Liquid Biopsy Biomarkers in Metastatic Castration-Resistant Prostate Cancer Treated with Second-Generation Antiandrogens: Ready for Clinical Practice? A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Literature Search
2.2. Inclusion and Exclusion Criteria
- Full-text articles and conference abstracts.
- Observational studies and clinical trials of patients with mCRPC treated with AR axis-targeting agents.
- Studies that used liquid biopsy to obtain the following biomaterials: circulating tumor cells, cell-free nucleic acids, and extracellular vesicles.
- Articles not in English.
- Specific study types: Case reports and case series, clinical trial protocols, editorials, opinion articles, surveys, in vitro, in silico, and animal-model studies, reviews, and pooled analyses (when the individual studies were already retrieved).
- Other study populations: Studies that grouped both mCRPC and nonmetastatic or castrate-sensitive PCa, studies of specific subtypes of mCRPC (i.e., neuroendocrine, small-cell, aggressive-variant PCa).
- Other or unclear treatment (other treatments than AR axis-targeting agents—ChT, PSMA-targeted radionuclide therapy, or combinations of AR axis-targeting agents and other treatments).
- Inappropriate or unclear timing of liquid biopsy (more than one month before or after the beginning of treatment).
- Articles not reporting time-to-event data: PFS/time to progression (clinical, radiographic, PSA, or unspecified) or OS.
- Preliminary results.
2.3. Handling of Abstracts
2.4. Quality Assessment
3. Results
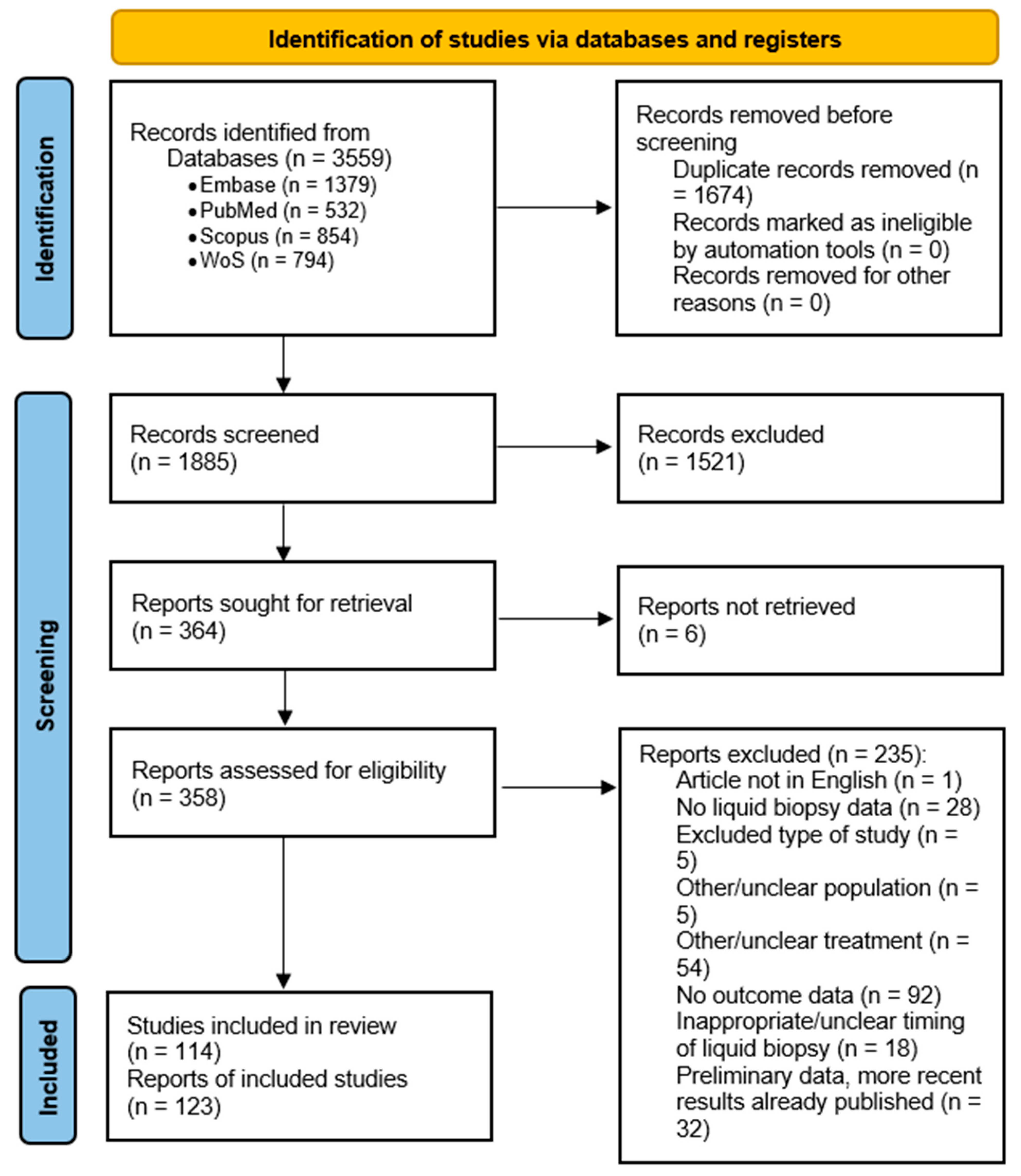
3.1. Search Results and Study Characteristics
3.2. Nonspecific Liquid Biopsy Markers
3.3. AR-Based Markers
3.4. Epigenetic Markers
3.5. DNA Damage Response and Homologous Recombination Response Pathways
3.6. Cell Cycle Regulation-Related Markers
3.7. PI3K Pathway
3.8. WNT Pathway
3.9. PSA (KLK3), PSMA, and PSCA
3.10. Other Individual Biomarkers
3.11. Multiple Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and Biology of Prostate Cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef]
- Cai, M.; Song, X.-L.; Li, X.-A.; Chen, M.; Guo, J.; Yang, D.-H.; Chen, Z.; Zhao, S.-C. Current Therapy and Drug Resistance in Metastatic Castration-Resistant Prostate Cancer. Drug Resist. Updates 2023, 68, 100962. [Google Scholar] [CrossRef]
- Patel, V.; Liaw, B.; Oh, W. The Role of Ketoconazole in Current Prostate Cancer Care. Nat. Rev. Urol. 2018, 15, 643–651. [Google Scholar] [CrossRef]
- Thakur, A.; Roy, A.; Ghosh, A.; Chhabra, M.; Banerjee, S. Abiraterone Acetate in the Treatment of Prostate Cancer. Biomed. Pharmacother. 2018, 101, 211–218. [Google Scholar] [CrossRef]
- Sharifi, N. The 5α-Androstanedione Pathway to Dihydrotestosterone in Castration-Resistant Prostate Cancer. J. Investig. Med. 2012, 60, 504–507. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a Second-Generation Antiandrogen for Treatment of Advanced Prostate Cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, S.; Lou, W.; Yang, J.C.; Armstrong, C.M.; Lombard, A.P.; D’Abronzo, L.S.; Evans, C.P.; Gao, A.C.; Liu, C. Cross-Resistance Among Next-Generation Antiandrogen Drugs Through the AKR1C3/AR-V7 Axis in Advanced Prostate Cancer. Mol. Cancer Ther. 2020, 19, 1708–1718. [Google Scholar] [CrossRef]
- Sugawara, T.; Baumgart, S.J.; Nevedomskaya, E.; Reichert, K.; Steuber, H.; Lejeune, P.; Mumberg, D.; Haendler, B. Darolutamide Is a Potent Androgen Receptor Antagonist with Strong Efficacy in Prostate Cancer Models. Int. J. Cancer 2019, 145, 1382–1394. [Google Scholar] [CrossRef]
- Shafi, A.A.; Putluri, V.; Arnold, J.M.; Tsouko, E.; Maity, S.; Roberts, J.M.; Coarfa, C.; Frigo, D.E.; Putluri, N.; Sreekumar, A.; et al. Differential Regulation of Metabolic Pathways by Androgen Receptor (AR) and Its Constitutively Active Splice Variant, AR-V7, in Prostate Cancer Cells. Oncotarget 2015, 6, 31997–32012. [Google Scholar] [CrossRef]
- Uo, T.; Plymate, S.R.; Sprenger, C.C. The Potential of AR-V7 as a Therapeutic Target. Expert Opin. Ther. Targets 2018, 22, 201–216. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Kim, V.S.; Yang, H.; Timilshina, N.; Breunis, H.; Emmenegger, U.; Gregg, R.; Hansen, A.R.; Tomlinson, G.; Alibhai, S.M.H. The Role of Frailty in Modifying Physical Function and Quality of Life over Time in Older Men with Metastatic Castration-Resistant Prostate Cancer. J. Geriatr. Oncol. 2023, 14, 101417. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Crocetto, F.; Russo, G.; Di Zazzo, E.; Pisapia, P.; Mirto, B.F.; Palmieri, A.; Pepe, F.; Bellevicine, C.; Russo, A.; La Civita, E.; et al. Liquid Biopsy in Prostate Cancer Management—Current Challenges and Future Perspectives. Cancers 2022, 14, 3272. [Google Scholar] [CrossRef]
- Ionescu, F.; Zhang, J.; Wang, L. Clinical Applications of Liquid Biopsy in Prostate Cancer: From Screening to Predictive Biomarker. Cancers 2022, 14, 1728. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid Biopsy in Cancer: Current Status, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Prostate Cancer (Version 1.2025). Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 29 March 2025).
- Wang, K.; Wang, X.; Pan, Q.; Zhao, B. Liquid Biopsy Techniques and Pancreatic Cancer: Diagnosis, Monitoring, and Evaluation. Mol. Cancer 2023, 22, 167. [Google Scholar] [CrossRef]
- Croitoru, V.M.; Cazacu, I.M.; Popescu, I.; Paul, D.; Dima, S.O.; Croitoru, A.E.; Tanase, A.D. Clonal Hematopoiesis and Liquid Biopsy in Gastrointestinal Cancers. Front. Med. 2021, 8, 772166. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Scherer, R.W.; Meerpohl, J.J.; Pfeifer, N.; Schmucker, C.; Schwarzer, G.; von Elm, E. Full Publication of Results Initially Presented in Abstracts. Cochrane Database Syst. Rev. 2018, 11, 1. [Google Scholar] [CrossRef]
- The Pros and Cons of Including Abstracts in Systematic Reviews: Findings from the Multiple Data Sources Study (MUDS)|Cochrane Colloquium Abstracts. Available online: https://abstracts.cochrane.org/2016-seoul/pros-and-cons-including-abstracts-systematic-reviews-findings-multiple-data-sources (accessed on 1 February 2025).
- Hackenbroich, S.; Kranke, P.; Meybohm, P.; Weibel, S. Include or Not to Include Conference Abstracts in Systematic Reviews? Lessons Learned from a Large Cochrane Network Meta-Analysis Including 585 Trials. Syst. Rev. 2022, 11, 178. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting Recommendations for Tumour MARKer Prognostic Studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef]
- Hopewell, S.; Clarke, M.; Moher, D.; Wager, E.; Middleton, P.; Altman, D.G.; Schulz, K.F.; CONSORT Group. CONSORT for Reporting Randomised Trials in Journal and Conference Abstracts. Lancet 2008, 371, 281–283. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Wise, D.; Kelvin, J.; Graf, R.; Schreiber, N.A.; McLaughlin, B.; Fernandez, L.; Rivera, N.; Petines, B.; Harvey, M.; Nguyen, L.; et al. Glucocorticoid Receptor (GR) Expression in Circulating Tumor Cells (CTCs) to Prognosticate Overall Survival (OS) for Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients (Pts) Treated with Androgen Receptor Signaling Inhibitors (ARSi). JCO 2017, 35, 194. [Google Scholar] [CrossRef]
- Oeyen, S.; Liégeois, V.; De Laere, B.; Buys, A.; Strijbos, M.; Dirix, P.; Meijnders, P.; Vermeulen, P.; Van Laere, S.; Dirix, L. Automated Enumeration and Phenotypic Characterization of CTCs and tdEVs in Patients with Metastatic Castration Resistant Prostate Cancer. Prostate Cancer Prostatic Dis. 2021, 24, 499–506. [Google Scholar] [CrossRef]
- Gu, T.; Li, J.; Chen, T.; Zhu, Q.; Ding, J. Circulating Tumor Cell Quantification during Abiraterone plus Prednisone Therapy May Estimate Survival in Metastatic Castration-Resistant Prostate Cancer Patients. Int. Urol. Nephrol. 2023, 55, 883–892. [Google Scholar] [CrossRef]
- Scher, H.I.; Armstrong, A.J.; Schonhoft, J.D.; Gill, A.; Zhao, J.L.; Barnett, E.; Carbone, E.; Lu, J.; Antonarakis, E.S.; Luo, J.; et al. Development and Validation of Circulating Tumour Cell Enumeration (Epic Sciences) as a Prognostic Biomarker in Men with Metastatic Castration-Resistant Prostate Cancer. Eur. J. Cancer 2021, 150, 83–94. [Google Scholar] [CrossRef]
- Lorenzo, G.D.; Zappavigna, S.; Crocetto, F.; Giuliano, M.; Ribera, D.; Morra, R.; Scafuri, L.; Verde, A.; Bruzzese, D.; Iaccarino, S.; et al. Assessment of Total, PTEN–, and AR-V7+ Circulating Tumor Cell Count by Flow Cytometry in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Enzalutamide. Clin. Genitourin. Cancer 2021, 19, e286–e298. [Google Scholar] [CrossRef]
- Fleisher, M.; Danila, D.C.; Fizazi, K.; Hirmand, M.; Selby, B.; Phung, D.; De Bono, J.S.; Scher, H.I. Circulating Tumor Cell (CTC) Enumeration in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Treated with Enzalutamide Post-Chemotherapy (Phase 3 AFFIRM Study). JCO 2015, 33, 5035. [Google Scholar] [CrossRef]
- Kohli, M.; Li, J.; Du, M.; Hillman, D.W.; Tan, W.; Carlson, R.; Wang, L.; Wang, L.; Liu, M.C.; Zhang, H.; et al. Circulating Tumor Cells (CTCs) and Plasma Cell Free DNA (cfDNA) Androgen Receptor Amplification (ARamp)-Based Prognosis in Metastatic Castration-Resistant Prostate Cancer (mCRPC). JCO 2017, 35, 152. [Google Scholar] [CrossRef]
- De Laere, B.; Oeyen, S.; Van Oyen, P.; Ghysel, C.; Ampe, J.; Ost, P.; Demey, W.; Hoekx, L.; Schrijvers, D.; Brouwers, B.; et al. Circulating Tumor Cells and Survival in Abiraterone- and Enzalutamide-Treated Patients with Castration-Resistant Prostate Cancer. Prostate 2018, 78, 435–445. [Google Scholar] [CrossRef]
- Steuber, T.; Stroelin, P.; Schlomm, T.; Heinzer, H.; Becker, A.; Budäus, L.; Pantel, K.; Riethdorf, S. 954 Evaluation of Circulating Tumor Cells to Predict Metastatic Progression in Men Treated with Abirateron Acetat for Castration Resistant Prostate Cancer: A Sub-Analysis of the German Named Patient Program. J. Urol. 2012, 187, e388. [Google Scholar] [CrossRef]
- Kohli, M.; Du, M.; Wang, L.; Huang, C.-C. Abstract 4588: Prognostic Association of Plasma Cell Free DNA (cfDNA) Copy Number Variation Based Algorithmic Score with Survival in Metastatic Castration Resistant Prostate Cancer (mCRPC). Cancer Res. 2018, 78, 4588. [Google Scholar] [CrossRef]
- Danila, D.C.; Anand, A.; Sung, C.C.; Heller, G.; Leversha, M.A.; Cao, L.; Lilja, H.; Molina, A.; Sawyers, C.L.; Fleisher, M.; et al. TMPRSS2-ERG Status in Circulating Tumor Cells as a Predictive Biomarker of Sensitivity in Castration-Resistant Prostate Cancer Patients Treated With Abiraterone Acetate. Eur. Urol. 2011, 60, 897–904. [Google Scholar] [CrossRef]
- Maruzzo, M.; Rossi, E.; Basso, U.; Facchinetti, A.; Anile, G.; Pierantoni, F.; Galuppo, S.; Evangelista, L.; Pizzirani, E.; Zamarchi, R.; et al. Prognostic and Predictive Role of CTCs and AR-V7+ CTCs Expression in Metastatic Catrate Resistant Prostate Cancer (mCRPC): A Feasibility Study. JCO 2018, 36, 367. [Google Scholar] [CrossRef]
- Kohli, M.; Li, J.; Du, M.; Hillman, D.W.; Dehm, S.M.; Tan, W.; Carlson, R.; Campion, M.B.; Wang, L.; Wang, L.; et al. Prognostic Association of Plasma Cell-Free DNA-Based Androgen Receptor Amplification and Circulating Tumor Cells in Pre-Chemotherapy Metastatic Castration-Resistant Prostate Cancer Patients. Prostate Cancer Prostatic Dis. 2018, 21, 411–418. [Google Scholar] [CrossRef]
- Giridhar, K.; Sanhueza, C.T.; Hillman, D.W.; Alkhateeb, H.; Carlson, R.; Tan, W.; Quevedo, F.; Pagliaro, L.C.; Costello, B.A.; Kohli, M. Prognostic Value of Chromogranin-a (CGA) Compared to Circulating Tumor Cells (CTCs) in Metastatic Castration Resistant Prostate Cancer (mCRPC). JCO 2018, 36, 249. [Google Scholar] [CrossRef]
- Pal, S.K.; He, M.; Chen, L.; Yang, L.; Pillai, R.; Twardowski, P.; Hsu, J.; Kortylewski, M.; Jones, J.O. Synaptophysin Expression on Circulating Tumor Cells in Patients with Castration Resistant Prostate Cancer Undergoing Treatment with Abiraterone Acetate or Enzalutamide. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 162.e1–162.e6. [Google Scholar] [CrossRef]
- Giridhar, K.; Sosa, C.; Hillman, D.W.; Sanhueza, C.T.; Wang, L.; Cheville, J.C.; Dehm, S.; Kohli, M. Whole Blood Androgen Receptor (AR) Variant (ARV12, ARV14) Expression and Overall Survival (OS) in Metastatic Castrate Resistant Prostate Cancer (mCRPC). JCO 2017, 35, 5058. [Google Scholar] [CrossRef]
- Morra, R.; Zappavigna, S.; Facchini, G.; Ribera, D.; Morelli, F.; Luce, A.; Iaccarino, S.; Izzo, M.; Scafuri, L.; Riccio, V.; et al. Circulating Tumor Cells Count in Prostate Cancer Patients Treated with Enzalutamide: The LANZA Study. Tumori J. 2019, 105, 1–216. [Google Scholar] [CrossRef]
- De Laere, B.; Oeyen, S.; Mayrhofer, M.; Whitington, T.; van Dam, P.-J.; Van Oyen, P.; Ghysel, C.; Ampe, J.; Ost, P.; Demey, W.; et al. TP53 Outperforms Other Androgen Receptor Biomarkers to Predict Abiraterone or Enzalutamide Outcome in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 1766–1773. [Google Scholar] [CrossRef]
- Fernandez-Perez, M.P.; Perez-Navarro, E.; Alonso-Gordoa, T.; Conteduca, V.; Font, A.; Vázquez-Estévez, S.; González-Del-Alba, A.; Wetterskog, D.; Antonarakis, E.S.; Mellado, B.; et al. A Correlative Biomarker Study and Integrative Prognostic Model in Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide. Prostate 2023, 83, 376–384. [Google Scholar] [CrossRef]
- Hirano, H.; Nagata, M.; Nagaya, N.; Nakamura, S.; Ashizawa, T.; Lu, Y.; Kawano, H.; Kitamura, K.; Sakamoto, Y.; Fujita, K.; et al. Bone Scan Index (BSI) Scoring by Using Bone Scintigraphy and Circulating Tumor Cells (CTCs): Predictive Factors for Enzalutamide Effectiveness in Patients with Castration-Resistant Prostate Cancer and Bone Metastases. Sci. Rep. 2023, 13, 8704. [Google Scholar] [CrossRef]
- Sepe, P.; Verzoni, E.; Miodini, P.; Claps, M.; Ratta, R.; Martinetti, A.; Mennitto, R.; Sottotetti, E.; Procopio, G.; Cappelletti, V.; et al. Could Circulating Tumor Cells and ARV7 Detection Improve Clinical Decisions in Metastatic Castration-Resistant Prostate Cancer? The Istituto Nazionale Dei Tumori (INT) Experience. Cancers 2019, 11, 980. [Google Scholar] [CrossRef]
- Francolini, G.; Loi, M.; Ciccone, L.P.; Detti, B.; Di Cataldo, V.; Pinzani, P.; Salvianti, F.; Salvatore, G.; Sottili, M.; Santini, C.; et al. Prospective Assessment of AR Splice Variant and Multi-Biomarker Expression on Circulating Tumor Cells of mCRPC Patients Undergoing Androgen Receptor Targeted Agents: Interim Analysis of PRIMERA Trial (NCT04188275). Med. Oncol. 2022, 39, 119. [Google Scholar] [CrossRef]
- Nakamura, S.; Nagata, M.; Nagaya, N.; Ashizawa, T.; Hirano, H.; Lu, Y.; Ide, H.; Horie, S. The Detection and Negative Reversion of Circulating Tumor Cells as Prognostic Biomarkers for Metastatic Castration-Resistant Prostate Cancer with Bone Metastases Treated by Enzalutamide. Cancers 2024, 16, 772. [Google Scholar] [CrossRef]
- Wüstmann, N.; Seitzer, K.; Humberg, V.; Vieler, J.; Grundmann, N.; Steinestel, J.; Tiedje, D.; Duensing, S.; Krabbe, L.-M.; Bögemann, M.; et al. Co-Expression and Clinical Utility of AR-FL and AR Splice Variants AR-V3, AR-V7 and AR-V9 in Prostate Cancer. Biomark. Res. 2023, 11, 37. [Google Scholar] [CrossRef]
- Nørgaard, M.; Bjerre, M.T.; Fredsøe, J.; Vang, S.; Jensen, J.B.; De Laere, B.; Grönberg, H.; Borre, M.; Lindberg, J.; Sørensen, K.D. Prognostic Value of Low-Pass Whole Genome Sequencing of Circulating Tumor DNA in Metastatic Castration-Resistant Prostate Cancer. Clin. Chem. 2023, 69, 386–398. [Google Scholar] [CrossRef]
- Conteduca, V.; Casadei, C.; Scarpi, E.; Brighi, N.; Schepisi, G.; Lolli, C.; Gurioli, G.; Toma, I.; Poti, G.; Farolfi, A.; et al. Baseline Plasma Tumor DNA (ctDNA) Correlates with PSA Kinetics in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Treated with Abiraterone or Enzalutamide. Cancers 2022, 14, 2219. [Google Scholar] [CrossRef]
- Efstathiou, E.; Attard, G.; Lucas, J.; Thomas, S.; Gormley, M.; Aguilar-Bonavides, C.; Flaig, T.W.; Franke, F.; Goodman, O.B.; Oudard, S.; et al. Blood Biomarkers and Association with Clinical Outcomes in Metastatic Castration-Resistant Prostate Cancer (mCRPC): Prespecified Longitudinal Analysis from the ACIS Study of Apalutamide (APA) or Placebo Combined with Abiraterone Acetate plus Prednisone (AAP). JCO 2022, 40, 142. [Google Scholar] [CrossRef]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef]
- Conteduca, V.; Scarpi, E.; Caroli, P.; Lolli, C.; Gurioli, G.; Brighi, N.; Poti, G.; Farolfi, A.; Altavilla, A.; Schepisi, G.; et al. Combining Liquid Biopsy and Functional Imaging Analysis in Metastatic Castration-Resistant Prostate Cancer Helps Predict Treatment Outcome. Mol. Oncol. 2022, 16, 538–548. [Google Scholar] [CrossRef]
- Tolmeijer, S.H.; Boerrigter, E.; Sumiyoshi, T.; Kwan, E.M.; Ng, S.W.S.; Annala, M.; Donnellan, G.; Herberts, C.; Benoist, G.E.; Hamberg, P.; et al. Early On-Treatment Changes in Circulating Tumor DNA Fraction and Response to Enzalutamide or Abiraterone in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2023, 29, 2835–2844. [Google Scholar] [CrossRef]
- Torquato, S.; Pallavajjala, A.; Goldstein, A.; Valda Toro, P.; Silberstein, J.L.; Lee, J.; Nakazawa, M.; Waters, I.; Chu, D.; Shinn, D.; et al. Genetic Alterations Detected in Cell-Free DNA Are Associated With Enzalutamide and Abiraterone Resistance in Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Du, M.; Tian, Y.; Tan, W.; Wang, L.; Wang, L.; Kilari, D.; Huang, C.-C.; Wang, L.; Kohli, M. Plasma Cell-Free DNA-Based Predictors of Response to Abiraterone Acetate/Prednisone and Prognostic Factors in Metastatic Castration-Resistant Prostate Cancer. Prostate Cancer Prostatic Dis. 2020, 23, 705–713. [Google Scholar] [CrossRef]
- Belic, J.; Graf, R.; Bauernhofer, T.; Cherkas, Y.; Ulz, P.; Waldispuehl-Geigl, J.; Perakis, S.; Gormley, M.; Patel, J.; Li, W.; et al. Genomic Alterations in Plasma DNA from Patients with Metastasized Prostate Cancer Receiving Abiraterone or Enzalutamide. Int. J. Cancer 2018, 143, 1236–1248. [Google Scholar] [CrossRef]
- Khalaf, D.; Annala, M.; Finch, D.L.; Oja, C.D.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; Beja, K.; Vandekerkhove, G.R.; Gleave, M.; et al. Phase 2 Randomized Cross-over Trial of Abiraterone + Prednisone (ABI+P) vs. Enzalutamide (ENZ) for Patients (Pts) with Metastatic Castration Resistant Prostate Cancer (mCPRC): Results for 2nd-Line Therapy. JCO 2018, 36, 5015. [Google Scholar] [CrossRef]
- Romanel, A.; Tandefelt, D.G.; Conteduca, V.; Jayaram, A.; Casiraghi, N.; Wetterskog, D.; Salvi, S.; Amadori, D.; Zafeiriou, Z.; Rescigno, P.; et al. Plasma AR and Abiraterone-Resistant Prostate Cancer. Sci. Transl. Med. 2015, 7, 312re10. [Google Scholar] [CrossRef]
- Jayaram, A.; Wingate, A.; Wetterskog, D.; Wheeler, G.; Sternberg, C.N.; Jones, R.; Berruti, A.; Lefresne, F.; Lahaye, M.; Thomas, S.; et al. Plasma Tumor Gene Conversions after One Cycle Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer: A Biomarker Analysis of a Multicenter International Trial. Ann. Oncol. 2021, 32, 726–735. [Google Scholar] [CrossRef]
- Fettke, H.; Kwan, E.M.; Bukczynska, P.; Ng, N.; Nguyen-Dumont, T.; Southey, M.C.; Davis, I.D.; Mant, A.; Parente, P.; Pezaro, C.; et al. Prognostic Impact of Total Plasma Cell-Free DNA Concentration in Androgen Receptor Pathway Inhibitor–Treated Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. Focus 2021, 7, 1287–1291. [Google Scholar] [CrossRef]
- Hendriks, R.J.; Dijkstra, S.; Smit, F.P.; Vandersmissen, J.; Van de Voorde, H.; Mulders, P.F.A.; van Oort, I.M.; Van Criekinge, W.; Schalken, J.A. Epigenetic Markers in Circulating Cell-Free DNA as Prognostic Markers for Survival of Castration-Resistant Prostate Cancer Patients. Prostate 2018, 78, 336–342. [Google Scholar] [CrossRef]
- Conteduca, V.; Scarpi, E.; Matteucci, F.; Caroli, P.; Ravaglia, G.; Fantini, L.; Gurioli, G.; Schepisi, G.; Wetterskog, D.; Menna, C.; et al. Multimodal Approach to Outcome Prediction in Metastatic Castration-Resistant Prostate Cancer by Integrating Functional Imaging and Plasma DNA Analysis. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Lorente, D.; Olmos, D.; Mateo, J.; Dolling, D.; Bianchini, D.; Seed, G.; Flohr, P.; Crespo, M.; Figueiredo, I.; Miranda, S.; et al. Circulating Tumour Cell Increase as a Biomarker of Disease Progression in Metastatic Castration-Resistant Prostate Cancer Patients with Low Baseline CTC Counts. Ann. Oncol. 2018, 29, 1554–1560. [Google Scholar] [CrossRef]
- Yip, S.; Fizazi, K.; Laird, D.; Matsubara, N.; Azad, A.; Joung, J.Y.; Fong, P.C.C.; Van Bruwaene, S.; Liu, G.; Voog, E.; et al. Exploration of Circulating Tumor Cell (CTC) Conversion and CTC0 as Prognostic Biomarkers for Efficacy in TALAPRO-2: Phase 3 Study of Talazoparib (TALA) + Enzalutamide (ENZA) vs. Placebo (PBO) + ENZA as First-Line (1L) Treatment in Patients (Pts) with Metastatic Castration-Resistant Prostate Cancer (mCRPC). JCO 2024, 42, 5023. [Google Scholar] [CrossRef]
- Gill, D.M.; Agarwal, N.; Hahn, A.W.; Johnson, E.; Poole, A.; Carroll, E.; Boucher, K.M.; Salama, M.E.; Agarwal, A.M. Impact of Circulating Tumor Cell (CTC) Nucleus Size on Outcomes with Abiraterone Acetate (AA) Therapy in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC). JCO 2017, 35, 253. [Google Scholar] [CrossRef]
- Del Re, M.; Biasco, E.; Crucitta, S.; Derosa, L.; Rofi, E.; Orlandini, C.; Miccoli, M.; Galli, L.; Falcone, A.; Jenster, G.W.; et al. The Detection of Androgen Receptor Splice Variant 7 in Plasma-Derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. Eur. Urol. 2017, 71, 680–687. [Google Scholar] [CrossRef]
- Markowski, M.C.; Wang, H.; Sullivan, R.; Rifkind, I.; Sinibaldi, V.; Schweizer, M.T.; Teply, B.A.; Ngomba, N.; Fu, W.; Carducci, M.A.; et al. A Multicohort Open-Label Phase II Trial of Bipolar Androgen Therapy in Men with Metastatic Castration-Resistant Prostate Cancer (RESTORE): A Comparison of Post-Abiraterone Versus Post-Enzalutamide Cohorts. Eur. Urol. 2021, 79, 692–699. [Google Scholar] [CrossRef]
- Sepe, P.; Procopio, G.; Pircher, C.C.; Basso, U.; Caffo, O.; Cappelletti, V.; Claps, M.; De Giorgi, U.; Fratino, L.; Guadalupi, V.; et al. A Phase II Study Evaluating the Efficacy of Enzalutamide and the Role of Liquid Biopsy for Evaluation of ARv7 in mCRPC Patients with Measurable Metastases Including Visceral Disease (Excalibur Study). Ther. Adv. Med. Oncol. 2024, 16, 17588359231217958. [Google Scholar] [CrossRef]
- Del Re, M.; Conteduca, V.; Crucitta, S.; Gurioli, G.; Casadei, C.; Restante, G.; Schepisi, G.; Lolli, C.; Cucchiara, F.; Danesi, R.; et al. Androgen Receptor Gain in Circulating Free DNA and Splicing Variant 7 in Exosomes Predict Clinical Outcome in CRPC Patients Treated with Abiraterone and Enzalutamide. Prostate Cancer Prostatic Dis. 2021, 24, 524–531. [Google Scholar] [CrossRef]
- Del Re, M.; Crucitta, S.; Sbrana, A.; Rofi, E.; Paolieri, F.; Gianfilippo, G.; Galli, L.; Falcone, A.; Morganti, R.; Porta, C.; et al. Androgen Receptor (AR) Splice Variant 7 and Full-Length AR Expression Is Associated with Clinical Outcome: A Translational Study in Patients with Castrate-Resistant Prostate Cancer. BJU Int. 2019, 124, 693–700. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Fedor, H.L.; Lotan, T.L.; Marzo, A.M.D.; et al. Abstract 2910: Androgen Receptor Splice Variant-7 Predicts Resistance to Enzalutamide in Patients with Castration-Resistant Prostate Cancer. Cancer Res. 2014, 74, 2910. [Google Scholar] [CrossRef]
- Seitz, A.K.; Thoene, S.; Bietenbeck, A.; Nawroth, R.; Tauber, R.; Thalgott, M.; Schmid, S.; Secci, R.; Retz, M.; Gschwend, J.E.; et al. AR-V7 in Peripheral Whole Blood of Patients with Castration-Resistant Prostate Cancer: Association with Treatment-Specific Outcome Under Abiraterone and Enzalutamide. Eur. Urol. 2017, 72, 828–834. [Google Scholar] [CrossRef]
- Todenhöfer, T.; Azad, A.; Stewart, C.; Gao, J.; Eigl, B.J.; Gleave, M.E.; Joshua, A.M.; Black, P.C.; Chi, K.N. AR-V7 Transcripts in Whole Blood RNA of Patients with Metastatic Castration Resistant Prostate Cancer Correlate with Response to Abiraterone Acetate. J. Urol. 2017, 197, 135–142. [Google Scholar] [CrossRef]
- Qu, F.; Xie, W.; Nakabayashi, M.; Zhang, H.; Jeong, S.H.; Wang, X.; Komura, K.; Sweeney, C.J.; Sartor, O.; Lee, G.-S.M.; et al. Association of AR-V7 and Prostate-Specific Antigen RNA Levels in Blood with Efficacy of Abiraterone Acetate and Enzalutamide Treatment in Men with Prostate Cancer. Clin. Cancer Res. 2017, 23, 726–734. [Google Scholar] [CrossRef]
- Gupta, S.; Hovelson, D.H.; Kemeny, G.; Halabi, S.; Foo, W.-C.; Anand, M.; Somarelli, J.A.; Tomlins, S.A.; Antonarakis, E.S.; Luo, J.; et al. Discordant and Heterogeneous Clinically Relevant Genomic Alterations in Circulating Tumor Cells vs. Plasma DNA from Men with Metastatic Castration Resistant Prostate Cancer. Genes Chromosomes Cancer 2020, 59, 225–239. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Luo, J.; Nanus, D.M.; Giannakakou, P.; Szmulewitz, R.Z.; Danila, D.C.; Healy, P.; Anand, M.; Berry, W.R.; Zhang, T.; et al. Prospective Multicenter Study of Circulating Tumor Cell AR-V7 and Taxane Versus Hormonal Treatment Outcomes in Metastatic Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2020, 4, 1285–1301. [Google Scholar] [CrossRef]
- Chung, J.-S.; Wang, Y.; Henderson, J.; Singhal, U.; Qiao, Y.; Zaslavsky, A.B.; Hovelson, D.H.; Spratt, D.E.; Reichert, Z.; Palapattu, G.S.; et al. Circulating Tumor Cell–Based Molecular Classifier for Predicting Resistance to Abiraterone and Enzalutamide in Metastatic Castration-Resistant Prostate Cancer. Neoplasia 2019, 21, 802–809. [Google Scholar] [CrossRef]
- Fettke, H.; Kwan, E.M.; Docanto, M.M.; Bukczynska, P.; Ng, N.; Graham, L.-J.K.; Mahon, K.; Hauser, C.; Tan, W.; Wang, X.H.; et al. Combined Cell-Free DNA and RNA Profiling of the Androgen Receptor: Clinical Utility of a Novel Multianalyte Liquid Biopsy Assay for Metastatic Prostate Cancer. Eur. Urol. 2020, 78, 173–180. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, G.; Zhao, X.; Zhao, J.; Chen, J.; Shen, P.; Chen, N.; Zeng, H. 650P Comparing the Predictive Value of Exosome, Circulating Tumor Cells, and Tumor Tissue in Detecting AR-V7 among Metastatic Castration-Resistant Prostate Cancer Treated with Abiraterone. Ann. Oncol. 2020, 31 (Suppl. 4), S530. [Google Scholar] [CrossRef]
- Schlack, K.; Seitzer, K.; Wüstmann, N.; Humberg, V.; Grundmann, N.; Steinestel, J.; Tiedje, D.; Rahbar, K.; Krabbe, L.-M.; Bögemann, M.; et al. Comparison of Circulating Tumor Cells and AR-V7 as Clinical Biomarker in Metastatic Castration-Resistant Prostate Cancer Patients. Sci. Rep. 2022, 12, 11846. [Google Scholar] [CrossRef]
- Maillet, D.; Allioli, N.; Peron, J.; Plesa, A.; Decaussin-Petrucci, M.; Tartas, S.; Ruffion, A.; Crouzet, S.; Rimokh, R.; Gillet, P.-G.; et al. Improved Androgen Receptor Splice Variant 7 Detection Using a Highly Sensitive Assay to Predict Resistance to Abiraterone or Enzalutamide in Metastatic Prostate Cancer Patients. Eur. Urol. Oncol. 2021, 4, 609–617. [Google Scholar] [CrossRef]
- Morgan, T.M.; Chung, J.-S.; Wang, Y.; Henderson, J.; Singhal, U.; Qiao, Y.; Zaslavsky, A.; Hovelson, D.H.; Alva, A.S.; Feng, F.Y.-C.; et al. Indentification of a CTC-Based Gene Expression Signature Predicting Resistance to Abiraterone and Enzalutamide in mCRPC. JCO 2017, 35, 5072. [Google Scholar] [CrossRef]
- Boerrigter, E.; Benoist, G.E.; van Oort, I.M.; Verhaegh, G.W.; van Hooij, O.; Groen, L.; Smit, F.; Oving, I.M.; de Mol, P.; Smilde, T.J.; et al. Liquid Biopsy Reveals KLK3 mRNA as a Prognostic Marker for Progression Free Survival in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing First-Line Abiraterone Acetate and Prednisone Treatment. Mol. Oncol. 2021, 15, 2453–2465. [Google Scholar] [CrossRef]
- Scher, H.I.; Graf, R.P.; Schreiber, N.A.; McLaughlin, B.; Lu, D.; Louw, J.; Danila, D.C.; Dugan, L.; Johnson, A.; Heller, G.; et al. Nuclear-Specific AR-V7 Protein Localization Is Necessary to Guide Treatment Selection in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 874–882. [Google Scholar] [CrossRef]
- Joncas, F.-H.; Lucien, F.; Rouleau, M.; Morin, F.; Leong, H.S.; Pouliot, F.; Fradet, Y.; Gilbert, C.; Toren, P. Plasma Extracellular Vesicles as Phenotypic Biomarkers in Prostate Cancer Patients. Prostate 2019, 79, 1767–1776. [Google Scholar] [CrossRef]
- Wang, S.; Du, P.; Cao, Y.; Tang, X.; Yang, X.; Ma, J.; Yu, Z.; Yang, Y. The Association of AR-V7 with Resistance to Abiraterone in Metastatic Castration-Resistant Prostate Cancer. J. Men’s Health 2022, 18, 1–8. [Google Scholar] [CrossRef]
- Conteduca, V.; Wetterskog, D.; Sharabiani, M.T.A.; Grande, E.; Fernandez-Perez, M.P.; Jayaram, A.; Salvi, S.; Castellano, D.; Romanel, A.; Lolli, C.; et al. Androgen Receptor Gene Status in Plasma DNA Associates with Worse Outcome on Enzalutamide or Abiraterone for Castration-Resistant Prostate Cancer: A Multi-Institution Correlative Biomarker Study. Ann. Oncol. 2017, 28, 1508–1516. [Google Scholar] [CrossRef]
- Gurioli, G.; Conteduca, V.; Lolli, C.; Schepisi, G.; Gargiulo, S.; Altavilla, A.; Casadei, C.; Scarpi, E.; De Giorgi, U. Plasma AR Copy Number Changes and Outcome to Abiraterone and Enzalutamide. Front. Oncol. 2020, 10, 567809. [Google Scholar] [CrossRef]
- Conteduca, V.; Scarpi, E.; Caroli, P.; Salvi, S.; Lolli, C.; Burgio, S.L.; Menna, C.; Schepisi, G.; Testoni, S.; Gurioli, G.; et al. Circulating Androgen Receptor Combined with 18F-Fluorocholine PET/CT Metabolic Activity and Outcome to Androgen Receptor Signalling-Directed Therapies in Castration-Resistant Prostate Cancer. Sci. Rep. 2017, 7, 15541. [Google Scholar] [CrossRef]
- Salvi, S.; Casadio, V.; Conteduca, V.; Lolli, C.; Gurioli, G.; Martignano, F.; Schepisi, G.; Testoni, S.; Scarpi, E.; Amadori, D.; et al. Circulating AR Copy Number and Outcome to Enzalutamide in Docetaxel-Treated Metastatic Castration-Resistant Prostate Cancer. Oncotarget 2016, 7, 37839–37845. [Google Scholar] [CrossRef]
- Salvi, S.; Casadio, V.; Conteduca, V.; Burgio, S.L.; Menna, C.; Bianchi, E.; Rossi, L.; Carretta, E.; Masini, C.; Amadori, D.; et al. Circulating Cell-Free AR and CYP17A1 Copy Number Variations May Associate with Outcome of Metastatic Castration-Resistant Prostate Cancer Patients Treated with Abiraterone. Br. J. Cancer 2015, 112, 1717–1724. [Google Scholar] [CrossRef]
- Azad, A.; Wyatt, A.; Volik, S.; Gleave, M.; Collins, C.; Chi, K. Genomic Alterations in Cell-Free Dna and Enzalutamide Resistance in Castration-Resistant Prostate Cancer. Asia-Pac. J. Clin. Oncol. 2016, 12, 44–51. [Google Scholar] [CrossRef][Green Version]
- Fettke, H.; Kwan, E.M.; Bukczynska, P.; Steen, J.A.; Docanto, M.; Ng, N.; Parente, P.; Mant, A.; Foroughi, S.; Pezaro, C.; et al. Independent Prognostic Impact of Plasma NCOA2 Alterations in Metastatic Castration-Resistant Prostate Cancer. Prostate 2021, 81, 992–1001. [Google Scholar] [CrossRef]
- Jayaram, A.; Wingate, A.; Wetterskog, D.; Conteduca, V.; Khalaf, D.; Sharabiani, M.T.A.; Calabrò, F.; Barwell, L.; Feyerabend, S.; Grande, E.; et al. Plasma Androgen Receptor Copy Number Status at Emergence of Metastatic Castration-Resistant Prostate Cancer: A Pooled Multicohort Analysis. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Kwan, E.M.; Dai, C.; Fettke, H.; Hauser, C.; Docanto, M.M.; Bukczynska, P.; Ng, N.; Foroughi, S.; Graham, L.-J.K.; Mahon, K.; et al. Plasma Cell–Free DNA Profiling of PTEN-PI3K-AKT Pathway Aberrations in Metastatic Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2021, 5, 622–637. [Google Scholar] [CrossRef]
- Buelens, S.; Claeys, T.; Dhondt, B.; Poelaert, F.; Vynck, M.; Yigit, N.; Thas, O.; Ost, P.; Vandesompele, J.; Lumen, N.; et al. Prognostic and Therapeutic Implications of Circulating Androgen Receptor Gene Copy Number in Prostate Cancer Patients Using Droplet Digital Polymerase Chain Reaction. Clin. Genitourin. Cancer 2018, 16, 197–205.e5. [Google Scholar] [CrossRef]
- Lolli, C.; De Lisi, D.; Conteduca, V.; Gurioli, G.; Scarpi, E.; Schepisi, G.; Ravaglia, G.; Menna, C.; Farolfi, A.; Altavilla, A.; et al. Testosterone Levels and Androgen Receptor Copy Number Variations in Castration-Resistant Prostate Cancer Treated with Abiraterone or Enzalutamide. Prostate 2019, 79, 1211–1220. [Google Scholar] [CrossRef]
- Dong, B.; Fan, L.; Yang, B.; Chen, W.; Li, Y.; Wu, K.; Zhang, F.; Dong, H.; Cheng, H.; Pan, J.; et al. Use of Circulating Tumor DNA for the Clinical Management of Metastatic Castration-Resistant Prostate Cancer: A Multicenter, Real-World Study. J. Natl. Compr. Cancer Netw. 2021, 19, 905–914. [Google Scholar] [CrossRef]
- Silberstein, J.; Luber, B.; Wang, H.; Lu, C.; Chen, Y.; Zhu, Y.; Taylor, M.N.; Carducci, M.A.; Eisenberger, M.A.; Luo, J.; et al. Clinical Significance of AR mRNA Quantification from Circulating Tumor Cells (CTCs) in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Treated with Abiraterone (Abi) or Enzalutamide (Enza). JCO 2017, 35, 132. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Zhang, N.; Saha, J.; Nevalaita, L.; Shell, S.A.; Garratt, C.; Ikonen, T.; Fizazi, K. Real-World Assessment of AR-LBD Mutations in Metastatic Castration-Resistant Prostate Cancer. JCO 2023, 41, 204. [Google Scholar] [CrossRef]
- Chi, K.; Azad, A.; Volik, S.; Haegert, A.; Zalcberg, J.; Bihan, S.L.; McConeghy, B.; Gleave, M.; Wyatt, A.; Collins, C. 2504 Genomic Predictive and Prognostic Factors from Plasma Cell-Free DNA (cfDNA) for Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients (Pts) Commencing Enzalutamide (ENZ). Eur. J. Cancer 2015, 51, S474–S475. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. JCO 2017, 35, 2149–2156. [Google Scholar] [CrossRef]
- Schlack, K.; Seitzer, K.; Boegemann, M.; Krabbe, L.-M.; Schrader, A.J.; Grundmann, N.; Tiedje, D.; Steinestel, J.; Bernemann, C. Combinatorial Expression of Androgen Receptor Splice Variants: No Predictive Value in Castration-Resistant Prostate Cancer Patients Treated with Enzalutamide (Enza) or Abiraterone (Abi). JCO 2020, 38, e17547. [Google Scholar] [CrossRef]
- Chi, K.N.; Annala, M.; Sunderland, K.; Khalaf, D.; Finch, D.; Oja, C.D.; Vergidis, J.; Zulfiqar, M.; Beja, K.; Vandekerkhove, G.; et al. A Randomized Phase II Cross-over Study of Abiraterone + Prednisone (ABI) vs. Enzalutamide (ENZ) for Patients (Pts) with Metastatic, Castration-Resistant Prostate Cancer (mCRPC). JCO 2017, 35, 5002. [Google Scholar] [CrossRef]
- Moses, M.; Niu, A.; Lilly, M.B.; Hahn, A.W.; Nussenzveig, R.; Ledet, E.; Manogue, C.; Cotogno, P.; Lewis, B.; Layton, J.; et al. Circulating-Tumor DNA as Predictor of Enzalutamide Response Post-Abiraterone Treatment in Metastatic Castration-Resistant Prostate Cancer. Cancer Treat. Res. Commun. 2020, 24, 100193. [Google Scholar] [CrossRef]
- Mizuno, K.; Sumiyoshi, T.; Okegawa, T.; Terada, N.; Ishitoya, S.; Miyazaki, Y.; Kojima, T.; Katayama, H.; Fujimoto, N.; Hatakeyama, S.; et al. Clinical Impact of Detecting Low-Frequency Variants in Cell-Free DNA on Treatment of Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 6164–6173. [Google Scholar] [CrossRef]
- Erb, H.H.H.; Sparwasser, P.; Diehl, T.; Hemmerlein-Thomas, M.; Tsaur, I.; Jüngel, E.; Sommer, U.; Baretton, G.B.; Haferkamp, A.; Neisius, A.; et al. AR-V7 Protein Expression in Circulating Tumour Cells Is Not Predictive of Treatment Response in mCRPC. Urol. Int. 2020, 104, 253–262. [Google Scholar] [CrossRef]
- Scher, H.I.; Graf, R.P.; Schreiber, N.A.; Jayaram, A.; Winquist, E.; McLaughlin, B.; Lu, D.; Fleisher, M.; Orr, S.; Lowes, L.; et al. Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer. JAMA Oncol. 2018, 4, 1179–1186. [Google Scholar] [CrossRef]
- Graf, R.P.; Hullings, M.; Barnett, E.S.; Carbone, E.; Dittamore, R.; Scher, H.I. Clinical Utility of the Nuclear-Localized AR-V7 Biomarker in Circulating Tumor Cells in Improving Physician Treatment Choice in Castration-Resistant Prostate Cancer. Eur. Urol. 2020, 77, 170–177. [Google Scholar] [CrossRef]
- De Laere, B.; van Dam, P.-J.; Whitington, T.; Mayrhofer, M.; Diaz, E.H.; Van den Eynden, G.; Vandebroek, J.; Del-Favero, J.; Van Laere, S.; Dirix, L.; et al. Comprehensive Profiling of the Androgen Receptor in Liquid Biopsies from Castration-Resistant Prostate Cancer Reveals Novel Intra-AR Structural Variation and Splice Variant Expression Patterns. Eur. Urol. 2017, 72, 192–200. [Google Scholar] [CrossRef]
- To, S.Q.; Kwan, E.; Fettke, H.; Mant, A.; Docanto, M.; Martelotto, L.; Bukczynska, P.; Ng, N.; Graham, L.-J.; Parente, P.; et al. Abstract 2593: AR-V7 and AR-V9 Expression Is Not Predictive of Response to AR-Axis Targeting Agents in Metastatic Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 2593. [Google Scholar] [CrossRef]
- Alahi, I.; Chauhan, P.S.; Shiang, A.L.; Webster, J.; Dang, H.X.; Greiner, L.; yang, B.; Ledet, E.M.; Babbra, R.K.; Feng, W.; et al. Abstract 6698: Combinatorial Genomic and Epigenomic Cell-Free DNA Analysis of High-Risk Metastatic Castration Resistant Prostate Cancer Reveals Prognostic Liquid Biopsy Signatures. Cancer Res. 2023, 83, 6698. [Google Scholar] [CrossRef]
- Shiang, A.; Chauhan, P.S.; Dang, H.X.; Webster, J.; Ledet, E.M.; Babbra, R.K.; Feng, W.; Harris, P.K.; Jaeger, E.B.; Miller, P.; et al. Liquid Biopsy AR/Enhancer Alteration Detection before AR-Targeted Therapy and Correlation with Survival in Metastatic Castrate-Resistant Prostate Cancer Patients. JCO 2022, 40, 171. [Google Scholar] [CrossRef]
- Scher, H.I.; Lu, D.; Schreiber, N.A.; Louw, J.; Graf, R.P.; Vargas, H.A.; Johnson, A.; Jendrisak, A.; Bambury, R.; Danila, D.; et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016, 2, 1441–1449. [Google Scholar] [CrossRef]
- Benoist, G.E.; van Oort, I.M.; Boerrigter, E.; Verhaegh, G.W.; van Hooij, O.; Groen, L.; Smit, F.; de Mol, P.; Hamberg, P.; Dezentjé, V.O.; et al. Prognostic Value of Novel Liquid Biomarkers in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide: A Prospective Observational Study. Clin. Chem. 2020, 66, 842–851. [Google Scholar] [CrossRef]
- Boerrigter, E.; Benoist, G.E.; van Oort, I.M.; Verhaegh, G.W.; van Hooij, O.; Groen, L.; Smit, F.; Oving, I.M.; de Mol, P.; Smilde, T.J.; et al. Abstract 1413: Exploring the Prognostic Value of microRNAs and Drug Exposure in Patients with Metastatic Castration Resistant Prostate Cancer Treated with Abiraterone: A Prospective Observational Study. Cancer Res. 2020, 80, 1413. [Google Scholar] [CrossRef]
- Zedan, A.H.; Osther, P.J.S.; Assenholt, J.; Madsen, J.S.; Hansen, T.F. Circulating miR-141 and miR-375 Are Associated with Treatment Outcome in Metastatic Castration Resistant Prostate Cancer. Sci. Rep. 2020, 10, 227. [Google Scholar] [CrossRef]
- Sharova, E.; Maruzzo, M.; Del Bianco, P.; Cavallari, I.; Pierantoni, F.; Basso, U.; Ciminale, V.; Zagonel, V. Prognostic Stratification of Metastatic Prostate Cancer Patients Treated With Abiraterone and Enzalutamide Through an Integrated Analysis of Circulating Free microRNAs and Clinical Parameters. Front. Oncol. 2021, 11, 626104. [Google Scholar] [CrossRef]
- Detassis, S.; Precazzini, F.; Grasso, M.; Del Vescovo, V.; Maines, F.; Caffo, O.; Campomenosi, P.; Denti, M.A. Plasma microRNA Signature as Companion Diagnostic for Abiraterone Acetate Treatment in Metastatic Castration-Resistant Prostate Cancer: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 5573. [Google Scholar] [CrossRef]
- Tao, W.; Luo, Z.-H.; He, Y.-D.; Wang, B.-Y.; Xia, T.-L.; Deng, W.-M.; Zhang, L.-X.; Tang, X.-M.; Meng, Z.-A.; Gao, X.; et al. Plasma Extracellular Vesicle circRNA Signature and Resistance to Abiraterone in Metastatic Castration-Resistant Prostate Cancer. Br. J. Cancer 2023, 128, 1320–1332. [Google Scholar] [CrossRef]
- Peter, M.R.; Bilenky, M.; Shi, Y.; Pu, J.; Kamdar, S.; Hansen, A.R.; Fleshner, N.E.; Sridhar, S.S.; Joshua, A.M.; Hirst, M.; et al. A Novel Methylated Cell-Free DNA Marker Panel to Monitor Treatment Response in Metastatic Prostate Cancer. Epigenomics 2022, 14, 811–822. [Google Scholar] [CrossRef]
- Filon, M.; Yang, B.; Purohit, T.A.; Schehr, J.; Singh, A.; Bigarella, M.; Lewis, P.; Denu, J.; Lang, J.; Jarrard, D.F. Development of a Multiplex Assay to Assess Activated P300/CBP in Circulating Prostate Tumor Cells. Oncotarget 2023, 14, 738–746. [Google Scholar] [CrossRef]
- Shevrin, D.H.; Yang, M.; Imas, P.; Gulukota, K.; Northshore University Healthsystem. Associations of Circulating Cell-Free DNA (cfDNA) and Clinical Outcomes in Metastatic Castrate-Resistant Prostate Cancer (mCRPC). JCO 2021, 39, 137. [Google Scholar] [CrossRef]
- Zhang, J.; Zimmermann, B.; Galletti, G.; Halabi, S.; Gjyrezi, A.; Yang, Q.; Gupta, S.; Sboner, A.; Anand, M.; George, D.J.; et al. Association of Circulating Tumor Cell RB1 Loss RNA Signature with Outcomes and Immune Phenotypes in Men with mCRPC. JCO 2022, 40, 139. [Google Scholar] [CrossRef]
- Gupta, S.; Halabi, S.; Yang, Q.; Roy, A.; Tubbs, A.; Gore, Y.; George, D.J.; Nanus, D.M.; Antonarakis, E.S.; Danila, D.C.; et al. PSMA-Positive Circulating Tumor Cell Detection and Outcomes with Abiraterone or Enzalutamide Treatment in Men with Metastatic Castrate-Resistant Prostate Cancer. Clin. Cancer Res. 2023, 29, 1929–1937. [Google Scholar] [CrossRef]
- Chung, J.-S.; Wang, Y.; James, H.; Singhal, U.; Qiao, Y.; Zaslavsky, A.; Hovelson, D.; Feng, F.; Palapattu, G.; Russell, T.; et al. PD71-06 CTC-Based Gene Expression for Predicting Resistance to Abiraterone and Enzalutamide in Mcrpc. J. Urol. 2017, 197, e1357. [Google Scholar] [CrossRef]
- Cho, H.; Cha, J.; Han, K.-H.; Chung, J.-S. Abstract 3699: Identifying Novel Resistance Biomarkers in Circulating Tumor Cell-Expressed Transcriptomes of Metastatic Castration-Resistant Prostate Cancer Patients Treated with Androgen Receptor Signaling Inhibitors. Cancer Res. 2024, 84, 3699. [Google Scholar] [CrossRef]
- Zhu, S.; Ni, Y.; Wang, Z.; Zhang, X.; Zhang, Y.; Zhao, F.; Dai, J.; Wang, Z.; Zhu, X.; Chen, J.; et al. Plasma Exosomal AKR1C3 mRNA Expression Is a Predictive and Prognostic Biomarker in Patients with Metastatic Castration-Resistant Prostate Cancer. Oncologist 2022, 27, e870–e877. [Google Scholar] [CrossRef]
- Maillet, D.; Allioli, N.; Péron, J.; Plesa, A.; Decaussin-Petrucci, M.; Tartas, S.; Sajous, C.; Ruffion, A.; Crouzet, S.; Freyer, G.; et al. Her2 Expression in Circulating Tumor Cells Is Associated with Poor Outcomes in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 6014. [Google Scholar] [CrossRef]
- Halabi, S.; Guo, S.; Park, J.J.; Nanus, D.M.; George, D.J.; Antonarakis, E.S.; Danila, D.C.; Szmulewitz, R.Z.; McDonnell, D.P.; Norris, J.D.; et al. The Impact of Circulating Tumor Cell HOXB13 RNA Detection in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Treated with Abiraterone or Enzalutamide. Clin. Cancer Res. 2024, 30, 1152–1159. [Google Scholar] [CrossRef]
- Ma, Y. OCT4-positive Circulating Tumor Cells May Predict a Poor Prognosis in Patients with Metastatic Castration-resistant Prostate Cancer Treated with Abiraterone plus Prednisone Therapy. Oncol. Lett. 2023, 26, 452. [Google Scholar] [CrossRef]
- Conteduca, V.; Del Re, M.; Scarpi, E.; Crucitta, S.; Gurioli, G.; Restante, G.; Cucchiara, F.; Lolli, C.; Brighi, N.; Schepisi, G.; et al. High Exosomal PD-L1 Expression in Relation to Lymph Node Progression in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Treated with Abiraterone (Abi) or Enzalutamide (Enza). JCO 2022, 40, e17038. [Google Scholar] [CrossRef]
- Zhu, S.; Ni, Y.; Sun, G.; Wang, Z.; Chen, J.; Zhang, X.; Zhao, J.; Zhu, X.; Dai, J.; Liu, Z.; et al. Exosomal TUBB3 mRNA Expression of Metastatic Castration-Resistant Prostate Cancer Patients: Association with Patient Outcome under Abiraterone. Cancer Med. 2021, 10, 6282–6290. [Google Scholar] [CrossRef]
- Brown, L.C.; Halabi, S.; Schonhoft, J.D.; Yang, Q.; Luo, J.; Nanus, D.M.; Giannakakou, P.; Szmulewitz, R.Z.; Danila, D.C.; Barnett, E.S.; et al. Circulating Tumor Cell Chromosomal Instability and Neuroendocrine Phenotype by Immunomorphology and Poor Outcomes in Men with mCRPC Treated with Abiraterone or Enzalutamide. Clin. Cancer Res. 2021, 27, 4077–4088. [Google Scholar] [CrossRef]
- de Bono, J.S.; Pantel, K.; Efstathiou, E.; Sternberg, C.N.; Gauna, D.C.; Fizazi, K.; Tombal, B.; Wülfing, C.; Schonhoft, J.D.; Tubbs, A.; et al. 614P Circulating Tumor Cell (CTC) Morphologic Sub-Types Present Prior to Treatment in the CARD Trial Identify Therapy Resistance. Ann. Oncol. 2021, 32, S653–S654. [Google Scholar] [CrossRef]
- Scher, H.I.; Graf, R.P.; Schreiber, N.A.; McLaughlin, B.; Jendrisak, A.; Wang, Y.; Lee, J.; Greene, S.; Krupa, R.; Lu, D.; et al. Phenotypic Heterogeneity of Circulating Tumor Cells Informs Clinical Decisions between AR Signaling Inhibitors and Taxanes in Metastatic Prostate Cancer. Cancer Res. 2017, 77, 5687–5698. [Google Scholar] [CrossRef]
- De Laere, B.; Crippa, A.; Ghysel, C.; Ost, P.; Rajan, P.; Eklund, M.; Dirix, L.; Grönberg, H.; Lindberg, J. Elevated Driver Mutational Burden or Number of Perturbed Pathways and Poor Response to Abiraterone or Enzalutamide in Metastatic Castration-Resistant Prostate Cancer. Ann. Oncol. 2019, 30, v30–v31. [Google Scholar] [CrossRef]
- De Laere, B.; Crippa, A.; Mortezavi, A.; Ghysel, C.; Rajan, P.; Eklund, M.; Wyatt, A.; Dirix, L.; Ost, P.; Grönberg, H.; et al. Increased Pathway Complexity Is a Prognostic Biomarker in Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 1588. [Google Scholar] [CrossRef]
- Isebia, K.T.; de Jong, A.; de Weerd, V.; Beaufort, C.; Hamberg, P.; Lolkema, M.P.; Wilting, S.M.; De Wit, R.; Martens, J.W.M.; Jansen, M.P.H.M. mFAST-SeqS Based Aneuploidy Score in Circulating Cell-Free DNA and Role as Early Response Marker in Patients with Metastatic Prostate Cancer Treated with Androgen Receptor Signaling Inhibitor. JCO 2023, 41, 5058. [Google Scholar] [CrossRef]
- Haas, N.B.; LaRiviere, M.J.; Buckingham, T.H.; Cherkas, Y.; Calara-Nielsen, K.; Foulk, B.; Patel, J.; Gross, S.; Smirnov, D.; Vaughn, D.J.; et al. Blood-Based Gene Expression Signature Associated with Metastatic Castrate-Resistant Prostate Cancer Patient Response to Abiraterone plus Prednisone or Enzalutamide. Prostate Cancer Prostatic Dis. 2021, 24, 448–456. [Google Scholar] [CrossRef]
- Groen, L.; Kloots, I.; Englert, D.; Seto, K.; Estafanos, L.; Smith, P.; Verhaegh, G.W.; Mehra, N.; Schalken, J.A. Transcriptome Profiling of Circulating Tumor Cells to Predict Clinical Outcomes in Metastatic Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 9002. [Google Scholar] [CrossRef]
- Halabi, S.; Luo, B.; Guo, S.S.; Knutson, T.; Lyman, J.; Kobilka, A.; Beltran, H.; Antonarakis, E.S.; Rosenberg, J.E.; Galsky, M.D.; et al. A Clinical-Genetic (CG) Circulating Tumor DNA (ctDNA)-Based Prognostic Model for Predicting Overall Survival (OS) in Men with Metastatic Castrate-Resistant Prostate Cancer (mCRPC) Treated with Potent Androgen Receptor Inhibition (Alliance). JCO 2024, 42, 5007. [Google Scholar] [CrossRef]
- McKay, R.R.; Kwak, L.; Crowdis, J.P.; Sperger, J.M.; Zhao, S.G.; Xie, W.; Werner, L.; Lis, R.T.; Zhang, Z.; Wei, X.X.; et al. Phase II Multicenter Study of Enzalutamide in Metastatic Castration-Resistant Prostate Cancer to Identify Mechanisms Driving Resistance. Clin. Cancer Res. 2021, 27, 3610–3619. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Lee, R.J.; Stott, S.L.; Ting, D.T.; Wittner, B.S.; Ulman, M.; Smas, M.E.; Lord, J.B.; Brannigan, B.W.; Trautwein, J.; et al. Androgen Receptor Signaling in Circulating Tumor Cells as a Marker of Hormonally Responsive Prostate Cancer. Cancer Discov. 2012, 2, 995–1003. [Google Scholar] [CrossRef]
- Okegawa, T.; Ninomiya, N.; Masuda, K.; Nakamura, Y.; Tambo, M.; Nutahara, K. AR-V7 in Circulating Tumor Cells Cluster as a Predictive Biomarker of Abiraterone Acetate and Enzalutamide Treatment in Castration-Resistant Prostate Cancer Patients. Prostate 2018, 78, 576–582. [Google Scholar] [CrossRef]
- Shi, Y.; Au, J.S.-K.; Thongprasert, S.; Srinivasan, S.; Tsai, C.-M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.-C. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non–Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef]
- Van-Duyne, G.; Blair, I.A.; Sprenger, C.; Moiseenkova-Bell, V.; Plymate, S.; Penning, T.M. The Androgen Receptor. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2023; Volume 123, pp. 439–481. [Google Scholar] [CrossRef]
- Vasseur, D.; Arbab, A.; Giudici, F.; Marzac, C.; Michiels, S.; Tagliamento, M.; Bayle, A.; Smolenschi, C.; Sakkal, M.; Aldea, M.; et al. Genomic Landscape of Liquid Biopsy Mutations in TP53 and DNA Damage Genes in Cancer Patients. npj Precis. Oncol. 2024, 8, 51. [Google Scholar] [CrossRef]
- Heath, A.P.; Ferretti, V.; Agrawal, S.; An, M.; Angelakos, J.C.; Arya, R.; Bajari, R.; Baqar, B.; Barnowski, J.H.B.; Burt, J.; et al. The NCI Genomic Data Commons. Nat. Genet. 2021, 53, 257–262. [Google Scholar] [CrossRef]
- Huang, M.-F.; Wang, Y.-X.; Chou, Y.-T.; Lee, D.-F. Therapeutic Strategies for RB1-Deficient Cancers: Intersecting Gene Regulation and Targeted Therapy. Cancers 2024, 16, 1558. [Google Scholar] [CrossRef]
- Sanchez, G.M.; Chigane, D.; Lin, M.; Xu, L.; Yellapantula, V.; Berry, J.L. Retinoblastoma: Aqueous Humor Liquid Biopsy. Taiwan J. Ophthalmol. 2025, 15, 55–61. [Google Scholar] [CrossRef]
- Lee, D.H.; Yoon, H.; Park, S.; Kim, J.S.; Ahn, Y.-H.; Kwon, K.; Lee, D.; Kim, K.H. Urinary Exosomal and Cell-Free DNA Detects Somatic Mutation and Copy Number Alteration in Urothelial Carcinoma of Bladder. Sci. Rep. 2018, 8, 14707. [Google Scholar] [CrossRef]
- Sahin, I.; Saat, H.; Aksoy, S.; Dizdar, O.; Erdem, H.B.; Bahsi, T. Liquid Biopsy: Novel Perspectives on the Importance and Spectrum of PIK3CA, PTEN and RET Mutations in Solid Tumors. Mol. Clin. Oncol. 2022, 16, 1. [Google Scholar] [CrossRef]
- Wang, Y.; Lieberman, R.; Pan, J.; Zhang, Q.; Du, M.; Zhang, P.; Nevalainen, M.; Kohli, M.; Shenoy, N.K.; Meng, H.; et al. miR-375 Induces Docetaxel Resistance in Prostate Cancer by Targeting SEC23A and YAP1. Mol. Cancer 2016, 15, 70. [Google Scholar] [CrossRef]
- Gan, J.; Liu, S.; Zhang, Y.; He, L.; Bai, L.; Liao, R.; Zhao, J.; Guo, M.; Jiang, W.; Li, J.; et al. MicroRNA-375 Is a Therapeutic Target for Castration-Resistant Prostate Cancer through the PTPN4/STAT3 Axis. Exp. Mol. Med. 2022, 54, 1290–1305. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Ye, M.; Dai, X.; Zhu, X.; Wei, W. The Diverse Roles of SPOP in Prostate Cancer and Kidney Cancer. Nat. Rev. Urol. 2020, 17, 339–350. [Google Scholar] [CrossRef]
- An, J.; Wang, C.; Deng, Y.; Yu, L.; Huang, H. Destruction of Full-Length Androgen Receptor by Wild-Type SPOP, but Not Prostate-Cancer-Associated Mutants. Cell Rep. 2014, 6, 657–669. [Google Scholar] [CrossRef]
- McGrath, C.B.; Shreves, A.H.; Shanahan, M.R.; Guard, H.E.; Nhliziyo, M.V.; Pernar, C.H.; Penney, K.L.; Lotan, T.L.; Fiorentino, M.; Mucci, L.A.; et al. Etiology of Prostate Cancer with the TMPRSS2:ERG Fusion: A Systematic Review of Risk Factors. Int. J. Cancer 2025, 156, 1898–1908. [Google Scholar] [CrossRef]
- Abramovic, I.; Ulamec, M.; Bojanac, A.K.; Bulic-Jakus, F.; Jezek, D.; Sincic, N. miRNA in Prostate Cancer: Challenges toward Translation. Epigenomics 2020, 12, 543–558. [Google Scholar] [CrossRef]
- Smith, Z.L.; Eggener, S.E.; Murphy, A.B. African-American Prostate Cancer Disparities. Curr. Urol. Rep. 2017, 18, 81. [Google Scholar] [CrossRef]
- Ahmad, H.S.; Zafer, S.; Qadri, H.M.; Bashir, A. Limitless Potential within Limited Resources: The Realm of Liquid Biopsy for Brain Tumors in Low-Middle-Income Countries. Brain Spine 2024, 4, 102817. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Wu, J.; Cheng, G.; Yang, H.; Hua, L.; Wang, Z. Prognostic Value of Circulating Tumor Cells in Castration Resistant Prostate Cancer: A Meta-Analysis. Urol. J. 2016, 13, 2881–2888. [Google Scholar] [CrossRef]
- Khan, T.; Becker, T.M.; Scott, K.F.; Descallar, J.; de Souza, P.; Chua, W.; Ma, Y. Prognostic and Predictive Value of Liquid Biopsy-Derived Androgen Receptor Variant 7 (AR-V7) in Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 868031. [Google Scholar] [CrossRef]
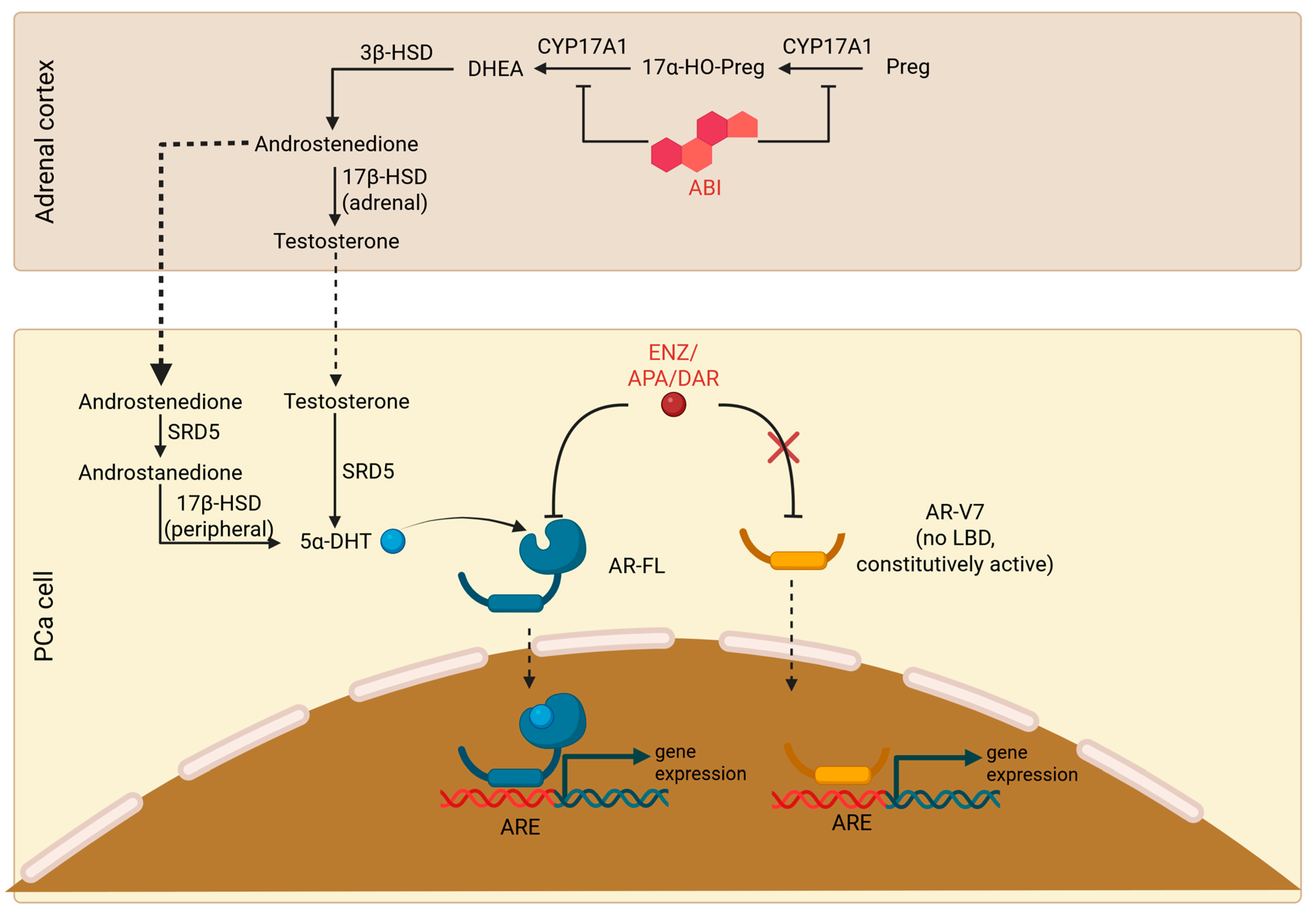
| Marker | Type | Outcome | Treatment | Previous CRPC Treatment | Population | Prevalence of Alteration | Significant in MV Analysis | Follows Checklist | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ≥1 CTC/7.5 mL blood (CellSearch®) | FT | PFS HR 2.51 (1.67–3.75), OS HR 5.02 (2.64–9.55) | ABI/ENZ | Any | Belgium | 69% | Yes | Yes | [30] |
| ≥1 CTC/7.5 mL blood (CellSearch®) | FT | rPFS HR 1.940 (1.01–3.73), OS HR 2.50 (1.12–5.59) | ABI | NR | China | 68.40% | Yes | No | [31] |
| ≥3 CTC/mL blood (CellSearch®) | FT | OS HR 2.3 (1.6–3.3) (discovery cohort) | ABI/ENZ | Prior ARSI or taxanes allowed | United States | 28.70% | Yes | Yes | [32] |
| ≥3 CTC/mL blood (CellSearch®) | FT | PFS HR 2.2 (1.4–3.3) OS HR 2.5 (1.6–3.9) (validation cohort) | ABI/ENZ | Prior ARSI or taxanes allowed | United States | 36% | Yes | Yes | [32] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | FT | rPFS HR 2.35 (1.14–4.84), OS HR 3.08 (1.45–6.54) | ENZ | Taxanes/ABI | Italy | 42.2% | No | Yes | [33] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | Abs | median rPFS 6.8 vs. 11 mo., median OS 13.6 mo. vs. not reached | ENZ | Post-DTX | United States | 49.50% | NR | Yes | [34] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | FT | rPFS HR 1.95 (1.03–3.70), OS HR 2.10 (0.845–5.20) | ABI | NR | China | 22.50% | Yes | No | [31] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | Abs | lower OS (p = 0.001) | ABI | NR | United States | 34.30% | NR | Yes | [35] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | FT | crPFS HR 2.475 (1.72–3.67), OS HR 4.75 (2.76–8.27) | ABI/ENZ | None/DTX/ARSI | Belgium | 40.60% | As continuous variable | Yes | [36] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | Abs | rPFS HR 3.8 (p = 0.02) | ABI | Prior ChT allowed | Germany | 27.6% of all; 40% of CTC+ | NR | Yes | [37] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | Abs | OS HR 1.92 (0.99–3.7) | ABI | No prior ChT | United States | NR | NR | Yes | [38] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | FT | median OS 48 vs. 122 wk. (p < 0.001) | ABI | Prior ARSI or ChT allowed | United States | 73% | NR | No | [39] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | Abs | Not significant | ABI/ENZ | NR | Italy | 32% | NR | Yes | [40] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | FT | OS HR 3.42 (1.53–7.65) | ABI | None (first-line) | United States | 39.10% | No | Yes | [41] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | Abs | OS HR 2.97 (p = 0.0012) | ABI | NR | United States | 39.40% | NR | Yes | [42] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | FT | median PFS ~3 mo. vs. not reached | ABI/ENZ | NR | United States | NR | NR | No | [43] |
| ≥5 CTC/7.5 mL blood (CellSearch®) | Abs | OS HR 3.42 (p = 0.02) | ABI | None (first-line) | United States | NR | NR | Yes | [44] |
| ≥5 CTC/7.5 mL blood (flow cytometry-based assay) | Abs | rPFS HR 2.29 (1.0165–5.145), OS HR 3.06 (1.135–8.27) | ENZ | NR | Italy | NR | NR | Yes | [45] |
| CTC as a continuous variable (CellSearch® assay) | FT | PFS HR 1.33 (1.14–1.55) (multivariate) | ABI/ENZ | Prior ARSI or ChT allowed | Belgium | NA | Yes | Yes | [46] |
| CTC+ (AdnaTest®, Venlo, The Netherlands) | FT | rPFS HR 5.21 (2.82–9.64), PSA PFS HR 3.37 (2.01–5.56), OS HR 4.68 (2.64–8.28) | ENZ | None | Spain | 36% | Yes | Yes | [47] |
| CTC+ (AdnaTest®) | FT | PFS HR 2.37 (0.97–5.71) | ENZ | None/DTX/ARSI | Japan | 56.50% | NR | No | [48] |
| CTC+ (AdnaTest®) | FT | rPFS HR 4.38 (1.7–11.3), PSA PFS HR 3.85 (1.64–9.01), OS HR 6.21 (1.38–28.17) | ABI/ENZ | Prior ARSI or ChT allowed | Italy | 56.80% | NR | No | [49] |
| CTC+ (AdnaTest®) | FT | median PFS 16 mo. vs. not reached (p = 0.02), median OS 29 mo. vs. not reached (p = 0.05) | ABI/ENZ | None (first-line) | Italy | 43.2% | NR | No | [50] |
| CTC+ (AdnaTest®) | FT | OS HR 3.03 (1.11–8.23) | ENZ | Prior ABI or DTX allowed | Japan | 60.50% | NR | Yes | [51] |
| CTC+ (Dynabeads®) | FT | PFS HR 3.97 (1.52–10.37), OS HR 5.24 (1.86–14.81) | ABI/ENZ | Prior ARSI or ChT allowed | Germany | 73.70% | NR | No | [52] |
| CTC+ (Epic Sciences®, San Diego, CA, USA) | Abs | OS HR 1.58 (0.31–7.90) (multivariate) | ABI/ENZ | NR | United States | 69% | NA | Yes | [29] |
| CTC conversion from ≥5/7.5 mL to <−5/7.5 m at week 12 | FT | OS HR 3.76 (p < 0.001) | ABI | Post-DTX | Multicenter | 18.1% | Yes | Yes | [68] |
| CTC conversion (≥5 to <5/7.5 mL) at week 9 (CellSearch®) | Abs | rPFS HR 0.16 (0.07–0.40) | ENZ | None (first-line) | Multicenter | NR | NR | Yes | [69] |
| CTC conversion from (≥5 to <5/7.5 mL) at week 17 (CellSearch®) | Abs | rPFS HR 0.26 (0.11–0.59) | ENZ | None (first-line) | Multicenter | NR | NR | Yes | [69] |
| CTC negativation (>0 to 0) at week 9 (CellSearch®) | Abs | rPFS HR 0.41 (0.24–0.69) | ENZ | None (first-line) | Multicenter | NR | NR | Yes | [69] |
| CTC negativation (>0 to 0) at week 17 (CellSearch®) | Abs | rPFS HR 0.36 (0.20–0.64) | ENZ | None (first-line) | Multicenter | NR | NR | Yes | [69] |
| No CTC negativation during treatment (AdnaTest®) | FT | OS HR 3.97 (1.36–11.67) | ENZ | Prior ABI or DTX allowed | Japan | 46.2% of initially CTC+ | Yes | Yes | [51] |
| Marker | Type | Outcome | Treatment | Previous CRPC Treatment | Population/Region | Prevalence of Alteration | Significant in MV Analysis | Follows Checklist | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ctDNA fraction > 18.0% | FT | PSA PFS HR 4.64 (1.53–14.06), OS HR 3.50 (1.14–10.77) (MV) | ABI/ENZ | Prior DTX allowed | Italy | 50% (median as threshold) | NA | No | [54] |
| ctDNA fraction high | Abs | OS HR 2.5 (p < 0.001) | APA + ABI | NR | Multicenter | 63% | Yes | Yes | [55] |
| ctDNA fraction > 2% | FT | TTP HR 3.56 (2.28–5.57), OS HR 12.92 (5.68–29.42) | ABI/ENZ | None (first line) | Canada | 20.9% | NR | Yes | [56] |
| ctDNA fraction > 30% | FT | TTP HR 2.05 (1.42–2.96), OS HR 7.51 (3.41–16.57) | ABI/ENZ | None (first line) | Canada | 57.2% | Yes | Yes | [56] |
| ctDNA fraction > 18.8% | FT | PFS HR 1.91 (1.13–3.21), OS HR 2.34 (1.32–4.12) (MV) | ABI/ENZ | Prior ARSIs or taxanes allowed | Italy | 50% (median as threshold) | NA | Yes | [57] |
| ctDNA fraction > 1% | FT | PFS HR 2.48 (1.45–4.23), OS HR 3.56 (1.89–6.72) | ABI/ENZ | No prior ChT | Netherlands | 59.3% | NR | Yes | [58] |
| ctDNA fraction > 30% | FT | PFS HR 6.26 (3.03–12.94), OS HR 8.12 (3.75–17.61) | ABI/ENZ | No prior ChT | Netherlands | 19.8% | NR | Yes | [58] |
| ctDNA mutant allele fraction > 7% | FT | PFS HR 1.76 (1.03–3.01), OS HR 2.92 (1.4–6.11) | ABI/ENZ | Prior ChT or ARSI allowed | United States | 43.50% | NR | Yes | [59] |
| ctDNA fraction high | FT | OS HR 1.04 (1.01–1.07) | ABI | None (first-line) | United States | NR | NR | Yes | [60] |
| ctDNA genomic complexity high (equivalent to >10% ctDNA) | FT | PFS HR 5.59 (1.03–30.28) | ABI/ENZ | Prior DTX or ARSI allowed | Austria + United States | 40% | NR | Yes | [61] |
| ctDNA fraction ≥2% | Abs | TTPP HR 2.04 (1.43–2.90), OS HR 4.07 (2.40–6.91) | ABI/ENZ | NR | Canada | NR | NR | No | [62] |
| ctDNA fraction ≥18.1% | FT | rPFS HR 1.83 (1.15–2.94), OS HR 2.23 (1.21–4.09) | ABI | Prior ENZ/orteronel/taxanes allowed | Italy + United Kingdom | 50% (median as threshold) | Yes | Yes | [63] |
| ctDNA positive | FT | rPFS HR 1.8 (1.20–2.96), OS HR 3.01 (1.88–4.83) | ABI | NR | Multicenter | 46.9% | Yes | Yes | [64] |
| ctDNA fraction 3.2–21.1% relative to <3.2% | FT | PSA PFS HR 1.80 (1.13–2.87), OS HR 1.94 (1.07–3.52) | ABI/ENZ | Prior DTX in castration-sensitive context allowed | Denmark | NR | Yes | Yes | [53] |
| ctDNA fraction >21.1% relative to <3.2% | FT | PSA PFS HR 4.075 (2.50–6.65), OS HR 5.08 (2.77–9.31) | ABI/ENZ | Prior DTX in castration-sensitive context allowed | Denmark | NR | Yes | Yes | [53] |
| cfDNA concentration > 3.4 ng/mL | FT | median OS ~19 mo. vs. not reached (p = 0.01) | ABI/ENZ | None (first line) | Netherlands | 50% (median as threshold) | NR | No | [66] |
| cfDNA concentration > 38.5 ng/mL | FT | OS HR 4.24 (2.64–6.79) | ABI/ENZ | Prior DTX allowed | Italy | 50.50% | NR | Yes | [67] |
| cfDNA concentration > 8.4 ng/mL | FT | PSA PFS HR 2.0 (1.1–3.5), crPFS HR 2.4 (1.3–4.2), OS HR 3.3 (1.7–6.6) | ABI/ENZ | Prior ARSI/ChT allowed | Australia | 50% (median as threshold) | Yes | No | [65] |
| Article Type | Source | Outcome | Treatment | Previous CRPC Treatment | Population/Region | Prevalence of Alteration | Significant in MV Analysis | Follows Checklist | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| FT | CTC | rPFS 7.4 mo. vs. 14.6 mo. PSA-PFS 8.2 mo. vs. 12.6 mo., OS 25.8 mo. vs. 25.4 mo. | ENZ | None (first-line) | Spain | 16% of CTC+ (5.8% of total) | NR | Yes | [47] |
| FT | CTC | median crPFS 4.3 mo. vs. 6.1 mo. (p = 0.11) | BAT + ABI/ENZ | ABI/ENZ | United States | 19.2% | NR | No | [72] |
| FT | CTC | Worse PFS (p = 0.005), Worse OS (p = 0.0055) | ENZ | None (first-line) | Italy | 24% | NR | No | [73] |
| FT | Exosomal RNA | median PFS 5.4 mo. vs. 24.3 mo. (p < 0.0001), median OS 16.2 mo. vs. not reached (p < 0.0001) | ABI/ENZ | None (first-line) | Italy | 36% | Yes | No | [74] |
| FT | Exosomal RNA | median PFS 4 mo. vs 24 mo. (p < 0.0001), median OS 9 mo. vs. not reached (p < 0.0001) | ABI/ENZ | Prior taxanes allowed, no prior ARSI | Italy | 22% | NR | No | [75] |
| Abs | CTC | PSA PFS HR 7.4 (2.7–20.6) | ENZ | NR | United States | 38.7% | Yes | Yes | [76] |
| FT | CTC | PSA PFS HR 7.4 (2.7–20.6), crPFS HR 8.5 (2.8–25.5), OS HR 6.9 (1.7–28.1) | ENZ | Prior taxanes or ARSI allowed | United States | 39% | PSA PFS and crPFS | Yes | [12] |
| FT | CTC | PSA PFS HR 6.1 (3.9–66.0) crPFS HR 16.5 (3.3–82.9), OS HR 12.7 (1.3–125.3) | ABI | Prior taxanes or ARSI allowed | United States | 19% | PSA PFS and crPFS | Yes | [12] |
| FT | Whole-blood mRNA | median PSA PFS 2.4 mo. vs. 3.7 mo. (p < 0.001), median cPFS 2.7 mo. vs. 5.5 mo. (p < 0.001), median OS 4 mo. vs. 13.9 mo. (p < 0.001) | ABI/ENZ | Prior DTX, CBZ, or ABI allowed | Germany | 18% | Yes | No | [77] |
| FT | Whole-blood mRNA | PSA PFS HR 8.8 (2.3–29.8), OS HR 6.8 (1.8–22.0) | ABI | Prior DTX or CBZ allowed | Canada | 11.1% | Yes | No | [78] |
| FT | CTC | rPFS HR 5.05 (2.4–10.64), OS HR 2.25 (1.1–4.58) | ENZ | Prior taxanes or ABI allowed | Italy | 48.9% | Yes | Yes | [33] |
| FT | Whole-blood mRNA | TTF HR 1.73 (0.83–3.60) (3rd vs. 1st and 2nd tertile) (MV) | ABI | Prior taxanes or ABI allowed | United States | 33% | NA | Yes | [79] |
| FT | Whole-blood mRNA | TTF HR 2.08 (0.83–5.24) (3rd vs. 1st and 2nd tertile) (MV) | ENZ | Prior taxanes or ABI allowed | United States | 33% | NA | Yes | [79] |
| FT | cfDNA | rPFS HR 3.2 (1.4–7.4) | ABI/ENZ | Prior ABI/ENZ allowed | United States | 18% of pts. with AR-V7-negative CTCs | NR | Yes | [80] |
| FT | CTC (in-house assay) | rPFS HR 2.3 (1.5–3.5), OS HR 2.8 (1.7–4.5) | ABI/ENZ | Prior ABI/ENZ allowed | United States | 24% | Yes | Yes | [81] |
| FT | CTC (Epic Sciences® assay, San Diego, CA, USA) | rPFS HR 2.2 (1.2–4.3), OS HR 3.1 (1.6–5.9) | ABI/ENZ | Prior ABI/ENZ allowed | United States | 10% | Yes | Yes | [81] |
| FT | CTC | PFS HR 8.56 (2.40–30.43) | ENZ | None/DTX/ARSI | Japan | 8.7% (15.4% of CTC+) | NR | No | [48] |
| FT | CTC | median PSA PFS ~55 vs. ~145 days (p = 0.011), median crPFS ~30 vs. ~200 days (p = 0.004) | ABI/ENZ | NR | United States | NR | NR | No | [82] |
| FT | cfDNA and cfRNA | PSA PFS HR 2.1 (0.81–5.9), crPFS HR 2.4 (0.82–6.9), OS HR 3.5 (1.1–11) | ABI/ENZ | Prior ARSI or ChT allowed | Australia | 16.4% | No | Yes | [83] |
| Abs | Exosomal RNA | PSA PFS HR 4.08 (1.13–14.69) | ABI | NR | China | 39.1% | NR | No | [84] |
| Abs | CTC | PSA PFS 1.00 (0.24–4.18) | ABI | NR | China | 36.4% | NR | No | [84] |
| FT | CTC | crPFS HR 2.685 (1.44–5.02), OS HR 2.95 (1.63–5.345) | ABI/ENZ | Prior ARSI or ChT allowed | Germany | 50.8% (61.1% of CTC+) | No | Yes | [85] |
| FT | CTC | rPFS HR 4.00 (1.67–9.41), PSA PFS HR 2.98 (1.34–6.61), OS HR 11.1 (2.43–50.63) | ABI/ENZ | Prior ARSI or ChT allowed | Italy | 45.9% | NR | No | [49] |
| FT | CTC (conventional AdnaTest®) | rPFS HR 2.1 (0.6–7), PSA PFS HR 3.1 (1.0–9.2) | ABI/ENZ | Prior ChT/ARSI/radium allowed | France | 22% | Only PSA PFS | No | [86] |
| FT | CTC (highly sensitive assay) | rPFS HR 7.3 (1.6–33), PSA PFS HR 10.8 (2.4–48.2) | ABI/ENZ | Prior ChT/ARSI/radium allowed | France | 56.1% | Yes | No | [86] |
| Abs | CTC | PSA PFS HR 4.35 (1.27–14.91), crPFS HR 5.43 (1.52–19.35) | ABI/ENZ | NR | United States | NR | NR | Yes | [87] |
| FT | Plasma RNA | PFS HR 1.37 (0.53–3.57) | ABI | Prior DTX allowed | Netherlands | 11.3% | NR | Yes | [88] |
| FT | CTC | PFS HR 4.27 (1.77–10.27) | ABI/ENZ/APA | Prior ARSI/taxanes allowed | United States | NR | Yes | Yes | [89] |
| FT | Exosomal RNA | median PFS 16.0 vs. 28.0 mo. (p = 0.049), median OS 22.9 vs. 32.9 mo. (p = 0.111) | ABI/ENZ | None (first-line) | Canada | 34.3% | NR | No | [90] |
| FT | CTC | median PFS 13 mo. vs. 16 mo. (p = 0.89), median OS NR vs. 29 mo.(p = 0.33) (relative to CTC+/AR-V7-) | ABI/ENZ | None (first-line) | Italy | 6.8% | NR | No | [50] |
| Abs | CTC | PFS log-rank p = 0.055, OS log-rank p = 0.02 | ABI/ENZ | NR | Italy | 14.3% | NR | Yes | [40] |
| FT | CTC | median rPFS ~200 vs. ~430 days (p = 0.037), median PSA PFS ~130 vs. ~455 days (p < 0.0001), median cPFS ~215 zs ~425 days (p = 0.012), median cancer-specific survival ~1085 vs. ~1585 days (p = 0.474) | ABI | None (first-line) | China | 35.8% | Yes | Yes | [91] |
| FT | CTC | median PFS 3 mo. vs. 20 mo. (p < 0.001), median OS 8 mo. vs. not reached (p < 0.001) | ABI/ENZ | Prior ABI or taxanes allowed | Italy | 38.9% | No | Yes | [71] |
| Marker | Article Type | Source | Outcome | Treatment | Previous CRPC Treatment | Population/Region | Prevalence of Alteration | Significant in MV Analysis | Follows Checklist | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| AR gain | FT | cfDNA | rPFS HR 9.83 (4.5–21.44), PSA PFS HR 4.03 (1.87–8.72), OS HR 6.65 (3.18–13.91) | ENZ | None (first line) | Spain | 11% | Yes | Yes | [47] |
| FT | cfDNA | median PFS 4.8 mo. vs. 24.3 mo. (p < 0.0001), median OS 8.17 mo. vs. not reached (p < 0.0001) | ABI/ENZ | None | Italy | 14% | Yes | No | [74] | |
| FT | cfDNA | PFS HR 2.18 (1.08–4.39), OS HR 3.98 (1.74–9.10) | ABI/ENZ | No prior taxanes | Italy + United Kingdom | NR | Yes | Yes | [92] | |
| FT | cfDNA | PFS HR 1.95 (1.23–3.11) OS HR 3.81 (2.28–6.37) | ABI/ENZ | Post-DTX | Italy + United Kingdom | NR | Yes | Yes | [92] | |
| FT | CTC | rPFS HR 1.6 (0.8–3.2) | ABI/ENZ | Prior ABI/ENZ allowed | United States | 45% | NR | Yes | [80] | |
| FT | cfDNA | PSA PFS HR 1.72 (1.05–2.81), OS HR 1.44 (0.86–2.40) (multivariate) | ABI/ENZ | Prior DTX allowed | Italy | 33% | NA | No | [54] | |
| FT | cfDNA | median PFS 5.4 mo. vs. 9.1 mo. (p = 0.0005), median OS 9.1 mo. vs. 27.3 mo. (p < 0.0001) | ABI/ENZ | Pre- or post-DTX | Italy | 21% | NR | No | [93] | |
| FT | cfDNA | PFS HR 2.68 (1.49–4.82), OS HR 2.09 (1.07–4.10) (MV) | ABI/ENZ | Post-DTX | Italy | 30% | NA | Yes | [94] | |
| FT | cfDNA | PFS HR 2.79 (1.55−5.02), OS HR 3.23 (1.64−6.35) | ENZ | DTX in all, ABI in 48% | Italy | 36% | Yes | Yes | [95] | |
| FT | cfDNA | PFS HR 3.73 (1.95–7.13), OS HR 4.68 (2.17–10.10) | ABI | Post-DTX | Italy | 30% | Yes | Yes | [96] | |
| FT | CTC | median PSA PFS ~70 vs. ~400 days (p = 0.006), median crPFS ~100 vs. ~655 days (p = 0.029) | ABI/ENZ | NR | United States | NR | NR | No | [82] | |
| Abs | cfDNA | lower OS (p < 0.0001) | ABI | NR | United States | 27% | NR | Yes | [35] | |
| FT | cfDNA | Any CN gain: TTP HR 2.05 (1.43–2.93), CN ≥ 8: TTP HR 2.65 (1.68–4.19), CN < 8: TTP HR 1.67 (1.07–2.62) | ABI/ENZ | None (first line) | Canada | Any CN: 33.2%, CN ≥ 8: 15.3%, CN < 8: 17.8% | No | Yes | [56] | |
| FT | cfDNA | PSA PFS HR 2.8 (1.3–6.1), crPFS HR 3.4 (1.4–8.2), OS HR 3.2 (1.2–8.5) | ABI/ENZ | Prior ARSI or ChT allowed | Australia | 39% | Yes | Yes | [83] | |
| FT | cfDNA | PFS HR 2.07 (1.2–3.57), OS HR 3.26 (1.52–7) | ABI/ENZ | Prior ARSI or ChT allowed | United States | 52% | No | Yes | [59] | |
| Abs | cfDNA | PFS HR 2.92 (1.59–5.37) | ENZ | NR | Canada | NR | NR | No | [97] | |
| FT | cfDNA | OS HR 1.56 (0.99–2.46) | ABI | None (first-line) | United States | NR | No | Yes | [60] | |
| FT | cfDNA | PFS HR 2.2 (1.2–4.4), OS HR 2.9 (1.5–5.7) | ABI/ENZ | Prior ARSI or ChT allowed | Australia | 22% | Only PFS | No | [98] | |
| FT | cfDNA | OS HR 2.64 (1.60–4.35) | ABI/ENZ | Prior DTX allowed | Italy | 26% | Yes | Yes | [67] | |
| FT | cfDNA | rPFS HR 2.0 (0.97–4.405), cPFS HR 1.95 (0.897–3.87), OS HR 2.37 (1.07–5.25) (MV) | ABI/ENZ | None (first-line) | Multicenter | 17% | NA | Yes | [99] | |
| FT | cfDNA | rPFS HR 3.52 (2.02–6.13), OS HR 7.09 (3.34–15.05) | ABI | Prior ENZ/orteronel/taxanes allowed | Italy + United Kingdom | 37% | Yes | Yes | [63] | |
| FT | cfDNA | cPFS HR 2.5 (1.3–4.8), OS HR 1.9 (0.99–3.8) | ABI/ENZ | Prior ARSI or ChT allowed | Australia | 42% | Yes | Yes | [100] | |
| FT | cfDNA | rPFS HR 1.98 (1.23–3.18), OS HR 1.96 (1.17–3.29) | ABI | NR | Multicenter | 21.1% | No | Yes | [64] | |
| FT | cfDNA | PFS HR 6.67 (1.52–33.33), OS HR 8.33 (0.96–100) | ABI | Prior treatment allowed | Belgium | NR | Yes | Yes | [101] | |
| FT | cfDNA | PFS HR 4.76 (1.41–16.67) OS HR 4.55 (0.94–20) | ENZ | Prior treatment allowed | Belgium | NR | Yes | Yes | [101] | |
| FT | cfDNA | OS HR 5.25 (2.21–12.46) | ABI | None (first-line) | United States | 37% | No | Yes | [41] | |
| FT | cfDNA | median PFS 5.3 mo. vs. 9 mo.(p = 0.0001) | ABI | Prior DTX | Italy | 38.9% | Yes | Yes | [102] | |
| FT | cfDNA | median PFS 2.8 mo. vs. 4.9 mo. (p = 0.0001) | ENZ | Prior DTX | Italy | 32.40% | Yes | Yes | [102] | |
| FT | cfDNA | PFS HR 3.91 (1.29–11.28) | ABI | Prior treatment allowed | China | 9% | NR | No | [103] | |
| AR over-expression | Abs | CTC | PSA PFS HR 3.61 (1.06–12.29), crPFS HR 4.91 (1.38–17.48) | ABI/ENZ | NR | United States | NR | NR | Yes | [87] |
| FT | CTC | median PFS 16 mo. vs. 13 mo. (p = 0.99), median OS 29 mo. vs. not reached (p = 0.72) | ABI/ENZ | None (first-line) | Italy | 27.3% | NR | No | [50] | |
| Abs | CTC | AR-FL negative vs. below median vs. above median: median PSA PFS 9.6 vs. 6.2 vs. 2.5 mo., median cPFS 11.1 vs. 8.7 vs. 3.2 mo., median OS 33.3 vs. 18.0 vs. 11.3 mo. (all p < 0.001) (multivariate) | ABI/ENZ | NR | United States | AR-FL positive: 52% | NA | Yes | [104] | |
| FT | Exosomal RNA | AR high vs. intermediate vs. low: median PFS 4 mo. vs. 18 mo. vs. 22 mo. (p = 0.014) | ABI/ENZ | Prior taxanes allowed, no prior ARSI | Italy | NR | NR | No | [75] |
| Source | Best Studied Biomarkers | Relatively Well-Studied Biomarkers | Understudied Biomarkers or Contradictory Evidence | Biomarkers not Supported by Current Evidence |
|---|---|---|---|---|
| cfDNA | AR-V7, AR gain, ctDNA fraction | More promising: PTEN alteration/loss, RB1 alteration/loss, TP53 alteration/loss Less studied or conflicting evidence: cfDNA concentration, AR point mutations, unspecified AR alterations, PIK3CA gain, PI3K pathway alterations, WNT pathway alterations, genome-wide aneuploidy | APC, BRAF, BRCA2 gain, BRCA2 alteration, CDK12, CYP17A1, MET, MYC, NCOA2, NCOR1, NF1, NKX3-1, OPHN1, ZFHX3, GSTP1, AR or enhancer alterations, AR or BRAF alteration, AR plus NCOA2 alteration, AR-V7 or V9, BRCA2 or ATM alterations, SPOP or CDH1 alteration, DDR pathway alterations, HRR pathway alterations, chr8 and 13q deletion, chr7 and 9q amplification, No. of driver gene mutations/no. of pathways affected | SPOP, DNA methylation patterns at baseline |
| CTC | AR-V7, AR gain or overexpression, CTC counts | Nuclear localized AR-V7, unspecified AR-V PTEN alteration/loss KLK3 (PSA), PSMA | ANXA2, BMP7, GAS6, GR, HER2, HOXB13, NKX3-1, OCT4, PSCA, RUNX2, SOX2, SPINK1, SYP, TSPAN8, WNT5B, CTC clusters/AR-V7 high, KLK3 high/AR-V7 high, PSA+/PSMA+ high, chromosomal instability phenotype, phenotypic heterogeneity | MYCN, TMPRSS2-ERG fusion |
| Exosomal or plasma RNA | AR-V7, AR overexpression | Unspecified AR alterations, KLK3, miR-375 | AKR1C3, hsa_circ_0113177, 0127731, 0002048, 0097211, 0116020, 0002910 miR-21, -103a, -141, -221, -223, -3687 NAALADL2-AS2, PD-L1, TUBB3, AR-V7 or V9, tdEV concentration | |
| Whole-blood RNA | AR-V7 | KLK3 | AR-V12 or V14, FOXA1, GRHL2, HOXB13, KLK2 | TMPRSS2-ERG fusion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badulescu, A.-V.; Rahota, R.; Vigdorovits, A.; Pop, O.L. Liquid Biopsy Biomarkers in Metastatic Castration-Resistant Prostate Cancer Treated with Second-Generation Antiandrogens: Ready for Clinical Practice? A Systematic Review. Cancers 2025, 17, 2482. https://doi.org/10.3390/cancers17152482
Badulescu A-V, Rahota R, Vigdorovits A, Pop OL. Liquid Biopsy Biomarkers in Metastatic Castration-Resistant Prostate Cancer Treated with Second-Generation Antiandrogens: Ready for Clinical Practice? A Systematic Review. Cancers. 2025; 17(15):2482. https://doi.org/10.3390/cancers17152482
Chicago/Turabian StyleBadulescu, Andrei-Vlad, Razvan Rahota, Alon Vigdorovits, and Ovidiu Laurean Pop. 2025. "Liquid Biopsy Biomarkers in Metastatic Castration-Resistant Prostate Cancer Treated with Second-Generation Antiandrogens: Ready for Clinical Practice? A Systematic Review" Cancers 17, no. 15: 2482. https://doi.org/10.3390/cancers17152482
APA StyleBadulescu, A.-V., Rahota, R., Vigdorovits, A., & Pop, O. L. (2025). Liquid Biopsy Biomarkers in Metastatic Castration-Resistant Prostate Cancer Treated with Second-Generation Antiandrogens: Ready for Clinical Practice? A Systematic Review. Cancers, 17(15), 2482. https://doi.org/10.3390/cancers17152482







