Fuelling the Fight from the Gut: Short-Chain Fatty Acids and Dexamethasone Synergise to Suppress Gastric Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Drug Preparation
2.2. Cell Culture
2.3. Cell Viability Assays
2.4. Synergy
2.5. Flow Cytometry Analyses of Apoptotic Profiles
2.6. ROS Production Analysis
2.7. Liquid Chromatography-Mass Spectrometry, Label Free Quantification Bottom-Up Proteomics Analysis
2.7.1. Cell Culture, Treatment, and Protein Extraction
2.7.2. Sample Preparation
2.7.3. Liquid Chromatography-Mass Spectrometry Data Independent Analysis
2.7.4. Data Processing
2.8. Statistical Analysis
3. Results and Discussion
3.1. Antiproliferative Activity of Postbiotic Combinations, Standard Immunotherapy and Standard Chemotherapy
3.2. Synergistic Potential of APB with Dex Against the AGS Gastric Adenocarcinoma Cells
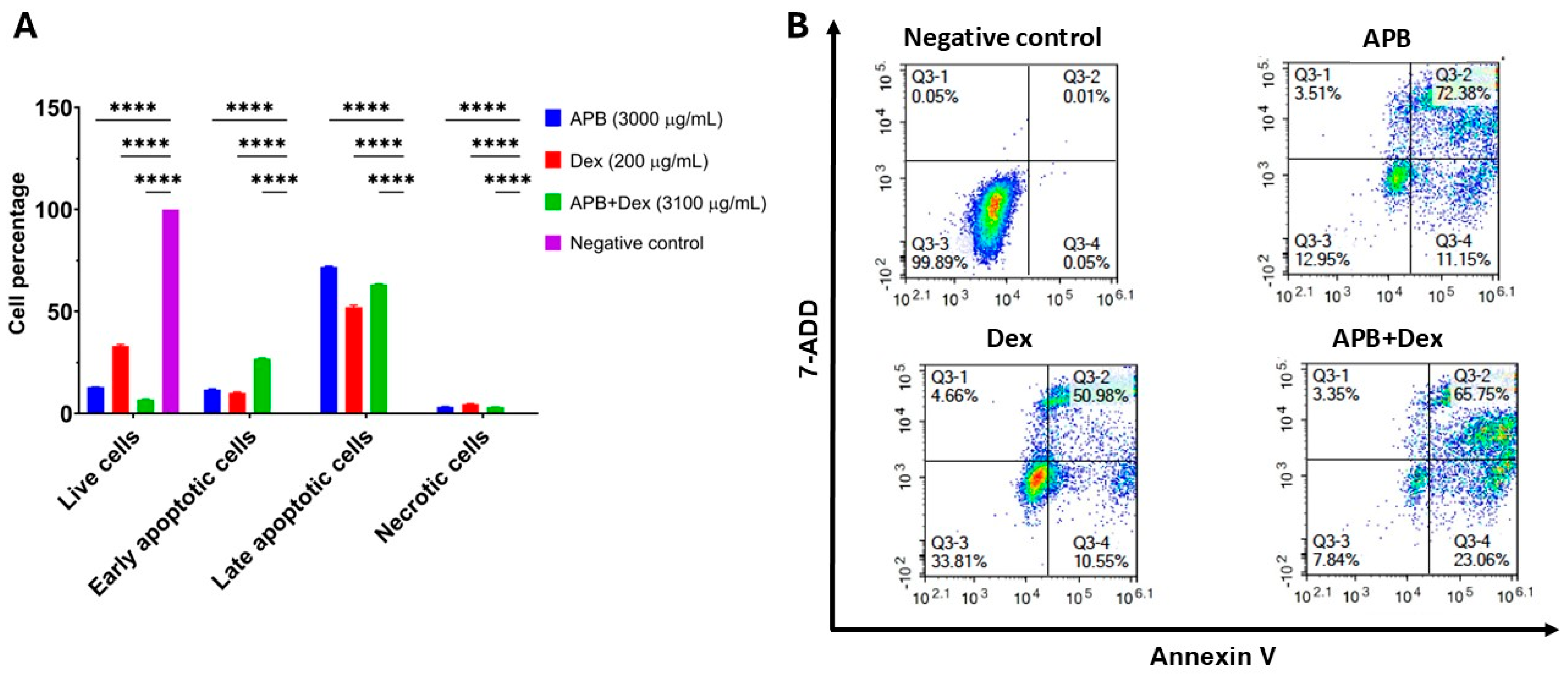
3.3. Flow Cytometric Analyses of Apoptotic Profiles of Mono and Combination Therapies
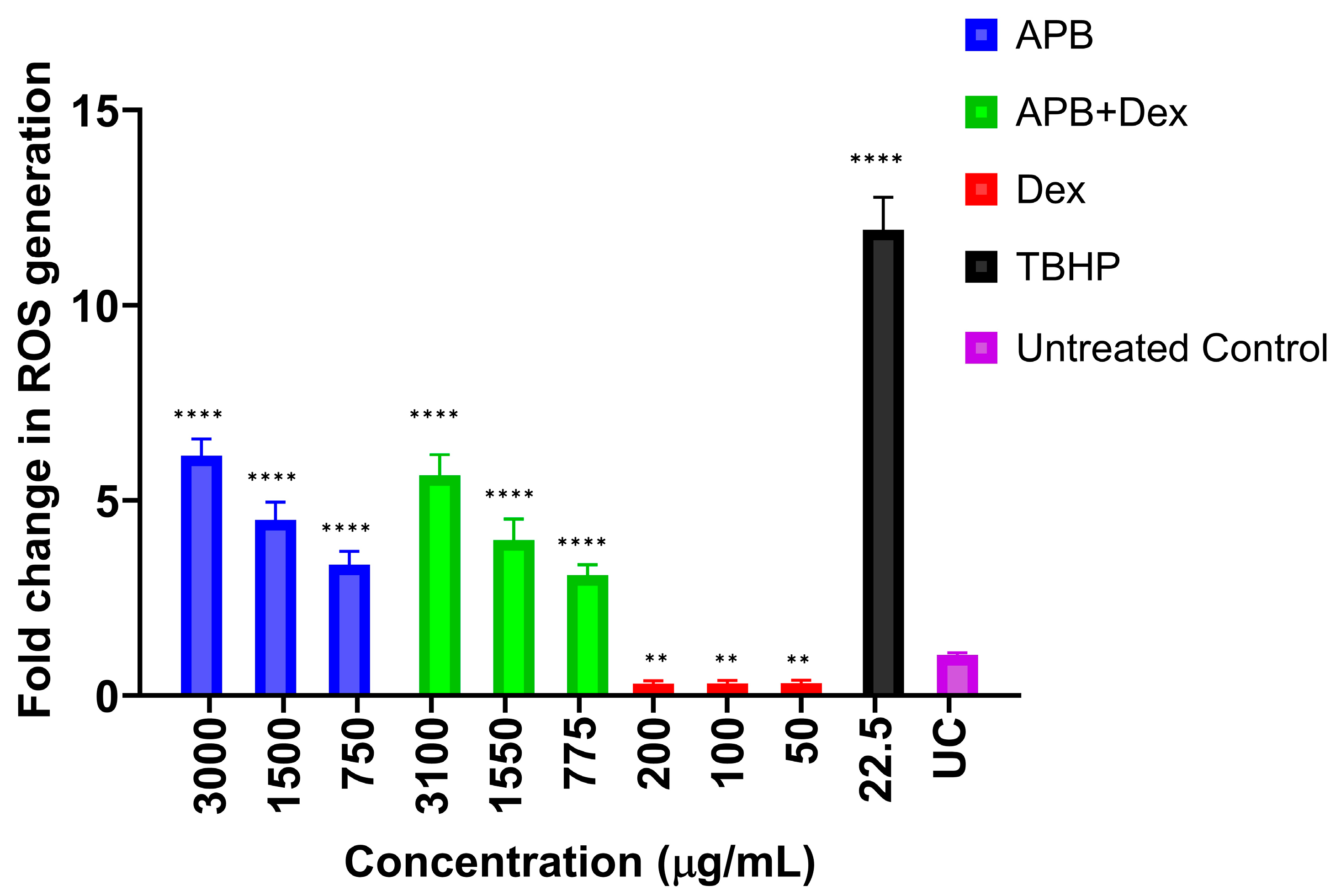
3.4. ROS Production in the AGS Cells After Treatment with Different Concentrations of APB, Dex and APB+Dex
3.5. Proteomics Study of the AGS Cells Treated with the Synergistic Combination vs. Mono Treatments
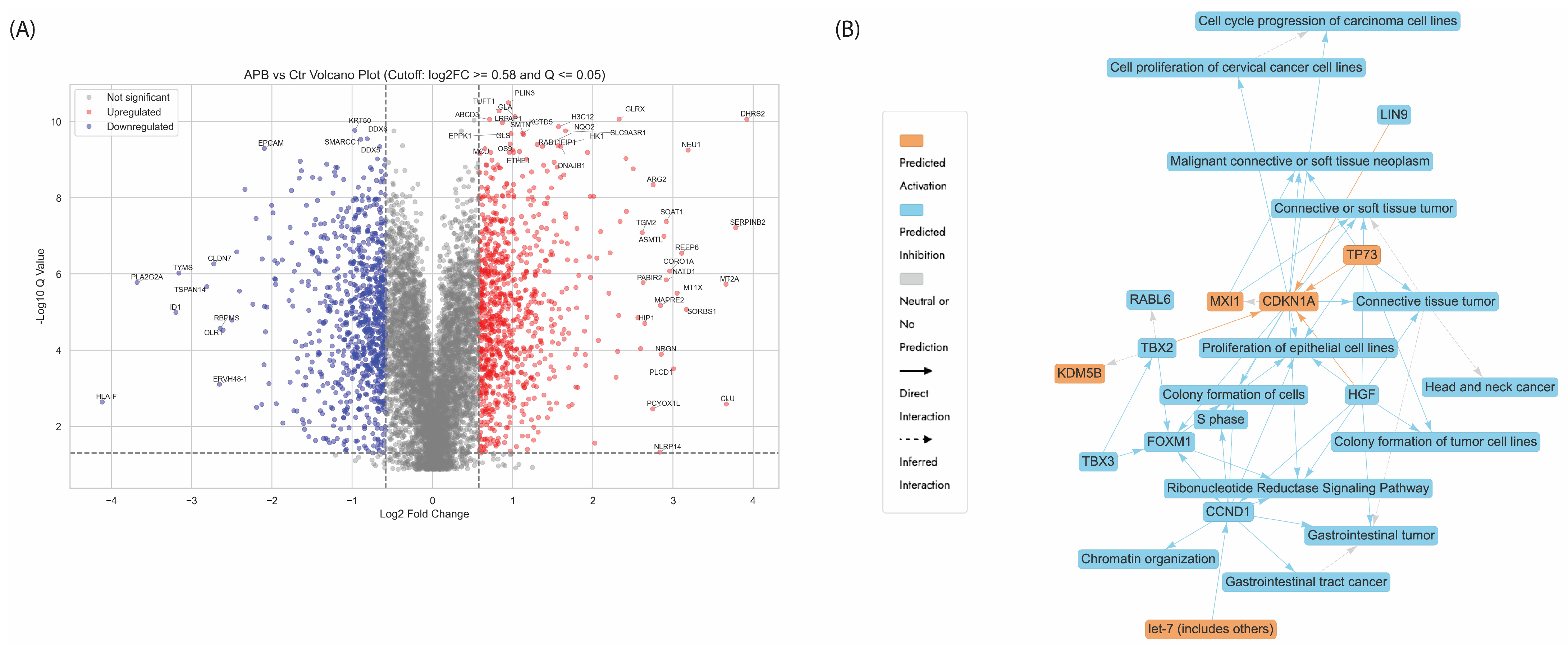
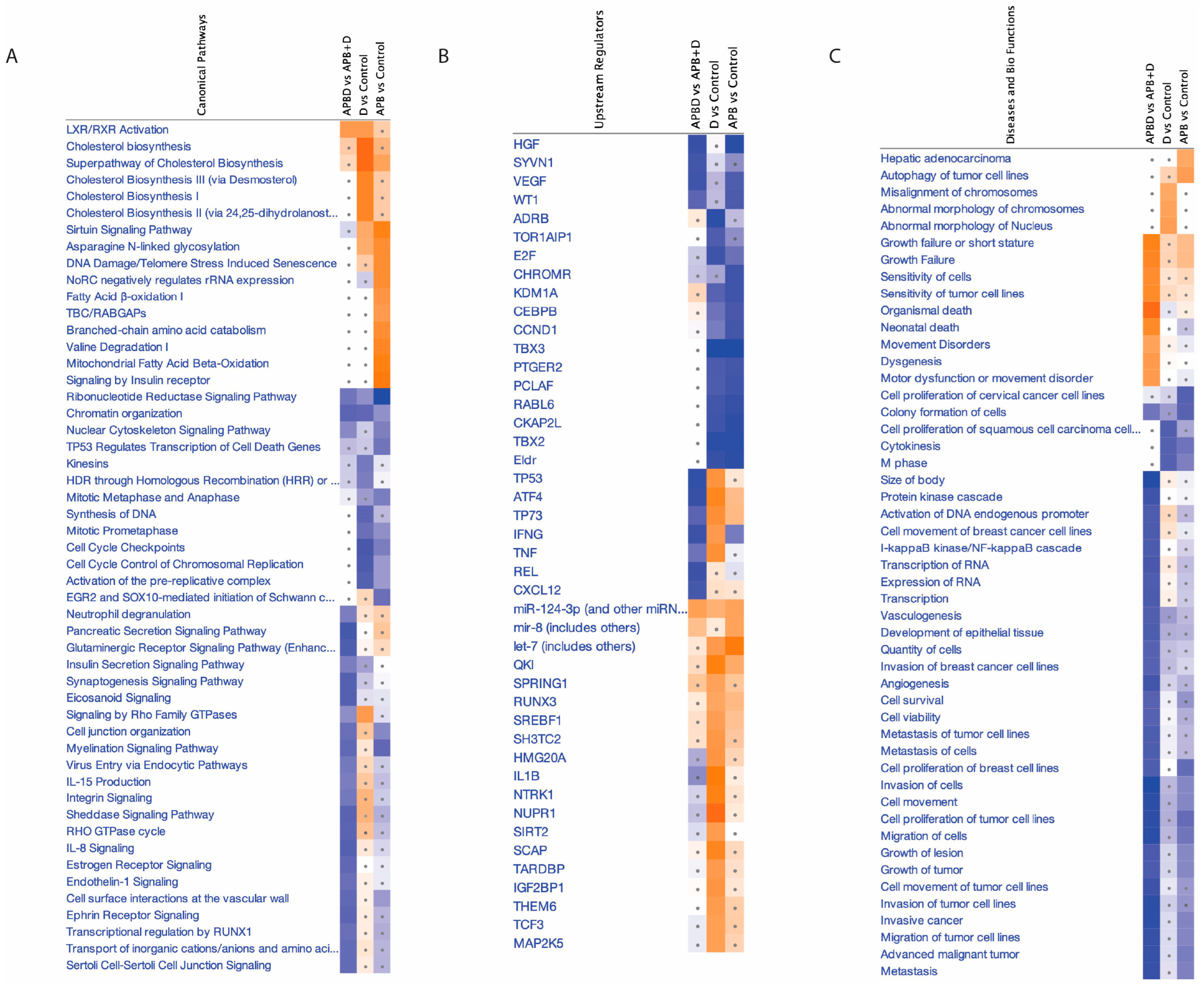
3.5.1. Enrichment Analyses of Differentially Expressed Proteins (DEPs) in APB-Treated AGS Cells Compared to the Untreated Control Cells
- -
- Chromatin Organisation
- -
- Ribonucleotide Reductase Signalling Pathway
- -
- Regulation of endogenous retroelements
- -
- Cell Cycle Checkpoint
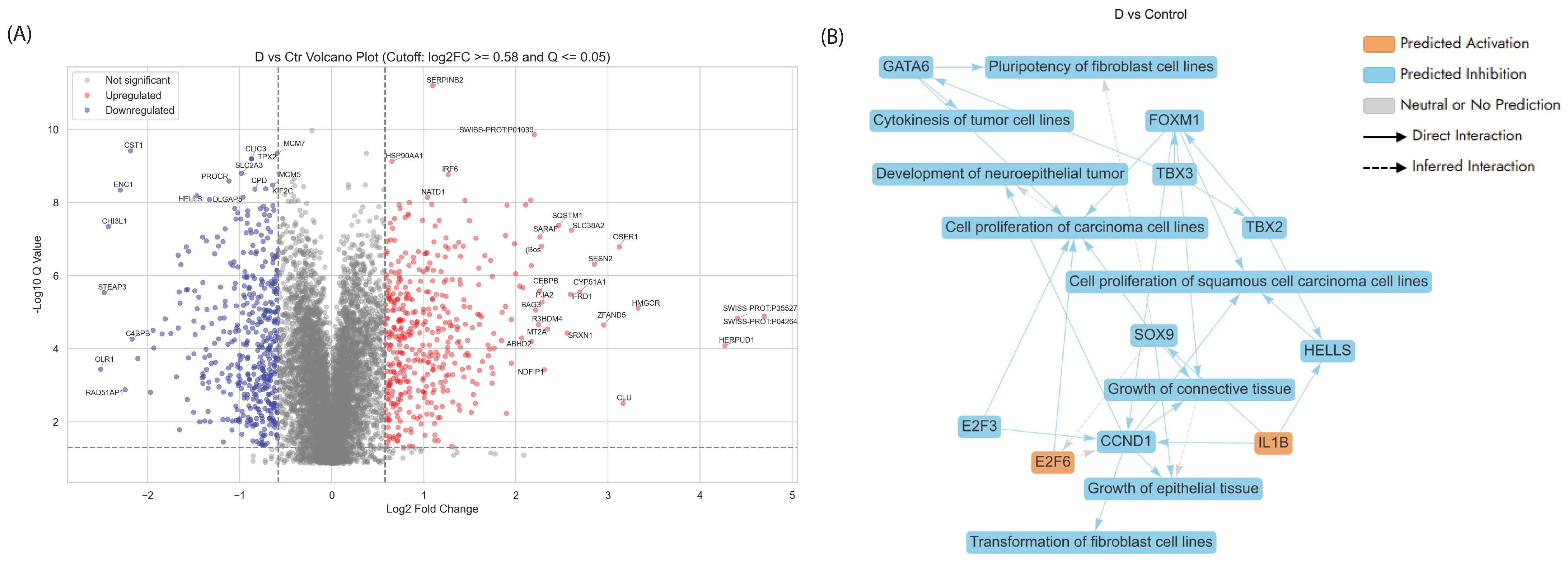
3.5.2. Enriched Pathways of DEPs in Dex Treated AGS Gastric Adenocarcinoma Cells Compared to Control
- -
- Activation of SREBF-Mediated Cholesterol Biosynthesis in Dex-Treated AGS Cells
- -
- Proteins related to the cell cycle
- -
- Ferroptosis
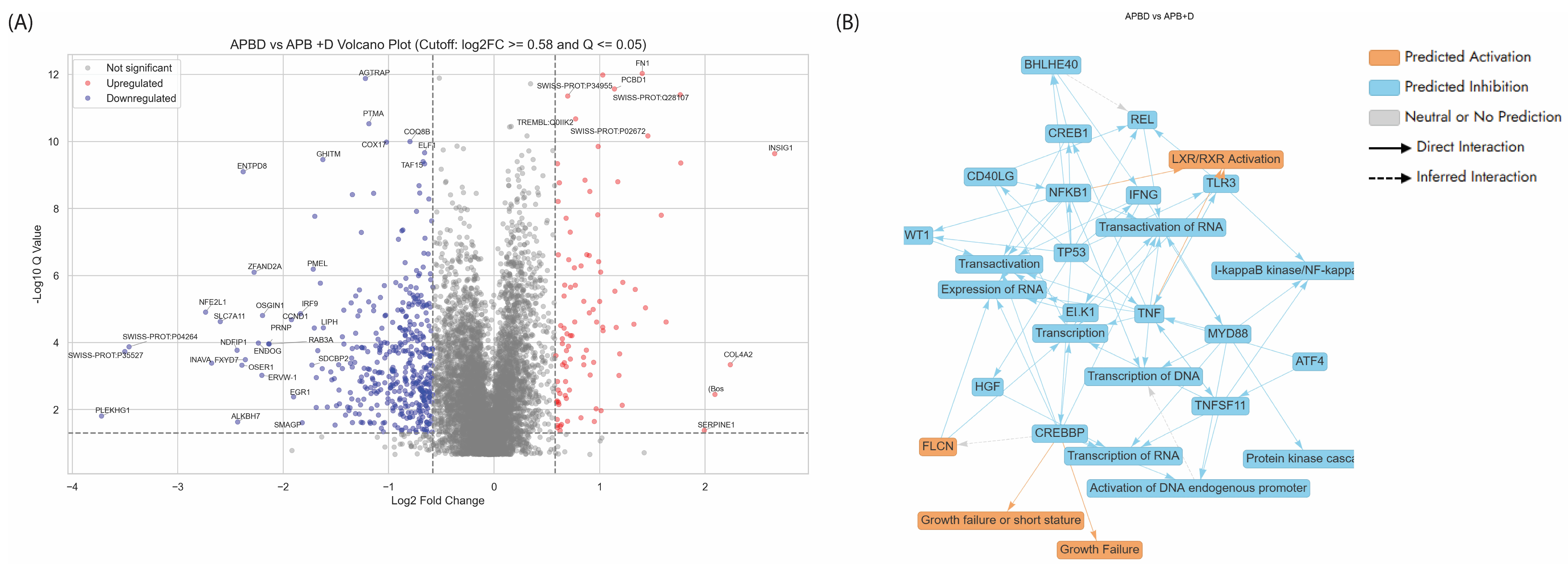
3.5.3. Enriched Pathways Using DEPs of APB+Dex Combination Treated AGS Cells vs. Mono Treatments
| Gene | Protein | Log2FC | Role | Ref |
|---|---|---|---|---|
| OSER1 | Oxidative stress-responsive serine-rich protein 1 | −2.39 | Noted for its role in the negative regulation of intracellular signal transduction. | [120] |
| DKK1 | Dickkopf-related protein 1 | −1.54 | Overexpressed in cancer, affecting Wnt signalling pathways. Overexpression correlates with poor survival in gastric cancer. | [121] |
| FOXO1 | Forkhead box protein O1 | −1.21 | A tumour suppressor transcription factor linked to cancer. | [122] |
| EPHA4 | Ephrin type-A receptor 4 | −1.07 | A receptor tyrosine kinase promoting cancer progression. | [123] |
| ABL1 ABL2 | Tyrosine kinase ABL1 and ABL2 | −0.79 −0.76 | Proto-oncogenes involved in cell differentiation, division, and adhesion and is linked various cancers, especially leukaemia. Altered signalling associated with gastric cancer. | [124] |
| MET | Hepatocyte growth factor receptor | −0.78 | A proto-oncogene involved in several cancers, including gastric cancer. Overexpression and mutations linked to poor prognosis in gastric cancer. | [125] |
| SRC | Proto-oncogene tyrosine-protein kinase Src | −0.68 | Associated with numerous cancers through oncogenic signalling. Promotes gastric cancer progression through activation of oncogenic pathways. | [126] |
| STAT1 | Signal transducer and activator of transcription 1-alpha/beta | 0.85 | Influences cancer progression and immune responses | [127] |
| DUSP10 | Dual specificity protein phosphatase 10 | 1.33 | Regulates pathways connected to cancer development. | [128] |
| CDKN2D | Cyclin-dependent kinase 4 inhibitor D | 1.18 | A cyclin-dependent kinase inhibitor linked to multiple cancers. | [129] |
| EPCAM | Epithelial cell adhesion molecule | −1.04 | Cell adhesion and signalling; upregulated in gastric tumours for proliferation and metastasis. | [36] |
| GNAQ | Guanine nucleotide-binding protein G(q) subunit alpha | −0.64 | Oncogene in G-protein signalling; implicated in tumour progression. | [130] |
| GNAS | Guanine nucleotide-binding protein G(s) subunit alpha isoforms XLas | −0.73 | Oncogenic signalling driver; mutated in some gastric cancers. | [130] |
| MAFG | Transcription factor MafG | −0.65 | Transcription factor in oxidative stress response; linked to oncogenesis. | [131] |
| TSPAN1, TSPAN6, TSPAN8, TSPAN14, TSPAN15, TSPAN31 | Tetraspanin | −0.78 to −1.49 | Roles in cell signalling, adhesion, and metastasis. | [132] |
- -
- Proteins Related to Homeostasis and Tumour Microenvironment Regulation
- -
- Proteins related to Transcription Regulation and Cell Growth Modulation
- -
- Proteins related to amino acid transport across the plasma membrane
4. Conclusions, Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burz, C.; Pop, V.; Silaghi, C.; Lupan, I.; Samasca, G. Prognosis and Treatment of Gastric Cancer: A 2024 Update. Cancers 2024, 16, 1708. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Gastroenterol. Rev./Przegląd Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Cheng, Q.; Xie, J.; Duan, Y.; Li, J.; She, Z.; Lu, W.; Chen, Y. The Psychological Distress of Gastrointestinal Cancer Patients and Its Association with Quality of Life among Different Genders. Support. Care Cancer 2024, 32, 329. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA A Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.H.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Al Fatease, A.; Alamri, A.H.; et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576. [Google Scholar] [CrossRef]
- Korkmaz, A.I.; Bal, C.; Eraslan, E.C.; Sevindik, M.; Akgul, H. Biological Activities of Agrocybe Praecox (Spring Fieldcap Mushroom). Prospect. Pharm. Sci. 2023, 21, 33–39. [Google Scholar] [CrossRef]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The Role of Key Gut Microbial Metabolites in the Development and Treatment of Cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]
- Eladwy, R.A.; Vu, H.T.; Shah, R.; Li, C.G.; Chang, D.; Bhuyan, D.J. The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond. Int. J. Mol. Sci. 2023, 24, 1716. [Google Scholar] [CrossRef]
- Chambers, L.M.; Rhoades, E.L.E.; Bharti, R.; Braley, C.; Tewari, S.; Trestan, L.; Alali, Z.; Bayik, D.; Lathia, J.D.; Sangwan, N.; et al. Disruption of the Gut Microbiota Confers Cisplatin Resistance in Epithelial Ovarian Cancer. Cancer Res. 2022, 82, 4654–4669. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a Promising Therapeutic Target in Cancer: From Pathogenesis to Clinic. Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef]
- Son, M.-Y.; Cho, H.-S. Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers. J. Microbiol. Biotechnol. 2023, 33, 849–856. [Google Scholar] [CrossRef]
- Filippone, A.; Casili, G.; Scuderi, S.A.; Mannino, D.; Lanza, M.; Campolo, M.; Paterniti, I.; Capra, A.P.; Colarossi, C.; Bonasera, A.; et al. Sodium Propionate Contributes to Tumor Cell Growth Inhibition through Ppar-γ Signaling. Cancers 2022, 15, 217. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Akbari, S.K.A.; Yousefimashouf, R.; Karampoor, S. Role of Microbiota-Derived Short-Chain Fatty Acids in Cancer Development and Prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef]
- Twycross, R. The Risks and Benefits of Corticosteroids in Advanced Cancer. Drug Saf. 1994, 11, 163–178. [Google Scholar] [CrossRef]
- Eladwy, R.A.; Alsherbiny, M.A.; Chang, D.; Fares, M.; Li, C.-G.; Bhuyan, D.J. The Postbiotic Sodium Butyrate Synergizes the Antiproliferative Effects of Dexamethasone Against the AGS Gastric Adenocarcinoma Cells. Front. Nutr. 2024, 11, 1372982. [Google Scholar] [CrossRef]
- Dissanayake, I.H.; Alsherbiny, M.A.; Chang, D.; Li, C.G.; Bhuyan, D.J. Antiproliferative Effects of Australian Native Plums against the Mcf7 Breast Adenocarcinoma Cells and Uplc-Qtof-Im-Ms-Driven Identification of Key Metabolites. Food Biosci. 2023, 54, 102864. [Google Scholar] [CrossRef]
- Alsherbiny, M.A.; Bhuyan, D.J.; Low, M.N.; Chang, D.; Li, C.G. Synergistic Interactions of Cannabidiol with Chemotherapeutic Drugs in Mcf7 Cells: Mode of Interaction and Proteomics Analysis of Mechanisms. Int. J. Mol. Sci. 2021, 22, 10103. [Google Scholar] [CrossRef] [PubMed]
- Jaye, K.; Alsherbiny, M.A.; Chang, D.; Li, C.-G.; Bhuyan, D.J. Mechanistic Insights into the Anti-Proliferative Action of Gut Microbial Metabolites against Breast Adenocarcinoma Cells. Int. J. Mol. Sci. 2023, 24, 15053. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’COnnell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, P.; Liu, Y.; Qi, M.; Dong, W. Combining Sodium Butyrate with Cisplatin Increases the Apoptosis of Gastric Cancer in Vivo and in Vitro Via the Mitochondrial Apoptosis Pathway. Front. Pharmacol. 2021, 12, 708093. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Liang, H.; Wang, W.; Li, B.; Liu, T.; Huang, Y.; Zhang, Z.; Qin, Y.; Zhou, X.; et al. The Roles and Applications of Short-Chain Fatty Acids Derived from Microbial Fermentation of Dietary Fibers in Human Cancer. Front. Nutr. 2023, 10, 1243390. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Sudaarsan, A.S.K.; Ghosh, A.R. Appraisal of Postbiotics in Cancer Therapy. Front. Pharmacol. 2024, 15, 1436021. [Google Scholar] [CrossRef]
- Martín-García, D.; García-Aranda, M.; Redondo, M. Therapeutic Potential of Clusterin Inhibition in Human Cancer. Cells 2024, 13, 665. [Google Scholar] [CrossRef]
- Vange, P.; Bruland, T.; Munkvold, B.; Røyset, E.S.; Gleave, M.; Bakke, I. Subtle Protective Roles of Clusterin in Gastric Metaplasia after Acute Oxyntic Atrophy. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 246–250.e1. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Guan, H.; Xiao, Q.; Wu, Z.; Shi, J.; Zhang, F.; Gao, P.; Song, Y.; Wang, Z. HIP1R Acts as a Tumor Suppressor in Gastric Cancer by Promoting Cancer Cell Apoptosis and Inhibiting Migration and Invasion through Modulating Akt. J. Clin. Lab. Anal. 2020, 34, e23425. [Google Scholar] [CrossRef] [PubMed]
- Schroder, W.A.; Major, L.D.; Le, T.T.; Gardner, J.; Sweet, M.J.; Janciauskiene, S.; Suhrbier, A. Tumor Cell-Expressed Serpinb2 Is Present on Microparticles and Inhibits Metastasis. Cancer Med. 2014, 3, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Hong, S. Multi-Faceted Roles of DNAjb Protein in Cancer Metastasis and Clinical Implications. Int. J. Mol. Sci. 2022, 23, 14970. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Schmitz, J.C.; Lin, X.; Tai, N.; Yan, W.; Farrell, M.; Bailly, M.; Chen, T.-M.; Chu, E. Thymidylate Synthase as a Translational Regulator of Cellular Gene Expression. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2002, 1587, 174–182. [Google Scholar] [CrossRef]
- Voeller, D.; Rahman, L.; Zajac-Kaye, M. Elevated Levels of Thymidylate Synthase Linked to Neoplastic Transformation of Mammalian Cells. Cell Cycle 2004, 3, 1003–1005. [Google Scholar] [CrossRef]
- Guijarro, M.V.; Nawab, A.; Dib, P.; Burkett, S.; Luo, X.; Feely, M.; Nasri, E.; Seifert, R.P.; Kaye, F.J.; Zajac-Kaye, M. Tyms Promotes Genomic Instability and Tumor Progression in Ink4a/Arf Null Background. Oncogene 2023, 42, 1926–1939. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Sun, S.; Chen, Z.; Xiang, S.; Ding, Z.; Huang, Z.; Zhang, B. Understanding the Versatile Roles and Applications of Epcam in Cancers: From Bench to Bedside. Exp. Hematol. Oncol. 2022, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Wu, M.; Smiljanic, S.; Kaskun, O.; Ghannad-Zadeh, K.; Celebre, A.; Isaev, K.; Morrissy, A.S.; Guan, J.; Tong, J.; et al. Id1 Is Critical for Tumorigenesis and Regulates Chemoresistance in Glioblastoma. Cancer Res. 2019, 79, 4057–4071. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, C.; Zhao, J.; Ren, G.; Zhang, F.; Liu, X.; Cao, S.; Xu, Y.; Xia, Z. High Expression of Pla2g2a in Fibroblasts Plays a Crucial Role in the Early Progression of Carotid Atherosclerosis. J. Transl. Med. 2024, 22, 967. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, I.; Oshima, T. Claudins and Gastric Cancer: An Overview. Cancers 2022, 14, 290. [Google Scholar] [CrossRef]
- Nie, M.; Wang, Y.; Yu, Z.; Li, X.; Deng, Y.; Wang, Y.; Yang, D.; Li, Q.; Zeng, X.; Ju, J.; et al. AURKB Promotes Gastric Cancer Progression Via Activation of CCND1 Expression. Aging 2020, 12, 1304–1321. [Google Scholar] [CrossRef]
- Broude, E.V.; Demidenko, Z.N.; Vivo, C.; Swift, M.E.; Davis, B.M.; Blagosklonny, M.V.; Roninson, I.B. P21 (Cdkn1a) Is a Negative Regulator of P53 Stability. Cell Cycle 2007, 6, 1467–1470. [Google Scholar] [CrossRef]
- Bao, C.; Guo, L. Tp73-As1 Promotes Gastric Cancer Proliferation and Invasion by Regulation Mir-27b-3p/Tmed5 Axis. J. Cancer 2022, 13, 1324–1335. [Google Scholar] [CrossRef]
- Koh, S.A.; Lee, K.H. Function of Hepatocyte Growth Factor in Gastric Cancer Proliferation and Invasion. Yeungnam Univ. J. Med. 2020, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, L.; Li, Q.; Li, W.; Björkholm, M.; Jia, J.; Xu, D. Foxm1 Is up-Regulated in Gastric Cancer and Its Inhibition Leads to Cellular Senescence, Partially Dependent on P27kip1. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2009, 218, 419–427. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Qi, F.-Y.; Zhang, J.-G.; Pang, J.-R.; Ren, H.-M.; Shen, D.-D.; Zhao, L.-J.; Qi, L.; Liu, H.-M.; Zheng, Y.-C. Identification of the Upstream Regulators of Kdm5b in Gastric Cancer. Life Sci. 2022, 298, 120458. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, N.; Wicha, M.S.; Luo, M. The Roles of the Let-7 Family of Micrornas in the Regulation of Cancer Stemness. Cells 2021, 10, 2415. [Google Scholar] [CrossRef]
- Morikawa, T.; Hino, R.; Uozaki, H.; Maeda, D.; Ushiku, T.; Shinozaki, A.; Sakatani, T.; Fukayama, M. Expression of Ribonucleotide Reductase M2 Subunit in Gastric Cancer and Effects of RRM2 Inhibition in Vitro. Hum. Pathol. 2010, 41, 1742–1748. [Google Scholar] [CrossRef]
- Mjelle, R.; Hegre, S.A.; Aas, P.A.; Slupphaug, G.; Drabløs, F.; Sætrom, P.; Krokan, H.E. Cell Cycle Regulation of Human DNA Repair and Chromatin Remodeling Genes. DNA Repair 2015, 30, 53–67. [Google Scholar] [CrossRef]
- Topchu, I.; Pangeni, R.P.; Bychkov, I.; Miller, S.A.; Izumchenko, E.; Yu, J.; Golemis, E.; Karanicolas, J.; Boumber, Y. The Role of Nsd1, Nsd2, and Nsd3 Histone Methyltransferases in Solid Tumors. Cell. Mol. Life Sci. 2022, 79, 285. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, I.; Funata, S.; Nagahama, K.; Isogaya, K.; Takeuchi, H.; Abe, N.; Shibahara, J. DNMT3A Overexpression Is Associated with Aggressive Behavior and Enteroblastic Differentiation of Gastric Adenocarcinoma. Ann. Diagn. Pathol. 2020, 44, 151456. [Google Scholar] [CrossRef] [PubMed]
- Meehan, W.J.; Samant, R.S.; Hopper, J.E.; Carrozza, M.J.; Shevde, L.A.; Workman, J.L.; Eckert, K.A.; Verderame, M.F.; Welch, D.R. Breast Cancer Metastasis Suppressor 1 (Brms1) Forms Complexes with Retinoblastoma-Binding Protein 1 (Rbp1) and the Msin3 Histone Deacetylase Complex and Represses Transcription. J. Biol. Chem. 2004, 279, 1562–1569. [Google Scholar] [CrossRef]
- Guo, X.-L.; Cui, P.-L.; Wang, Y.-B.; Liang, P.-X.; Zhang, Y.-N.; Xu, Y.-Q. Effect of BRMS1 Expression on Proliferation, Migration and Adhesion of Mouse Forestomach Carcinoma. Asian Pac. J. Trop. Med. 2015, 8, 724–730. [Google Scholar] [CrossRef]
- Bhat, V.; Koneru, M.; Knapp, K.; Joneja, U.; Morrison, J.; Hong, Y.K. Identification and Treatment of Smarca4 Deficient Poorly Differentiated Gastric Carcinoma. Am. Surg. 2023, 89, 4987–4989. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Oda, Y. Oncogenic Roles of Smarcb 1/Ini 1 and Its Deficient Tumors. Cancer Sci. 2017, 108, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, D.; Yang, S.; Liang, J.; Wang, X.; Rao, Q. Clinicopathological Significance, Related Molecular Changes and Tumor Immune Response Analysis of the Abnormal Swi/Snf Complex Subunit Pbrm1 in Gastric Adenocarcinoma. Pathol. Oncol. Res. 2022, 28, 1610479. [Google Scholar] [CrossRef]
- Foskolou, I.P.; Jorgensen, C.; Leszczynska, K.B.; Olcina, M.M.; Tarhonskaya, H.; Haisma, B.; D’aNgiolella, V.; Myers, W.K.; Domene, C.; Flashman, E.; et al. Ribonucleotide Reductase Requires Subunit Switching in Hypoxia to Maintain DNA Replication. Mol. Cell 2017, 66, 206–220.e9. [Google Scholar] [CrossRef] [PubMed]
- O’rEilly, L.A.; Putoczki, T.L.; Mielke, L.A.; Low, J.T.; Lin, A.; Preaudet, A.; Herold, M.J.; Yaprianto, K.; Tai, L.; Kueh, A.; et al. Loss of Nf-κB1 Causes Gastric Cancer with Aberrant Inflammation and Expression of Immune Checkpoint Regulators in a Stat-1-Dependent Manner. Immunity 2018, 48, 570–583.e8. [Google Scholar] [CrossRef]
- Riquelme, I.; Tapia, O.; Espinoza, J.A.; Leal, P.; Buchegger, K.; Sandoval, A.; Bizama, C.; Araya-Orostica, J.C.; Peek, R.M.; Roa, J.C. The Gene Expression Status of the Pi3k/Akt/Mtor Pathway in Gastric Cancer Tissues and Cell Lines. Pathol. Oncol. Res. 2016, 22, 797–805. [Google Scholar] [CrossRef]
- Weng, X.; Zhang, H.; Ye, J.; Kan, M.; Liu, F.; Wang, T.; Deng, J.; Tan, Y.; He, L.; Liu, Y. Hypermethylated Epidermal Growth Factor Receptor (Egfr) Promoter Is Associated with Gastric Cancer. Sci. Rep. 2015, 5, 10154. [Google Scholar] [CrossRef]
- Zhao, L.-P.; Xue, C.; Zhang, J.-W.; Hu, Z.-H.; Zhao, Y.-Y.; Zhang, J.; Huang, Y.; Zhao, H.-Y.; Zhang, L. Expression of Rrm1 and Its Association with Resistancy to Gemcitabine-Based Chemotherapy in Advanced Nasopharyngeal Carcinoma. Chin. J. Cancer 2012, 31, 476–483. [Google Scholar] [CrossRef]
- Jiang, K.; Deng, M.; Du, W.; Liu, T.; Li, J.; Zhou, Y. Functions and Inhibitors of Chk1 in Cancer Therapy. Med. Drug Discov. 2024, 22, 100185. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, T.; Song, L.; Fu, H.; Luo, H.; Wu, J.; Zhao, S.; Zhang, T.; Guo, L.; Jin, L.; et al. Pras40 Hyperexpression Promotes Hepatocarcinogenesis. EBioMedicine 2020, 51, 102604. [Google Scholar] [CrossRef]
- Ooi, A.; Oyama, T.; Nakamura, R.; Tajiri, R.; Ikeda, H.; Fushida, S.; Dobashi, Y. Gene Amplification of Ccne1, Ccnd1, and Cdk6 in Gastric Cancers Detected by Multiplex Ligation-Dependent Probe Amplification and Fluorescence in Situ Hybridization. Hum. Pathol. 2017, 61, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.-H.; Yang, J.W.; Jung, E.-J.; Ju, Y.-T.; Jeong, C.-Y.; Kim, J.-Y.; Park, T.; Kim, T.-H.; Park, M.; et al. Smarcd3 Overexpression Promotes Epithelial–Mesenchymal Transition in Gastric Cancer. Cancers 2024, 16, 2282. [Google Scholar] [CrossRef]
- Kudaravalli, S.; Hollander, P.D.; Mani, S.A. Role of P38 Map Kinase in Cancer Stem Cells and Metastasis. Oncogene 2022, 41, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, Y.; Zhuang, Z.; Wang, D.; Ye, Z.; Jing, J.; Cheng, X. Advancements and Obstacles of Parp Inhibitors in Gastric Cancer. Cancers 2023, 15, 5114. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yan, W.; Li, J.; Yan, M.; Liu, B.; Yang, Z.; Yu, B. PHF10 Inhibits Gastric Epithelium Differentiation and Induces Gastric Cancer Carcinogenesis. Cancer Gene Ther. 2024, 31, 1511–1524. [Google Scholar] [CrossRef]
- Grundy, E.E.; Diab, N.; Chiappinelli, K.B. Transposable Element Regulation and Expression in Cancer. FEBS J. 2022, 289, 1160–1179. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Cho, Y.; Kang, H.; Yim, Y.-S.; Kim, S.-J.; Song, J.; Chun, K.-H. Targeting the Wee1 Kinase as a Molecular Targeted Therapy for Gastric Cancer. Oncotarget 2016, 7, 49902–49916. [Google Scholar] [CrossRef]
- Hino, K.; Nishina, T.; Kajiwara, T.; Bando, H.; Nakamura, M.; Kadowaki, S.; Minashi, K.; Yuki, S.; Ohta, T.; Hara, H.; et al. Association of Erbb2 Copy Number and Gene Coalterations with Trastuzumab Efficacy and Resistance in Human Epidermal Growth Factor Receptor 2–Positive Esophagogastric and Gastric Cancer. JCO Precis. Oncol. 2022, 6, e2200135. [Google Scholar] [CrossRef]
- Xing, K.; Gu, B.; Zhang, P.; Wu, X. Dexamethasone Enhances Programmed Cell Death 1 (Pd-1) Expression During T Cell Activation: An Insight into the Optimum Application of Glucocorticoids in Anti-Cancer Therapy. BMC Immunol. 2015, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Kakumoto, K.; Ikeda, J.-I.; Okada, M.; Morii, E.; Oneyama, C.; Ouchi, T. Mlst8 Promotes Mtor-Mediated Tumor Progression. PLoS ONE 2015, 10, e0119015. [Google Scholar] [CrossRef]
- Gallo, A.; Ronzio, M.; Bezzecchi, E.; Mantovani, R.; Dolfini, D. Nf-Y Subunits Overexpression in Gastric Adenocarcinomas (Stad). Sci. Rep. 2021, 11, 674. [Google Scholar] [CrossRef]
- Liu, P.-F.; Chen, C.-F.; Shu, C.-W.; Chang, H.-M.; Lee, C.-H.; Liou, H.-H.; Ger, L.-P.; Chen, C.-L.; Kang, B.-H. UBE2C is a Potential Biomarker for Tumorigenesis and Prognosis in Tongue Squamous Cell Carcinoma. Diagnostics 2020, 10, 674. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, F.; Liu, M.; Zhao, Q. UBE2C mRNA Expression Controlled by Mir-300 and Hur Determines Its Oncogenic Role in Gastric Cancer. Biochem. Biophys. Res. Commun. 2021, 534, 597–603. [Google Scholar] [CrossRef]
- Chen, P.; He, Z.; Wang, J.; Xu, J.; Jiang, X.; Chen, Y.; Liu, X.; Jiang, J. Hypoxia-Induced Zwint Mediates Pancreatic Cancer Proliferation by Interacting with P53/P21. Front. Cell Dev. Biol. 2021, 9, 682131. [Google Scholar] [CrossRef]
- Marks, J.R. Refining the Role of Brca1 in Combating Oxidative Stress. Breast Cancer Res. 2013, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hunter, T. Roles of Chk1 in Cell Biology and Cancer Therapy. Int. J. Cancer 2014, 134, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Shen, Q.; Zhang, P.; Tao, R.; Chang, W.; Li, R.; Xie, G.; Liu, W.; Zhang, L.; Kapoor, P.; et al. Chk1 Inhibition Potentiates the Therapeutic Efficacy of Parp Inhibitor Bmn673 in Gastric Cancer. Am. J. Cancer Res. 2017, 7, 473–483. [Google Scholar] [PubMed]
- Matheson, C.J.; Backos, D.S.; Reigan, P. Targeting Wee1 Kinase in Cancer. Trends Pharmacol. Sci. 2016, 37, 872–881. [Google Scholar] [CrossRef]
- Iliaki, S.; Beyaert, R.; Afonina, I.S. Polo-like Kinase 1 (Plk1) Signaling in Cancer and Beyond. Biochem. Pharmacol. 2021, 193, 114747. [Google Scholar] [CrossRef]
- Kanaji, S.; Saito, H.; Tsujitani, S.; Matsumoto, S.; Tatebe, S.; Kondo, A.; Ozaki, M.; Ito, H.; Ikeguchi, M. Expression of Polo-Like Kinase 1 (Plk1) Protein Predicts the Survival of Patients with Gastric Carcinoma. Oncology 2006, 70, 126–133. [Google Scholar] [CrossRef]
- Li, Q.; Tong, D.; Jing, X.; Ma, P.; Li, F.; Jiang, Q.; Zhang, J.; Wen, H.; Cui, M.; Huang, C.; et al. Mad2l1 Is Transcriptionally Regulated by Tead4 and Promotes Cell Proliferation and Migration in Colorectal Cancer. Cancer Gene Ther. 2023, 30, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.-B.; Li, X.-Z.; Zeng, S.; Liu, C.; Yang, S.-M.; Yang, L.; Hu, C.-J.; Bai, J.-Y. Regulation of the Master Regulator Foxm1 in Cancer. Cell Commun. Signal. 2018, 16, 57. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, X.; Shuang, F.; Chen, G. Dexamethasone Potentiates the Insulin-Induced Srebp-1c Expression in Primary Rat Hepatocytes. Food Sci. Hum. Wellness 2023, 12, 1519–1525. [Google Scholar] [CrossRef]
- Nakamura, T.; Iwase, A.; Bayasula, B.; Nagatomo, Y.; Kondo, M.; Nakahara, T.; Takikawa, S.; Goto, M.; Kotani, T.; Kiyono, T.; et al. Cyp51a1 Induced by Growth Differentiation Factor 9 and Follicle-Stimulating Hormone in Granulosa Cells Is a Possible Predictor for Unfertilization. Reprod. Sci. 2015, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Ershov, P.; Kaluzhskiy, L.; Mezentsev, Y.; Yablokov, E.; Gnedenko, O.; Ivanov, A. Enzymes in the Cholesterol Synthesis Pathway: Interactomics in the Cancer Context. Biomedicines 2021, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, M.; Zhang, K.; Zhang, J.; Gao, T.; Connell, D.O.; Yao, F.; Mu, C.; Cai, B.; Shang, Y.; et al. Nedd4 Ubiquitylates Vdac2/3 to Suppress Erastin-Induced Ferroptosis in Melanoma. Nat. Commun. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xing, N.; Xiahou, Z.; Yan, J.; Lin, Z.; Zhang, J. Unraveling the Intricacies of Glioblastoma Progression and Recurrence: Insights into the Role of Nfyb and Oxidative Phosphorylation at the Single-Cell Level. Front. Immunol. 2024, 15, 1368685. [Google Scholar] [CrossRef]
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the ‘Hallmarks of Cancer’. FEBS J. 2015, 282, 224–258. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qi, S.; Chen, L.; Zhu, J.; Liang, L.; Chen, X.; Zhang, H.; Zhuo, L.; Zhao, S.; Liu, S.; et al. The Roles and Mechanisms of Srebp1 in Cancer Development and Drug Response. Genes Dis. 2024, 11, 100987. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, L.; Kurosaki, M.; Garattini, S.-K.; Gianni’, M.; Fasola, G.; Rossit, L.; Prisciandaro, M.; Di Bartolomeo, M.; Bolis, M.; Rizzo, P.; et al. Anti-Tumor Activity of All-Trans Retinoic Acid in Gastric-Cancer: Gene-Networks and Molecular Mechanisms. J. Exp. Clin. Cancer Res. 2023, 42, 298. [Google Scholar] [CrossRef] [PubMed]
- Lauder, I.; Zaitoun, A.M.; Aherne, W.A. The Effects of Dexamethasone on the Cell Kinetics of a Murine Malignant Lymphoma. J. Pathol. 1979, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Maiyar, A.C.; Ge, Y.; Firestone, G.L. Glucocorticoids Induce a G1/G0 Cell Cycle Arrest of Con8 Rat Mammary Tumor Cells That Is Synchronously Reversed by Steroid Withdrawal or Addition of Transforming Growth Factor-Alpha. Mol. Endocrinol. 1993, 7, 1121–1132. [Google Scholar] [CrossRef][Green Version]
- He, W.; Meng, J. Cdc20: A Novel Therapeutic Target in Cancer. Am. J. Transl. Res. 2023, 15, 678. [Google Scholar][Green Version]
- Jalali, P.; Samii, A.; Rezaee, M.; Shahmoradi, A.; Pashizeh, F.; Salehi, Z. Ube2c: A Pan-Cancer Diagnostic and Prognostic Biomarker Revealed through Bioinformatics Analysis. Cancer Rep. 2024, 7, e2032. [Google Scholar] [CrossRef]
- Mylka, V.; Deckers, J.; Ratman, D.; De Cauwer, L.; Thommis, J.; De Rycke, R.; Impens, F.; Libert, C.; Tavernier, J.; Berghe, W.V.; et al. The Autophagy Receptor Sqstm1/P62 Mediates Anti-Inflammatory Actions of the Selective Nr3c1/Glucocorticoid Receptor Modulator Compound a (Cpda) in Macrophages. Autophagy 2018, 14, 2049–2064. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Y.; Ji, Z.; Liu, D.; Chen, X.; Chen, M.; Chen, K.; Lin, H.; Chen, Y.; Li, Z. Identification of C4bpa as Biomarker Associated with Immune Infiltration and Prognosis in Breast Cancer. Transl. Cancer Res. 2024, 13, 25–45. [Google Scholar] [CrossRef]
- Quadros, E.V.; Nakayama, Y.; Sequeira, J.M. Saporin Conjugated Monoclonal Antibody to the Transcobalamin Receptor Tcblr/Cd320 Is Effective in Targeting and Destroying Cancer Cells. J. Cancer Ther. 2013, 4, 1074. [Google Scholar] [CrossRef]
- Yan, H.-F.; Zou, T.; Tuo, Q.-Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and Links with Diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2020, 31, 107–125. [Google Scholar] [CrossRef]
- Li, H.; Jiang, S.; Yang, C.; Yang, S.; He, B.; Ma, W.; Zhao, R. Long-Term Dexamethasone Exposure Down-Regulates Hepatic Tfr1 and Reduces Liver Iron Concentration in Rats. Nutrients 2017, 9, 617. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Tang, S.-Q. Insulin-Induced Gene: A New Regulator in Lipid Metabolism. Peptides 2010, 31, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, S.; de Baaij, J.H.; Ferreira, P.; Germann, R.; de Klerk, J.B.; Lavrijsen, M.; van Zeeland, F.; Venselaar, H.; Kluijtmans, L.A.; Hoenderop, J.G.; et al. Mutations in Pcbd1 Cause Hypomagnesemia and Renal Magnesium Wasting. J. Am. Soc. Nephrol. 2014, 25, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, H.-Y.; Wang, Y.-P.; Bai, S.-Y.; Pu, K.; Zheng, Y.; Guo, Q.-H.; Guan, Q.-L.; Ji, R.; Zhou, Y.-N. COL4A Family: Potential Prognostic Biomarkers and Therapeutic Targets for Gastric Cancer. Transl. Cancer Res. 2020, 9, 5218–5232. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, W.; Li, X.; Zhang, L.; Gao, Y.; Peng, Q.; Du, C.; Jiang, N. AGTRAP Is a Prognostic Biomarker Correlated with Immune Infiltration in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 713017. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Liu, J.; Ma, X.; Zhao, J.; Su, Z.; Ahmad, S. Knockdown of Gastrin Promotes Apoptosis of Gastric Cancer Cells by Decreasing Ros Generation. BioMed Res. Int. 2021, 2021, 5590037. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Guo, C.; Li, X.; Zhang, B.; Huang, L. TAF15 Promotes Cell Proliferation, Migration and Invasion of Gastric Cancer Via Activation of the Raf1/Mek/Erk Signalling Pathway. Sci. Rep. 2023, 13, 5846. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Li, G.; Jin, Q.; Zhang, S. Human Gastric Cancer Decellularized Scaffold Promotes Epithelial-Mesenchymal Transition of Gastric Cancer Cells in Vitro with the Involvement of Elf1. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4945444 (accessed on 23 July 2025).
- Liu, X.; Xu, C.; Xiao, W.; Yan, N. Unravelling the Role of Nfe2l1 in Stress Responses and Related Diseases. Redox Biol. 2023, 65, 102819. [Google Scholar] [CrossRef]
- Wang, B.; Guo, H.; Yu, H.; Chen, Y.; Xu, H.; Zhao, G. The Role of the Transcription Factor Egr1 in Cancer. Front. Oncol. 2021, 11, 642547. [Google Scholar] [CrossRef]
- Graziano, F.; Fischer, N.W.; Bagaloni, I.; Di Bartolomeo, M.; Lonardi, S.; Vincenzi, B.; Perrone, G.; Fornaro, L.; Ongaro, E.; Aprile, G.; et al. Tp53 Mutation Analysis in Gastric Cancer and Clinical Outcomes of Patients with Metastatic Disease Treated with Ramucirumab/Paclitaxel or Standard Chemotherapy. Cancers 2020, 12, 2049. [Google Scholar] [CrossRef]
- Kim, M.S.; Yoo, N.J.; Lee, S.H. Expressional and Mutational Analysis of Crebbp Gene in Gastric and Colorectal Cancers with Microsatellite Instability. Pathol. Oncol. Res. 2013, 20, 221–222. [Google Scholar] [CrossRef]
- Chaithongyot, S.; Jantaree, P.; Sokolova, O.; Naumann, M. Nf-κB in Gastric Cancer Development and Therapy. Biomedicines 2021, 9, 870. [Google Scholar] [CrossRef]
- Du, Q.; Zhu, C.; Shang, Q.; Mao, H.; Li, X.; Huang, Y.; Du, N. A Study on the Correlation of Myd88 Expression with Gastric Cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 4836. [Google Scholar]
- Talukdar, P.D.; Chatterji, U. Transcriptional Co-Activators: Emerging Roles in Signaling Pathways and Potential Therapeutic Targets for Diseases. Signal Transduct. Target. Ther. 2023, 8, 427. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. Nf-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. Ifnγ: Signalling, Epigenetics and Roles in Immunity, Metabolism, Disease and Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Kim, J.; Kim, M.A.; Choi, J.; Kim, W.H. Increased Hgf Expression Induces Resistance to C-Met Tyrosine Kinase Inhibitors in Gastric Cancer. Anticancer Res. 2017, 37, 1127–1138. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Guan, Y.; Zhang, Q. Long Noncoding Rna Oser1-As1 Promotes the Malignant Properties of Non-Small Cell Lung Cancer by Sponging Microrna-433-3p and Thereby Increasing Smad2 Expression. Oncol. Rep. 2020, 44, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Chen, Z.; Wang, L.; Zhang, Z.-K.; Tan, X.; Liu, S.; Zhang, B.-T.; Lu, A.; Yu, Y.; Zhang, G. Dickkopf-1: A Promising Target for Cancer Immunotherapy. Front. Immunol. 2021, 12, 658097. [Google Scholar] [CrossRef]
- Ebrahimnezhad, M.; Natami, M.; Bakhtiari, G.H.; Tabnak, P.; Ebrahimnezhad, N.; Yousefi, B.; Majidinia, M. Foxo1, a Tiny Protein with Intricate Interactions: Promising Therapeutic Candidate in Lung Cancer. Biomed. Pharmacother. 2023, 169, 115900. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Lee, Y.-E.; Tian, Y.-F.; Sun, D.-P.; Sheu, M.-J.; Li, C.-F.; Lee, S.-W.; Lin, L.-C.; Chang, I.-W.; Wang, C.-T.; et al. High Expression of Epha4 Predicted Lesser Degree of Tumor Regression after Neoadjuvant Chemoradiotherapy in Rectal Cancer. J. Cancer 2017, 8, 1089–1096. [Google Scholar] [CrossRef]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of Abl Family Kinases in Cancer: From Leukaemia to Solid Tumours. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar] [CrossRef]
- Kim, E.S.; Salgia, R. Met Pathway as a Therapeutic Target. J. Thorac. Oncol. 2009, 4, 444–447. [Google Scholar] [CrossRef]
- Pelaz, S.G.; Tabernero, A. Src: Coordinating Metabolism in Cancer. Oncogene 2022, 41, 4917–4928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z. Stat1 in Cancer: Friend or Foe? Discov. Med. 2017, 24, 19–29. [Google Scholar] [PubMed]
- Jiménez-Martínez, M.; Stamatakis, K.; Fresno, M. The Dual-Specificity Phosphatase 10 (Dusp10): Its Role in Cancer, Inflammation, and Immunity. Int. J. Mol. Sci. 2019, 20, 1626. [Google Scholar] [CrossRef]
- Zhang, K.; Ni, X.; Ma, X.; Sun, R.; Qiu, J.; Luo, C. Linc01012 Upregulation Promotes Cervical Cancer Proliferation and Migration Via Downregulation of Cdkn2d. Oncol. Lett. 2023, 25, 124. [Google Scholar] [CrossRef] [PubMed]
- Silva-Rodríguez, P.; Fernández-Díaz, D.; Bande, M.; Pardo, M.; Loidi, L.; Blanco-Teijeiro, M.J. Gnaq and Gna11 Genes: A Comprehensive Review on Oncogenesis, Prognosis and Therapeutic Opportunities in Uveal Melanoma. Cancers 2022, 14, 3066. [Google Scholar] [CrossRef]
- Vera-Puente, O.; Rodriguez-Antolin, C.; Salgado-Figueroa, A.; Michalska, P.; Pernia, O.; Reid, B.M.; Rosas, R.; Garcia-Guede, A.; Sacristán, S.; Jimenez, J.; et al. Mafg Is a Potential Therapeutic Target to Restore Chemosensitivity in Cisplatin-Resistant Cancer Cells by Increasing Reactive Oxygen Species. Transl. Res. 2018, 200, 1–17. [Google Scholar] [CrossRef]
- Marni, R.; Chakraborty, A.; Malla, R. Oncogenic Tetraspanins: Implications for Metastasis, Drug Resistance, Cancer Stem Cell Maintenance and Diagnosis of Leading Cancers in Females. Gene Rep. 2022, 27, 101548. [Google Scholar] [CrossRef]
- Mast, A.E.; Wolberg, A.S.; Gailani, D.; Garvin, M.R.; Alvarez, C.; Miller, J.I.; Aronow, B.; Jacobson, D. SARS-CoV-2 Suppresses Anticoagulant and Fibrinolytic Gene Expression in the Lung. eLife 2021, 10, e64330. [Google Scholar] [CrossRef]
- Shan, Q.; Zhang, C.; Li, Y.; Li, Q.; Zhang, Y.; Li, X.; Shi, J.; Hu, F. Slc7a11, a Potential Immunotherapeutic Target in Lung Adenocarcinoma. Sci. Rep. 2023, 13, 18302. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, J.; Cui, M.; Jing, R. Slc7a11 Promotes the Progression of Gastric Cancer and Regulates Ferroptosis through Pi3k/Akt Pathway. Pathol.-Res. Pract. 2023, 248, 154646. [Google Scholar] [CrossRef]
- Sun, F.; Sun, P.; Yang, X.; Hu, L.; Gao, J.; Tian, T. Inhibition of Fstl3 Abates the Proliferation and Metastasis of Renal Cell Carcinoma Via the Gsk-3β/β-Catenin Signaling Pathway. Aging 2021, 13, 22528–22543. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Li, J.-P.; Zhang, Y.; Nie, M.-J.; Zhang, Y.-H.; Liu, S.-L.; Zou, X. Fstl3 Is a Prognostic Biomarker in Gastric Cancer and Is Correlated with M2 Macrophage Infiltration. OncoTargets Ther. 2021, 14, 4099–4117. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Nishikawa, M.; Kobayashi, T.; Harlin, E.W.; Ito, T.; Sato, K.; Sugiyama, T.; Yamakawa, H.; Nagase, T.; Ueda, H. The Rho Guanine Nucleotide Exchange Factor Plekhg1 Is Activated by Interaction with and Phosphorylation by Src Family Kinase Member FYN. J. Biol. Chem. 2022, 298, 101579. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Qu, Y.-W.; Wang, L.; Jia, F.-H.; Chen, P.; Wang, Y.-P.; Liu, H.-F. Identification of Potential Hub Genes of Gastric Cancer. Medicine 2022, 101, e30741. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.P.; Susanti, S.; Ilyas, M. Investigating Tns4 in the Colorectal Tumor Microenvironment Using 3D Spheroid Models of Invasion. Adv. Biosyst. 2020, 4, e2000031. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, A.; Kaminishi, M.; Nakanishi, Y.; Sugimura, T.; Ushijima, T. Reduced Expression of the Insulin-Induced Protein 1 and P41 Arp2/3 Complex Genes in Human Gastric Cancers. Int. J. Cancer 2002, 100, 57–62. [Google Scholar] [CrossRef]
- Jakobsen, S.; Nielsen, C.U. Exploring Amino Acid Transporters as Therapeutic Targets for Cancer: An Examination of Inhibitor Structures, Selectivity Issues, and Discovery Approaches. Pharmaceutics 2024, 16, 197. [Google Scholar] [CrossRef] [PubMed]
| Conc. μg/mL 1:1 | Cell Growth Inhibition (%) of AGS Cells | Cell Viability (%) of HS738.St/Int | ||||
|---|---|---|---|---|---|---|
| PB | AP | AB | PB | AP | AB | |
| 1500 + 1500 | 99.22 ± 1.42 aw | 59.79 ± 4.32 ax | 99.49 ± 0.85 ay | 58.86 ± 9.52 ax | 82.39 ± 12.30 ax | 61.08 ± 13.45 az |
| 750 + 750 | 91.14 ± 1.78 aw | 37.67 ± 9.68 bx | 90.08 ± 1.86 ax | 78.04 ± 4.98 aw | 93.48 ± 12.89 ax | 84.38 ± 13.01 ax |
| 375 + 375 | 75.93 ± 1.92 bw | 29.31 ± 6.50 bx | 76.62 ± 2.81 by | 81.09 ± 10.51 aw | 107.71 ± 33.81 ay | 102.51 ± 17.25 ay |
| 187.5 + 187.5 | 51.36 ± 7.50 cw | 23.87 ± 6.76 bw | 49.95 ± 7.31 cx | 100.62 ± 16.88 aw | 143.38 ± 26.78 ax | 103.77 ± 10.35 ax |
| 93.75 + 93.75 | 25.11 ± 8.21 dw | 19.20 ± 7.46 bx | 24.28 ± 7.26 dy | 127.45 ± 26.75 ax | 149.37 ± 9.05 ay | 122.20 ± 16.51 az |
| 46.875 + 46.875 | 9.79 ± 7.25 ew | 11.98 ± 12.23 bx | 12.01 ± 8.06 ey | 138.92 ± 9.91 ax | 175.62 ± 4.32 ay | 149.29 ± 21.90 az |
| IC50 | 421.23 ± 15.31 | 1141.13 ± 362.00 | 446.53 ± 19.55 | NA | NA | NA |
| Conc. μg/mL | Cell Growth Inhibition (%) of AGS Cells | Conc. μg/mL | Cell Growth Inhibition (%) of AGS Cells | Conc. μg/mL APB + Dex | Cell Growth Inhibition (%) of AGS Cells | Cell Viability (%) of HS738.St/Int |
|---|---|---|---|---|---|---|
| APB | Dex | APB + Dex | ||||
| 3000 | 95.65 ± 7.90 ax | 200 | 80.46 ± 8.08 ax | 3000 + 100 | 103.17 ± 3.06 ax | 68.57 ± 11.74 ax |
| 1500 | 86.25 ± 8.42 ax | 100 | 39.38 ± 24.51 bx | 1500 + 50 | 87.81 ± 5.57 bx | 85.91 ± 10.59 ax |
| 750 | 65.54 ± 4.91 bx | 50 | - | 750 + 25 | 62.42 ± 5.34 cx | 110.88 ± 14.35 ay |
| 375 | 40.28 ± 8.05 cx | 25 | - | 375+ 12.5 | 43.49 ± 10.93 dx | 115.29 ± 16.81 ay |
| 187.5 | 22.20 ± 8.55 dx | 12.5 | - | 187.5 + 6.25 | 28.70 ± 11.91 ex | 134.46 ± 24.72 ay |
| 93.75 | 19.20 ± 10.38 dx | 6.25 | - | 93.75 + 3.13 | 16.46 ± 9.71 fx | 159.21 ± 14.16 ay |
| IC50 | 568.33 ± 82.56 | IC50 | 86.60 ± 11.85 | IC50 | 643.30 ± 58.26 | NA |
| Combination Index (CI) Values at | ||||
|---|---|---|---|---|
| Combinations | IC50 | IC75 | IC90 | IC95 |
| AP 1:1 (1500 μg/mL A + 1500 μg/mL P) | 3.53 | 7.28 | 17.13 | 31.66 |
| AB 1:1 (1500 μg/mL A + 1500 μg/mL B) | 1.14 | 1.37 | 1.80 | 2.19 |
| BP 1:1 (1500 μg/mL B + 1500 μg/mL P) | 1.41 | 1.68 | 2.11 | 2.51 |
| APB + Dex (3000 μg/mL APB + 100 μg/mL Dex) | 0.76 | 0.48 | 0.31 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eladwy, R.A.; Fares, M.; Chang, D.; Alsherbiny, M.A.; Li, C.-G.; Bhuyan, D.J. Fuelling the Fight from the Gut: Short-Chain Fatty Acids and Dexamethasone Synergise to Suppress Gastric Cancer Cells. Cancers 2025, 17, 2486. https://doi.org/10.3390/cancers17152486
Eladwy RA, Fares M, Chang D, Alsherbiny MA, Li C-G, Bhuyan DJ. Fuelling the Fight from the Gut: Short-Chain Fatty Acids and Dexamethasone Synergise to Suppress Gastric Cancer Cells. Cancers. 2025; 17(15):2486. https://doi.org/10.3390/cancers17152486
Chicago/Turabian StyleEladwy, Radwa A., Mohamed Fares, Dennis Chang, Muhammad A. Alsherbiny, Chun-Guang Li, and Deep Jyoti Bhuyan. 2025. "Fuelling the Fight from the Gut: Short-Chain Fatty Acids and Dexamethasone Synergise to Suppress Gastric Cancer Cells" Cancers 17, no. 15: 2486. https://doi.org/10.3390/cancers17152486
APA StyleEladwy, R. A., Fares, M., Chang, D., Alsherbiny, M. A., Li, C.-G., & Bhuyan, D. J. (2025). Fuelling the Fight from the Gut: Short-Chain Fatty Acids and Dexamethasone Synergise to Suppress Gastric Cancer Cells. Cancers, 17(15), 2486. https://doi.org/10.3390/cancers17152486








