: Gender differences. Epidemiol. Health 2022, 44, e2022012. [Google Scholar] [CrossRef]
- Tijhuis, M.A.; De Jong-Gierveld, J.; Feskens, E.J.; Kromhout, D. Changes in and factors related to loneliness in older men. The Zutphen Elderly Study. Age Ageing 1999, 28, 491–495. [Google Scholar] [CrossRef]
- Almeida, O.P.; Alfonso, H.; Hankey, G.J.; Flicker, L. Depression, antidepressant use and mortality in later life: The Health In Men Study. PLoS ONE 2010, 5, e11266. [Google Scholar] [CrossRef]
- Cheong, C.Y.; Yap, P.; Gwee, X.; Chua, D.Q.L.; Wee, S.L.; Yap, K.B.; Ng, T.P. Physical and functional measures predicting long-term mortality in community-dwelling older adults: A comparative evaluation in the Singapore Longitudinal Ageing Study. Aging 2021, 13, 25038–25054. [Google Scholar] [CrossRef]

| Definition | Stage |
|---|---|
| - No functional dependence in ADLs or IADLs | Fit |
| - No relevant comorbidities | |
| - No geriatric syndromes | |
| - Dependence in one or more IADLs but not in ADLs | Vulnerable |
| - Comorbidities present but not severe or life-threatening | |
| - May have mild depression or mild memory disorder, but not other significant geriatric syndromes | |
| - Age > 84 years | Frail |
| - Dependence in one or more ADLs | |
| - Any significant geriatric syndrome | |
| - 3 or more grade 3 comorbidities or one grade 4 |
| p-Value | Females (n = 3222) | Males (n = 3800) | Total Sample (n = 7022) | Variable |
|---|---|---|---|---|
| 0.04 | 78.7 ± 13.5 | 78.0 ± 12.4 | 78.3 ± 12.9 | Age |
| 0.95 | 24.20 ± 6.07 | 24.21 ± 6.04 | 24.20 ± 6.06 | BMI |
| 0.38 | 2.45 ± 1.66 | 2.48 ± 1.70 | 2.47 ± 1.68 | No. of comorbidities |
| <0.001 | Civil status | |||
| 1117 (34.7%) | 412 (10.8%) | 1529 (21.8%) | Widow/widower | |
| 1490 (46.2%) | 3027 (79.7%) | 4517 (64.3%) | Married | |
| <0.001 | Cohabitation status | |||
| 861 (29.5%) | 399 (11.0%) | 1260 (19.2%) | Alone | |
| 381 (13.1%) | 546 (15.0%) | 927 (14.2%) | With partner/spouse | |
| 1637 (56.1%) | 2668 (73.5%) | 4305 (65.7%) | With other family members | |
| 40 (1.4%) | 18 (0.5%) | 58 (0.9%) | NH | |
| <0.001 | Cancer site | |||
| 827 (25.7%) | 964 (25.4%) | 1791 (25.5%) | Colorectal | |
| 549 (17.0%) | 818 (21.5%) | 1367 (19.5%) | Upper digestive tract and liver | |
| 770 (23.9%) | 12 (0.3%) | 782 (11.1%) | Breast | |
| 153 (4.7%) | 863 (22.7%) | 1016 (14.5%) | Urinary tract | |
| 251 (7.8%) | 273 (7.2%) | 524 (7.5%) | Hematological | |
| 72 (2.2%) | 105 (2.8%) | 177 (2.5%) | Brain | |
| 139 (4.3%) | 179 (4.7%) | 318 (4.5%) | Skin | |
| 53 (1.6%) | 123 (3.2%) | 176 (2.5%) | Lung | |
| 408 (12.7%) | 463 (12.2%) | 871 (12.4%) | Other * | |
| <0.001 | Tumor status | |||
| 1129 (35.0%) | 1544 (40.6%) | 2673 (39.9%) | Localized | |
| 761 (23.6%) | 1146 (30.2%) | 1907 (28.4%) | Advanced | |
| <0.001 | Treatment | |||
| 1207 (37.4%) | 920 (24.2%) | 2127 (31.7%) | Surgery | |
| 1153 (70.0%) | 932 (60.3%) | 2085 (65.3%) | Chemotherapy/ Endocrine/Targeted therapy | |
| <0.001 | 674 (20.9%) | 988 (26.0%) | 1662 (23.8%) | Metastasis |
| <0.001 | Karnofsky performance scale | |||
| 936 (29.1%) | 1226 (32.3%) | 2162 (30.8%) | 80 | |
| 909 (28.2%) | 1279 (33.7%) | 2188 (31.2%) | 90–100 | |
| Geriatric multidimensional evaluation | ||||
| 0.04 | 21.92 ± 1.29 | 24.21 ± 3.50 | 23.25 ± 1.34 | MNA |
| 0.19 | 5.33 ± 2.01 | 5.36 ± 1.27 | 5.35 ± 1.65 | ADL |
| 0.02 | 6.27 ± 2.16 | 6.39 ± 2.14 | 6.33 ± 2.15 | IADL |
| <0.001 | 4.64 ± 2.87 | 4.13 ± 2.82 | 4.36 ± 2.85 | GDS |
| 0.09 | 26.69 ± 1.77 | 28.15 ± 5.31 | 27.49 ± 4.56 | MMSE |
| 0.99 | 1.00 (0.0; 2.00) | 1.00 (0.00; 2.00) | 1.00 (0.00; 2.00) | CIRS-CI |
| <0.001 | 3.67 ± 2.77 | 3.99 ± 3.02 | 4.60 ± 3.26 | Total drugs |
| <0.001 | Final multidimensional evaluation | |||
| 874 (27.1%) | 898 (23.6%) | 1772 (25.2%) | Frail | |
| 1077 (33.4%) | 1355 (35.7%) | 2432 (34.6%) | Fit | |
| 1150 (35.7%) | 1353 (35.6%) | 2503 (35.6%) | Vulnerable |
| Adjusted Analysis | Unadjusted Analysis | Variable | ||||||
|---|---|---|---|---|---|---|---|---|
| p-Value | 95% IC | HR | p-Value | 95% IC | HR | |||
| Upper | Lower | Upper | Lower | |||||
| Cohabitative status | ||||||||
| 0.49 | 1.4 | 0.85 | 1.09 | 0.69 | 4.87 | 0.09 | 0.67 | Alone |
| <0.001 | 2.07 | 1.35 | 1.67 | 0.8 | 5.65 | 0.11 | 0.78 | With other family members/carers |
| Multidimensional evaluation | ||||||||
| 0.28 | 1.05 | 0.98 | 0.99 | 0.27 | 1.05 | 0.98 | 0.99 | MNA |
| <0.001 | 0.96 | 0.85 | 0.91 | 0.001 | 0.96 | 0.86 | 0.91 | IADL |
| 0.33 | 1.05 | 0.98 | 1.01 | 0.58 | 1.04 | 0.98 | 1.01 | MMSE |
| 0.04 | 1.07 | 1.01 | 1.04 | 0.04 | 1.07 | 1.01 | 1.03 | GDS |
| 0.52 | 1.03 | 0.87 | 0.92 | 0.03 | 1.18 | 1.09 | 1.03 | CIRS-CI |
| 0.45 | 1.05 | 0.98 | 1.01 | 0.76 | 1.04 | 0.97 | 1.01 | Total no. drugs |
| 0.79 | 1.45 | 0.75 | 1.05 | 0.81 | 1.31 | 0.81 | 1.03 | Being Frail at CGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergo, E.; De Rui, M.; Ceolin, C.; Iannizzi, P.; Curreri, C.; Devita, M.; Ruffini, C.; Chiusole, B.; Feltrin, A.; Sergi, G.; et al. Beyond Treatment Decisions: The Predictive Value of Comprehensive Geriatric Assessment in Older Cancer Patients. Cancers 2025, 17, 2489. https://doi.org/10.3390/cancers17152489
Bergo E, De Rui M, Ceolin C, Iannizzi P, Curreri C, Devita M, Ruffini C, Chiusole B, Feltrin A, Sergi G, et al. Beyond Treatment Decisions: The Predictive Value of Comprehensive Geriatric Assessment in Older Cancer Patients. Cancers. 2025; 17(15):2489. https://doi.org/10.3390/cancers17152489
Chicago/Turabian StyleBergo, Eleonora, Marina De Rui, Chiara Ceolin, Pamela Iannizzi, Chiara Curreri, Maria Devita, Camilla Ruffini, Benedetta Chiusole, Alessandra Feltrin, Giuseppe Sergi, and et al. 2025. "Beyond Treatment Decisions: The Predictive Value of Comprehensive Geriatric Assessment in Older Cancer Patients" Cancers 17, no. 15: 2489. https://doi.org/10.3390/cancers17152489
APA StyleBergo, E., De Rui, M., Ceolin, C., Iannizzi, P., Curreri, C., Devita, M., Ruffini, C., Chiusole, B., Feltrin, A., Sergi, G., & Brunello, A. (2025). Beyond Treatment Decisions: The Predictive Value of Comprehensive Geriatric Assessment in Older Cancer Patients. Cancers, 17(15), 2489. https://doi.org/10.3390/cancers17152489








