Engineered Bifidobacterium Strains Colonization at Tumor Sites: A Novel Approach to the Delivery of Cancer Treatments
Simple Summary
Abstract
1. Introduction
2. Bifidobacterium and Hypoxic Environment
3. Bifidobacterium Colonization in Tumors
4. Bifidobacterium-Mediated Gene Therapy
5. Bifidobacterium-Mediated Immunotherapy
6. Bifidobacterium-Mediated Nanoparticle Formulated Chemotherapy Delivery
7. Bifidobacterium-Mediated HIFU Synergistic Nanoparticles Delivery
8. Limitations and Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jalota, A.; Sahu, S.K.; Haque, S. Therapeutic antibodies for the prevention and treatment of cancer. J. Biomed. Sci. 2024, 31, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, Q.; Huang, X.; Koo, S.; Kong, N.; Tao, W. mRNA-based cancer therapeutics. Nat. Rev. Cancer 2023, 8, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Chen, X.; Dobrovolskaia, M.A.; Lammers, T. Cancer nanomedicine. Nat. Rev. Cancer 2022, 22, 550–556. [Google Scholar] [CrossRef]
- Ijaz, M.; Hasan, I.; Chaudhry, T.H.; Huang, B.R.; Zhang, L.; Hu, Z.; Tan, Q.; Guo, B. Bacterial derivatives mediated drug delivery in cancer therapy: A new generation strategy. J. Nanobiotechnol. 2024, 22, 510. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Zare, M.; Aghayari, M.; Afkhami, H.; Jafari, G.A. Therapeutic bacteria and viruses to combat cancer: Double-edged sword in cancer therapy: New insights for future. Cell Commun. Signal. 2024, 22, 239. [Google Scholar] [CrossRef]
- Liang, S.; Wang, C.; Shao, Y.; Wang, Y.; Xing, D.; Geng, Z. Recent advances in bacteria-mediated cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1026248. [Google Scholar] [CrossRef]
- Din, S.R.U.; Saeed, S.; Khan, S.U.; Arbi, F.M.; Xuefang, G.; Zhong, M. Bacteria-driven cancer therapy: Exploring advancements and challenges. Crit. Rev. Oncol. Hematol. 2023, 191, 104141. [Google Scholar] [CrossRef] [PubMed]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, Q.; Zhang, Y.; Liu, Z.; Zheng, Z.; Liu, S.; Meng, L.; Xin, Y.; Jiang, X. Targeting hypoxia in the tumor microenvironment: A potential strategy to improve cancer immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 24. [Google Scholar] [CrossRef]
- Xiao, S.; Shi, H.; Zhang, Y.; Fan, Y.; Wang, L.; Xiang, L.; Liu, Y.; Zhao, L.; Fu, S. Bacteria-driven hypoxia targeting delivery of chemotherapeutic drug proving outcome of breast cancer. J. Nanobiotechnol. 2022, 20, 178. [Google Scholar] [CrossRef]
- Manome, A.; Abiko, Y.; Kawashima, J.; Washio, J.; Fukumoto, S.; Takahashi, N. Acidogenic Potential of Oral Bifidobacterium and its High Fluoride Tolerance. Front. Microbiol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Sánchez, B.; Champomier-Vergès, M.C.; Collado Mdel, C.; Anglade, P.; Baraige, F.; Sanz, Y.; de los Reyes-Gavilán, C.G.; Margolles, A.; Zagorec, M. Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl. Environ. Microbiol. 2007, 20, 6450. [Google Scholar] [CrossRef]
- Kimura, N.T.; Taniguchi, S.; Aoki, K.; Baba, T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980, 40, 2061–2068. [Google Scholar]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 2, 269–274. [Google Scholar] [CrossRef]
- Cronin, M.; Morrissey, D.; Rajendran, S.; El Mashad, S.M.; van Sinderen, D.; O’Sullivan, G.C.; Tangney, M. Orally administered Bifidobacteria as vehicles for delivery of agents to systemic tumors. Mol. Ther. 2010, 7, 1397–1407. [Google Scholar] [CrossRef]
- Osswald, A.; Sun, Z.; Grimm, V.; Ampem, G.; Riegel, K.; Westendorf, A.M.; Sommergruber, W.; Otte, K.; Dürre, P.; Riedel, C.U. Three-dimensional tumor spheroids for in vitro analysis of bacteria as gene delivery vectors in tumor therapy. Microb. Cell Fact. 2015, 14, 199. [Google Scholar] [CrossRef]
- Yazawa, K.; Fujimori, M.; Nakamura, T.; Sasaki, T.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res. Treat. 2001, 2, 165–170. [Google Scholar] [CrossRef]
- Fujimori, M. Genetically engineered Bifidobacterium as a drug delivery system for systemic therapy of metastatic breast cancer patients. Breast Cancer 2006, 13, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sasaki, T.; Fujimori, M.; Yazawa, K.; Kano, Y.; Amano, J.; Taniguchi, S. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci. Biotechnol. Biochem. 2002, 11, 2362–2366. [Google Scholar] [CrossRef]
- Hidaka, A.; Hamaji, Y.; Sasaki, T.; Taniguchi, S.; Fujimori, M. Exogenous cytosine deaminase gene expression in Bifidobacterium breve I-53-8w for tumor-targeting enzyme/prodrug therapy. Biosci. Biotechnol. Biochem. 2007, 12, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, G.F.; Fan, Y.R.; Liu, W.H.; Liu, X.J.; Wang, J.J.; Xu, G.X. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: Selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003, 2, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Yamashita, Y.; Kanamori, M.; Endersby, R.; Bankiewicz, K.S.; Baker, S.J.; Bergers, G.; Pieper, R.O. The PTEN/Akt pathway dictates the direct alphaVbeta3-dependent growth-inhibitory action of an active fragment of tumstatin in glioma cells in vitro and in vivo. Cancer Res. 2006, 66, 11331–11340. [Google Scholar] [CrossRef]
- Wei, C.; Xun, A.Y.; Wei, X.X.; Yao, J.; Wang, J.Y.; Shi, R.Y.; Yang, G.H.; Li, Y.X.; Xu, Z.L.; Lai, M.G.; et al. Bifidobacteria Expressing Tumstatin Protein for Antitumor Therapy in Tumor-Bearing Mice. Technol. Cancer Res. Treat. 2016, 3, 498–508. [Google Scholar] [CrossRef]
- Shimizu, Y.; Isoda, K.; Taira, Y.; Taira, I.; Kondoh, M.; Ishida, I. Anti-tumor effect of a recombinant Bifidobacterium strain secreting a claudin-targeting molecule in a mouse breast cancer model. Eur. J. Pharmacol. 2020, 887, 173596. [Google Scholar] [CrossRef]
- Wang, L.; Vuletic, I.; Deng, D.; Crielaard, W.; Xie, Z.; Zhou, K.; Zhang, J.; Sun, H.; Ren, Q.; Guo, C. Bifidobacterium breve as a delivery vector of IL-24 gene therapy for head and neck squamous cell carcinoma in vivo. Gene Ther. 2017, 11, 699–705. [Google Scholar] [CrossRef]
- Kaliberov, S.A.; Kaliberova, L.N.; Buchsbaum, D.J. Combined ionizing radiation and sKDR gene delivery for treatment of prostate carcinomas. Gene Ther. 2005, 5, 407–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.J.; Zhu, H.; Ma, B.Y.; Zhao, F.; Mao, S.H.; Liu, T.G.; He, J.P.; Deng, L.C.; Yi, C.; Huang, Y. Inhibitory effect of Bifidobacterium infantis-mediated sKDR prokaryotic expression system on angiogenesis and growth of Lewis lung cancer in mice. BMC Cancer 2012, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Z.; Mao, S.; Ma, B.; Zhou, S.; Deng, L.; Liu, T.; Cui, D.; Zhao, Y.; He, J.; et al. Antitumor effect of sFlt-1 gene therapy system mediated by Bifidobacterium infantis on Lewis lung cancer in mice. Cancer Gene Ther. 2011, 12, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Shioya, K.; Matsumura, T.; Seki, Y.; Shimizu, H.; Nakamura, T.; Taniguchi, S. Potentiated antitumor effects of APS001F/5-FC combined with anti-PD-1 antibody in a CT26 syngeneic mouse model. Biosci. Biotechnol. Biochem. 2021, 85, 324. [Google Scholar] [CrossRef]
- Kikuchi, T.; Shimizu, H.; Akiyama, Y.; Taniguchi, S. In situ delivery and production system of trastuzumab scFv with Bifidobacterium. Biochem. Biophys. Res. Commun. 2017, 493, 306. [Google Scholar] [CrossRef]
- Gurung, P.; Lim, J.; Shrestha, R.; Kim, Y.W. Chlorin e6-associated photodynamic therapy enhances abscopal antitumor effects via inhibition of PD-1/PD-L1 immune checkpoint. Sci. Rep. 2023, 13, 4647. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Liu, J.; Wang, B.; Pu, G.; Li, J.; Huang, Y.; Chu, M. Nanodrug-loaded Bifidobacterium bifidum conjugated with anti-death receptor antibody for tumor-targeted photodynamic and sonodynamic synergistic therapy. Acta Biomater. 2022, 146, 341. [Google Scholar] [CrossRef]
- Li, J.; Wen, Q.; Dai, J.; Wang, B.; Lu, Y.; Wu, Z.; Fan, Y.; Zeng, F.; Chen, Y.; Zhang, Y.; et al. An oral bioactive chitosan-decorated doxorubicin nanoparticles/bacteria bioconjugates enhance chemotherapy efficacy in an in-situ breast cancer model. Int. J. Biol. Macromol. 2024, 267, 131428. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Liu, Y.; Wen, Q.; Lin, S.; Wen, Q.; Lu, Y.; Dai, J.; Li, J.; Xiao, S.; et al. Bacteria-Driven Tumor Microenvironment-Sensitive Nanoparticles Targeting Hypoxic Regions Enhances the Chemotherapy Outcome of Lung Cancer. Int. J. Nanomed. 2023, 18, 1299. [Google Scholar] [CrossRef]
- Wu, D.; Fu, K.; Zhang, W.; Li, Y.; Ji, Y.; Dai, Y.; Yang, G. Chitosan nanomedicines-engineered Bifidobacteria complexes for effective colorectal tumor-targeted delivery of SN-38. Int. J. Pharm. 2024, 659, 124283. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, M.; Cao, K.; Qi, Z.; Zhu, L.; Zhang, Z.; Hou, L. A self-guidance biological hybrid drug delivery system driven by anaerobes to inhibit the proliferation and metastasis of colon cancer. Asian J. Pharm. Sci. 2022, 6, 892–907. [Google Scholar] [CrossRef]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, L.; Tang, Y.; Wang, Y.; Li, N.; Wang, D.; Zhang, Z.; Lin, L.; Du, Y.; Ou, X.; et al. US/MR Bimodal Imaging-Guided Bio-Targeting Synergistic Agent for Tumor Therapy. Int. J. Nanomed. 2022, 17, 2943–2960. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, C.; Jiang, B.; Wang, L.; Jiang, F.; Wang, D.; Wang, Y.; Yang, H.; Ou, X.; Du, Y.; et al. Bifidobacterium bifidum-Mediated Specific Delivery of Nanoparticles for Tumor Therapy. Int. J. Nanomed. 2021, 16, 4643–4659. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zou, W.; Jiang, B.; Xu, D.; Luo, Y.; Xiong, J.; Yan, S.; Wang, Y.; Tang, Y.; Chen, C.; et al. Experimental Study of Retention on the Combination of Bifidobacterium with High-Intensity Focused Ultrasound (HIFU) Synergistic Substance in Tumor Tissues. Sci. Rep. 2019, 9, 6423. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, F.; Wang, L.; Tang, Y.; Zhang, Z.; Du, Y.; Zou, J. Polyethylenimine (PEI)-modified poly (lactic-co-glycolic) acid (PLGA) nanoparticles conjugated with tumor-homing bacteria facilitate high intensity focused ultrasound-mediated tumor ablation. Biochem. Biophys. Res. Commun. 2021, 571, 104–109. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Luo, Y.; Xiong, J.; Tang, Y.; Yang, H.; Wang, L.; Jiang, F.; Gao, X.; Xu, D.; et al. Experimental Study of Tumor Therapy Mediated by Multimodal Imaging Based on a Biological Targeting Synergistic Agent. Int. J. Nanomed. 2020, 15, 1871–1888. [Google Scholar] [CrossRef]
- Procaccianti, G.; Roggiani, S.; Conti, G.; Brigidi, P.; Turroni, S.; D’Amico, F. Bifidobacterium in anticancer immunochemotherapy: Friend or foe? Microbiome Res. Rep. 2023, 2, 24. [Google Scholar] [CrossRef]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188494. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Ho, C.L. Recent Development of Probiotic Bifidobacteria for Treating Human Diseases. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef]
- Shevtsov, M.; Pitkin, E.; Combs, S.E.; Meulen, G.V.; Preucil, C.; Pitkin, M. Comparison In Vitro Study on the Interface Between Skin and Bone Cell Cultures and Microporous Titanium Samples Manufactured with 3D Printing Technology Versus Sintered Samples. Nanomaterials 2024, 14, 1484. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Yu, J. The role of gut microbiota and metabolites in cancer chemotherapy. J. Adv. Res. 2024, 64, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, S.Y.; Yoon, Y.; Park, C.; Sohn, J.; Jeong, J.J.; Jeon, B.N.; Jang, M.; An, C.; Lee, S.; et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 2021, 6, 277. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97. [Google Scholar] [CrossRef] [PubMed]
- Flórez, A.B.; Sierra, M.; Ruas-Madiedo, P.; Mayo, B. Susceptibility of lactic acid bacteria, Bifidobacteria and other bacteria of intestinal origin to chemotherapeutic agents. Int. J. Antimicrob. Agents 2016, 48, 547–550. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef]
- Taniguchi, S. In Situ Delivery and Production System (iDPS) of Anti-Cancer Molecules with Gene-Engineered Bifidobacterium. J. Pers. Med. 2021, 11, 566. [Google Scholar] [CrossRef]
- Fang, J.; Liao, L.; Yin, H.; Nakamura, H.; Shin, T.; Maeda, H. Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei. J. Pharm. Sci. 2014, 10, 3235–3243. [Google Scholar] [CrossRef]
- Fang, J.; Long, L.; Maeda, H. Enhancement of Tumor-Targeted Delivery of Bacteria with Nitroglycerin Involving Augmentation of the EPR Effect. Methods Mol. Biol. 2016, 1409, 9–23. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 12, 727–743. [Google Scholar] [CrossRef]
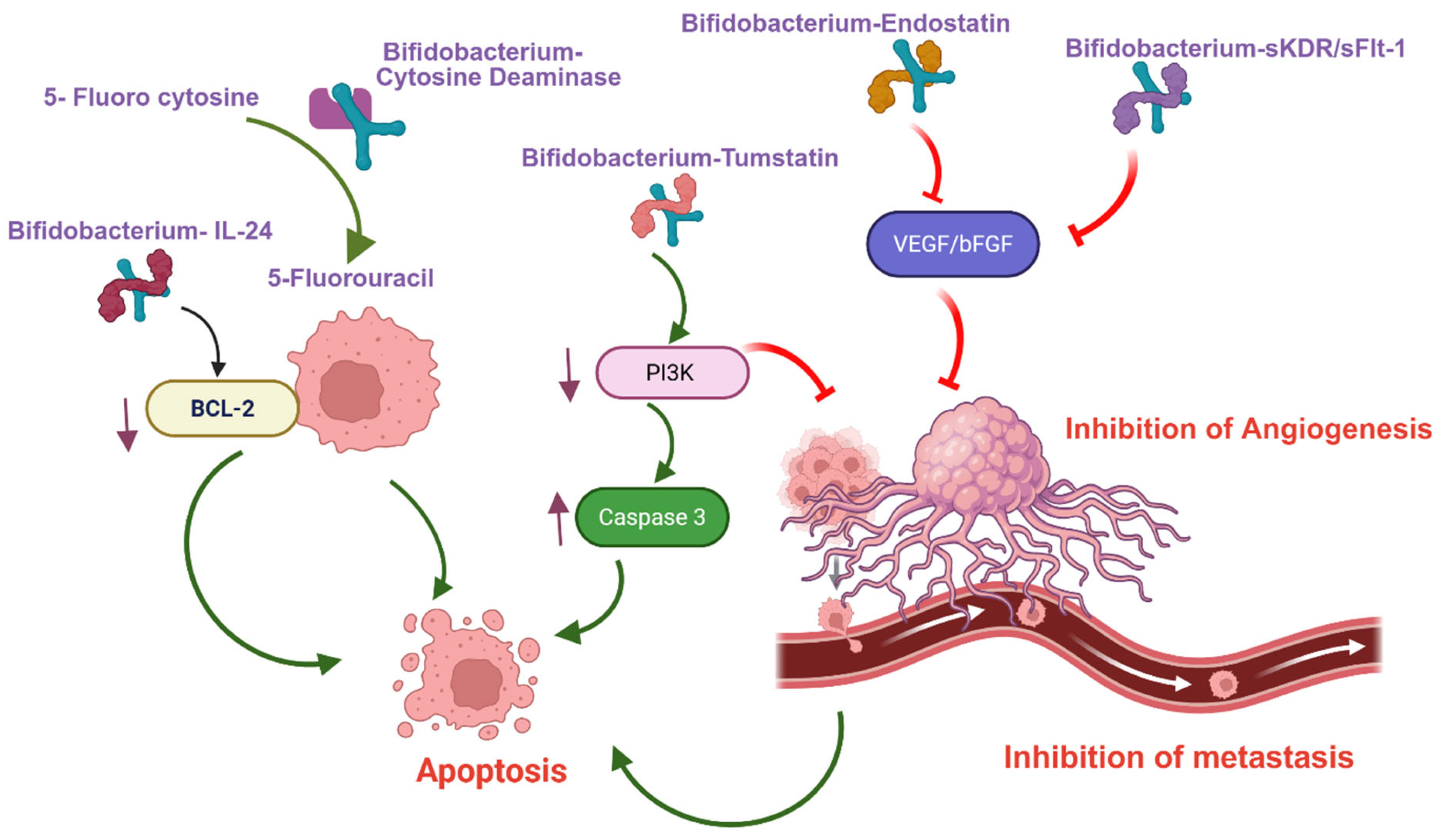

| Gene | Mode of Action | Bifidobacterium | Cancer | Observation (In Vivo) | Reference |
|---|---|---|---|---|---|
| Cytosine deaminase gene (CDG) | Conversion of the 5-fluorocytosine (5-FC) into active 5-FU | B. longum | Lung, Breast | [24] | |
| Cytosine deaminase gene (CDG) | Conversion of the 5-fluorocytosine (5-FC) into active 5-FU | B. breve (Higher CDG activity compared to B. longum) | Lung | [25] | |
| Endostatin | Angiogenesis inhibitor— downregulation of bFGF and VEGF | B. adolescentis | Liver | Inhibition of Tumor growth (69.9%) | [26] |
| Tumstatin | Angiogenesis inhibitor and promote apoptosis | B. longum | Colorectal | Inhibition of Tumor growth (75.21%) | [28] |
| Enterotoxin (C-terminal fragment of the Clostridium perfringens (C-CPE) (Claudin-4 blocker) | Claudin-4 inhibition—inhibits proliferation | B. longum | Breast (TNBC) | Inhibition of Tumor growth (49.4%) | [29] |
| Interleukin-24 | Promote apoptosis and/or autophagy | B. breve | Head and neck squamous | [30] | |
| Kinase insert domain receptor (sKDR) | Angiogenesis inhibitor (VEGF-mediated) | B. infantis | Lung | Inhibition of Tumor growth and higher survival (p ≤ 0.05) | [32,33] |
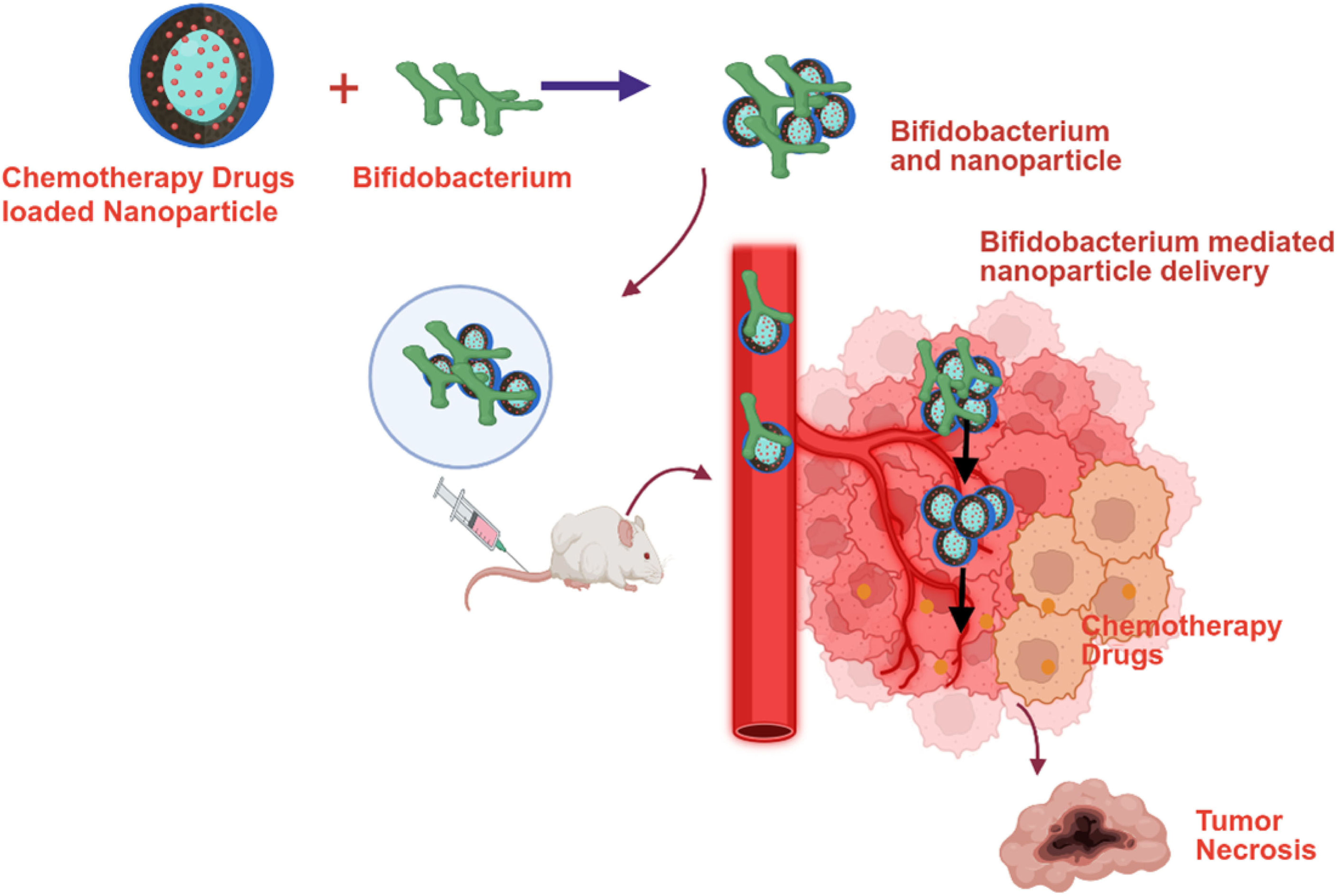
| Chemotherapy | Biohybrid | Bifidobacterium | Cancer | Observation (In Vivo) | Reference |
|---|---|---|---|---|---|
| Doxorubicin | Albumin-encapsulated doxorubicin coated with chitosan (Bif@BDC-NPs) | B. infantis | Breast | Inhibited tumor growth (94%) | [38] |
| Doxorubicin | Doxorubicin-loaded bovine serum albumin (Bif@DOX-NPs) | B. infantis | Breast | Inhibited tumor growth and prolonged the median survival of the tumor-bearing mice to 69 days | [15] |
| Paclitaxel | Polydopamine (PDA)-coated paclitaxel (Bif@PDA-PTX-NPs) | B. infantis | Lung | Inhibited tumor growth and prolonged the survival of tumor-bearing mice. | [39] |
| Irinotecan (CPT-11)-SN38 | Poly-L-glutamic acid SN38 (CS-L-PGA-SN38 NPs/B. bifi) | B. bifidum | Colorectal | Inhibited tumor growth (80%) | [40] |
| Doxorubicin & Endostatin | Iron alginate (FeAlg) gel with doxorubicin and endostatin (BI-ES-FeAlg/DOX) | B. infantis | Colorectal | Inhibited tumor growth (82%) | [41] |
| HIFU Synergist Nanoparticle | Biohybrid | Bifidobacterium | Observation (In Vivo) | Reference |
|---|---|---|---|---|
| Perfluorohexane (PFH) and superparamagnetic iron oxides (SPIO, Fe3O4) with cationic lipid (CL) | B. bifidum + PFH@CL/Fe3O4 NPs | B. bifidum | Higher coagulative necrosis and apoptosis | [43] |
| Aptamers CCFM641-5-functionalized Perfluorohexane (PFH) loaded poly(lactic-co-glycolic acid | Bifidobacterium+ AP-PFH/PLGA | B. bifidum | Tumor growth inhibition and prolong the survival (60 days) compared to docetaxel (30 days) | [44] |
| PEGylated cationic lipid nanoparticles (CL-NPs) | B. longum + CL-NPs | B. longum | Higher coagulative necrosis and apoptosis | [45] |
| Polyethylenimine (PEI)-modified poly(lactic-co-glycolic acid) nanoparticles loaded with sodium bicarbonate | B. bifidum + PEI-PLGA-NaHCO3 NPs | B. bifidum | Higher coagulative necrosis and apoptosis | [43] |
| Cationic lipid nanoparticles co-loaded with indocyanine green (ICG) and perfluorohexane (PFH) | B. longum + CL-ICG-PFH-NPs | B. longum | Higher coagulative necrosis volume and apoptosis | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amonkar, R.; Uy, A.A.; Ramirez, P.; Patel, H.; Jeong, J.J.; Shoyele, N.O.; Vaghela, V.; Lakshmikuttyamma, A. Engineered Bifidobacterium Strains Colonization at Tumor Sites: A Novel Approach to the Delivery of Cancer Treatments. Cancers 2025, 17, 2487. https://doi.org/10.3390/cancers17152487
Amonkar R, Uy AA, Ramirez P, Patel H, Jeong JJ, Shoyele NO, Vaghela V, Lakshmikuttyamma A. Engineered Bifidobacterium Strains Colonization at Tumor Sites: A Novel Approach to the Delivery of Cancer Treatments. Cancers. 2025; 17(15):2487. https://doi.org/10.3390/cancers17152487
Chicago/Turabian StyleAmonkar, Rhea, Ashley Ann Uy, Pablo Ramirez, Harina Patel, Jae Jin Jeong, Nicole Oyinade Shoyele, Vidhi Vaghela, and Ashakumary Lakshmikuttyamma. 2025. "Engineered Bifidobacterium Strains Colonization at Tumor Sites: A Novel Approach to the Delivery of Cancer Treatments" Cancers 17, no. 15: 2487. https://doi.org/10.3390/cancers17152487
APA StyleAmonkar, R., Uy, A. A., Ramirez, P., Patel, H., Jeong, J. J., Shoyele, N. O., Vaghela, V., & Lakshmikuttyamma, A. (2025). Engineered Bifidobacterium Strains Colonization at Tumor Sites: A Novel Approach to the Delivery of Cancer Treatments. Cancers, 17(15), 2487. https://doi.org/10.3390/cancers17152487







