Optimizing Treatment Precision: Role of Adaptive Radiotherapy in Modern Anal Cancer Management
Simple Summary
Abstract
1. Introduction
2. Fundamentals of Adaptive Radiotherapy
3. Radiation Delivery Techniques
4. Technical Considerations in Adaptive Radiotherapy
5. Implementation Challenges
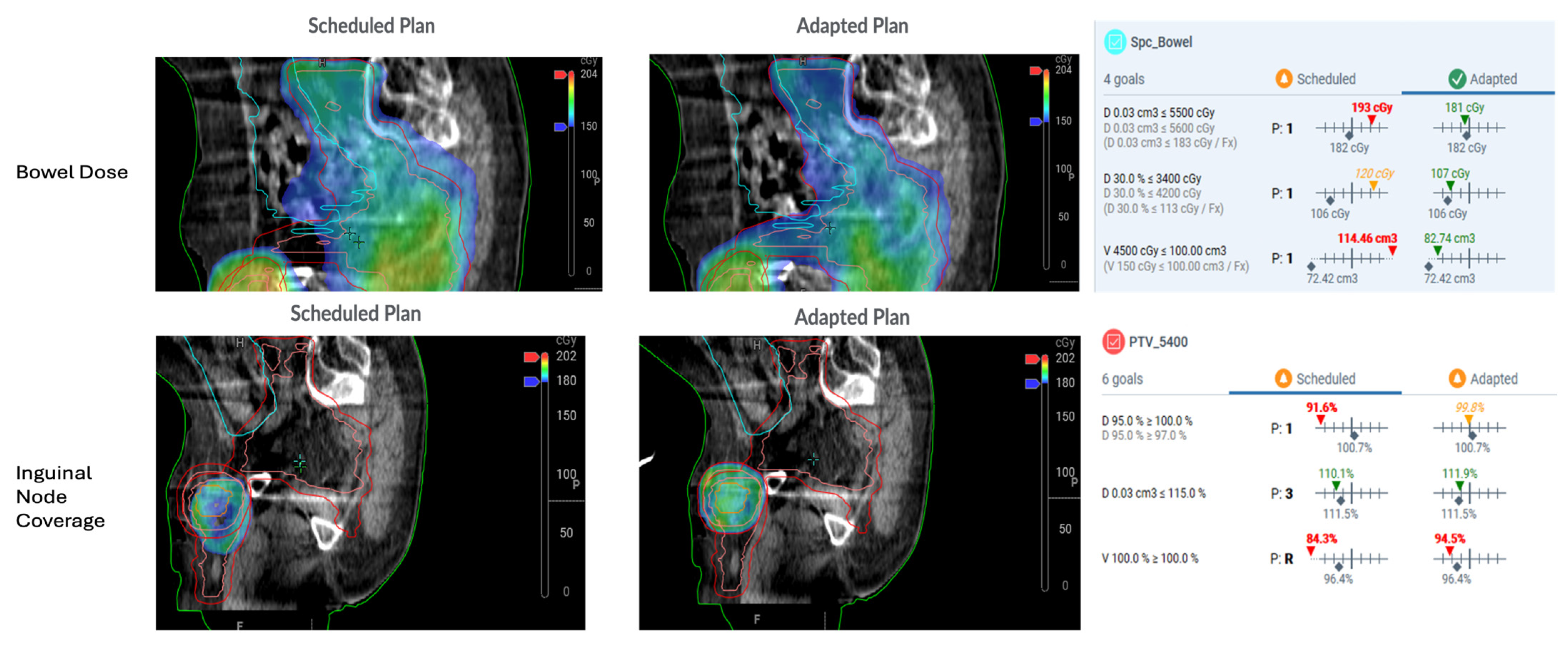
6. Clinical Treatment Planning for Adaptive Radiotherapy in Anal Cancer
7. Clinical Outcomes and Toxicity Benefits
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Feng, M.; Hallemeier, C.L.; Almada, C.; Aranha, O.; Dorth, J.; Felder, S.; Goodman, K.A.; Holliday, E.B.; Jethwa, K.R.; Kachnic, L.A.; et al. Radiation Therapy for Anal Squamous Cell Carcinoma: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2025, 15, 367–386. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Amin, M.A.; Aranha, O.; Benson, A.B., 3rd; Dorth, J.A.; Horowitz, D.P.; Kennecke, H.F.; Kim, S.; Kreppel, L.; et al. Systemic Therapy for Stage I-III Anal Squamous Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. 2025, 43, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Horowitz, D.P.; Kachnic, L.A. Acute and Chronic Complications After Treatment of Locoregional Anal Cancer: Prevention and Management Strategies. J. Natl. Compr. Canc. Netw. 2023, 21, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, C.J.S.; Christensen, P.; Pedersen, B.G.; Spindler, K.G. Anorectal function and radiation dose to pelvic floor muscles after primary treatment for anal cancer. Radiother. Oncol. 2021, 157, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, A.G.; Guren, M.G.; Vonen, B.; Wanderas, E.H.; Frykholm, G.; Wilsgaard, T.; Dahl, O.; Balteskard, L. Faecal incontinence after chemoradiotherapy in anal cancer survivors: Long-term results of a national cohort. Radiother. Oncol. 2013, 108, 55–60. [Google Scholar] [CrossRef]
- Bentzen, A.G.; Balteskard, L.; Wanderas, E.H.; Frykholm, G.; Wilsgaard, T.; Dahl, O.; Guren, M.G. Impaired health-related quality of life after chemoradiotherapy for anal cancer: Late effects in a national cohort of 128 survivors. Acta Oncol. 2013, 52, 736–744. [Google Scholar] [CrossRef]
- Kachnic, L.A.; Winter, K.; Myerson, R.J.; Goodyear, M.D.; Willins, J.; Esthappan, J.; Haddock, M.G.; Rotman, M.; Parikh, P.J.; Safran, H.; et al. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 27–33. [Google Scholar] [CrossRef]
- Kachnic, L.A.; Winter, K.A.; Myerson, R.J.; Goodyear, M.D.; Abitbol, A.A.; Streeter, O.E.; Augspurger, M.E.; Schefter, T.E.; Katz, A.W.; Fisher, B.J.; et al. Long-Term Outcomes of NRG Oncology/RTOG 0529: A Phase 2 Evaluation of Dose-Painted Intensity Modulated Radiation Therapy in Combination With 5-Fluorouracil and Mitomycin-C for the Reduction of Acute Morbidity in Anal Canal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 146–157. [Google Scholar] [CrossRef]
- Manfrida, S.; Fionda, B.; Mariani, S.; Luca, V.; Bertolini, R.; Barbaro, B.; Chiloiro, G.; Frascino, V.; Tagliaferri, L.; Gambacorta, M.A. High-tailored Anal Canal Radiotherapy (HIT-ART): Outcomes of a 10-Year Single Center Clinical Experience. In Vivo 2024, 38, 1306–1315. [Google Scholar] [CrossRef]
- Lavrova, E.; Garrett, M.D.; Wang, Y.F.; Chin, C.; Elliston, C.; Savacool, M.; Price, M.; Kachnic, L.A.; Horowitz, D.P. Adaptive Radiation Therapy: A Review of CT-based Techniques. Radiol. Imaging Cancer 2023, 5, e230011. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.R.; Moughan, J.; Myerson, R.; Abitbol, A.; Doncals, D.E.; Johnson, D.; Schefter, T.E.; Chen, Y.; Fisher, B.; Michalski, J.; et al. Predictors of Radiation Therapy-Related Gastrointestinal Toxicity From Anal Cancer Dose-Painted Intensity Modulated Radiation Therapy: Secondary Analysis of NRG Oncology RTOG 0529. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, D.P.; Wang, Y.F.; Lee, A.; Riegel, A.C.; Pasetsky, J.; Elliston, C.; Spina, C.; Deutsch, I.; Ghiassi-Nejad, Z.; Yuan, Y.; et al. Practical Approach to Computed Tomography Guided Online Adaptive Radiation Therapy for Abdominopelvic Tumors. Pract. Radiat. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Storm, K.S.; Astrom, L.M.; Sibolt, P.; Behrens, C.P.; Persson, G.F.; Serup-Hansen, E. ROAR-A: Re-optimization based Online Adaptive Radiotherapy of anal cancer, a prospective phase II trial protocol. BMC Cancer 2024, 24, 374. [Google Scholar] [CrossRef]
- Huddart, R.; Hafeez, S.; Griffin, C.; Choudhury, A.; Foroudi, F.; Syndikus, I.; Hindson, B.; Webster, A.; McNair, H.; Birtle, A.; et al. Dose-escalated Adaptive Radiotherapy for Bladder Cancer: Results of the Phase 2 RAIDER Randomised Controlled Trial. Eur. Urol. 2025, 87, 60–70. [Google Scholar] [CrossRef]
- Kibrom, A.Z.; Knight, K.A. Adaptive radiation therapy for bladder cancer: A review of adaptive techniques used in clinical practice. J. Med. Radiat. Sci. 2015, 62, 277–285. [Google Scholar] [CrossRef]
- Keall, P.; Poulsen, P.; Booth, J.T. See, Think, and Act: Real-Time Adaptive Radiotherapy. Semin. Radiat. Oncol. 2019, 29, 228–235. [Google Scholar] [CrossRef]
- Tyagi, N.; Fontenla, S.; Zhang, J.; Cloutier, M.; Kadbi, M.; Mechalakos, J.; Zelefsky, M.; Deasy, J.; Hunt, M. Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys. Med. Biol. 2017, 62, 2961–2975. [Google Scholar] [CrossRef]
- Tyagi, N.; Fontenla, S.; Zelefsky, M.; Chong-Ton, M.; Ostergren, K.; Shah, N.; Warner, L.; Kadbi, M.; Mechalakos, J.; Hunt, M. Clinical workflow for MR-only simulation and planning in prostate. Radiat. Oncol. 2017, 12, 119. [Google Scholar] [CrossRef]
- Lombardo, E.; Dhont, J.; Page, D.; Garibaldi, C.; Kunzel, L.A.; Hurkmans, C.; Tijssen, R.H.N.; Paganelli, C.; Liu, P.Z.Y.; Keall, P.J.; et al. Real-time motion management in MRI-guided radiotherapy: Current status and AI-enabled prospects. Radiother. Oncol. 2024, 190, 109970. [Google Scholar] [CrossRef]
- Low, D.A.; Fallone, B.G.; Raaymakers, B.W. MRI-Guided Radiation Therapy Systems. Semin. Radiat. Oncol. 2024, 34, 14–22. [Google Scholar] [CrossRef]
- Kim, E.; Park, Y.K.; Zhao, T.; Laugeman, E.; Zhao, X.N.; Hao, Y.; Chung, Y.; Lee, H. Image quality characterization of an ultra-high-speed kilovoltage cone-beam computed tomography imaging system on an O-ring linear accelerator. J. Appl. Clin. Med. Phys. 2024, 25, e14337. [Google Scholar] [CrossRef]
- Robar, J.L.; Cherpak, A.; MacDonald, R.L.; Yashayaeva, A.; McAloney, D.; McMaster, N.; Zhan, K.; Cwajna, S.; Patil, N.; Dahn, H. Novel Technology Allowing Cone Beam Computed Tomography in 6 Seconds: A Patient Study of Comparative Image Quality. Pract. Radiat. Oncol. 2024, 14, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Price, M.J.; Elliston, C.D.; Munbodh, R.; Spina, C.S.; Horowitz, D.P.; Kachnic, L.A. Enhancing Safety in AI-Driven Cone Beam CT-based Online Adaptive Radiation Therapy: Development and Implementation of an Interdisciplinary Workflow. Adv. Radiat. Oncol. 2024, 9, 101399. [Google Scholar] [CrossRef] [PubMed]
- Yashayaeva, A.; MacDonald, R.L.; Robar, J.; Cherpak, A. Evaluation of a Metal Artifact Reduction Algorithm for Image Reconstruction on a Novel CBCT Platform. J. Appl. Clin. Med. Phys. 2024, 25, e14516. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, D.; Low, D.A. Technical Challenges of Real-Time Adaptive MR-Guided Radiotherapy. Front. Oncol. 2021, 11, 634507. [Google Scholar] [CrossRef]
- Menten, M.J.; Fast, M.F.; Nill, S.; Kamerling, C.P.; McDonald, F.; Oelfke, U. Lung stereotactic body radiotherapy with an MR-linac—Quantifying the impact of the magnetic field and real-time tumor tracking. Radiother. Oncol. 2016, 119, 461–466. [Google Scholar] [CrossRef][Green Version]
- Benitez, C.M.; Chuong, M.D.; Kunzel, L.A.; Thorwarth, D. MRI-Guided Adaptive Radiation Therapy. Semin. Radiat. Oncol. 2024, 34, 84–91. [Google Scholar] [CrossRef]
- Parikh, N.R.; Clark, M.A.; Patel, P.; Kafka-Peterson, K.; Zaide, L.; Ma, T.M.; Steinberg, M.L.; Cao, M.; Raldow, A.C.; Lamb, J.; et al. Time-Driven Activity-Based Costing of CT-Guided vs MR-Guided Prostate SBRT. Appl. Radiat. Oncol. 2021, 10, 33–40. [Google Scholar] [CrossRef]
- Yoon, S.M.; Luterstein, E.; Chu, F.I.; Cao, M.; Lamb, J.; Agazaryan, N.; Low, D.; Raldow, A.; Steinberg, M.L.; Lee, P. Clinical outcomes of stereotactic magnetic resonance image-guided adaptive radiotherapy for primary and metastatic tumors in the abdomen and pelvis. Cancer Med. 2021, 10, 5897–5906. [Google Scholar] [CrossRef]
- de Jong, R.; Visser, J.; van Wieringen, N.; Wiersma, J.; Geijsen, D.; Bel, A. Feasibility of Conebeam CT-based online adaptive radiotherapy for neoadjuvant treatment of rectal cancer. Radiat. Oncol. 2021, 16, 136. [Google Scholar] [CrossRef]
- Lee, A.; Pasetsky, J.; Lavrova, E.; Wang, Y.F.; Sedor, G.; Li, F.L.; Gallitto, M.; Garrett, M.; Elliston, C.; Price, M.; et al. CT-guided online adaptive stereotactic body radiotherapy for pancreas ductal adenocarcinoma: Dosimetric and initial clinical experience. Clin. Transl. Radiat. Oncol. 2024, 48, 100813. [Google Scholar] [CrossRef]
- Kurz, C.; Buizza, G.; Landry, G.; Kamp, F.; Rabe, M.; Paganelli, C.; Baroni, G.; Reiner, M.; Keall, P.J.; van den Berg, C.A.T.; et al. Medical physics challenges in clinical MR-guided radiotherapy. Radiat. Oncol. 2020, 15, 93. [Google Scholar] [CrossRef]
- Paganelli, C.; Portoso, S.; Garau, N.; Meschini, G.; Via, R.; Buizza, G.; Keall, P.; Riboldi, M.; Baroni, G. Time-resolved volumetric MRI in MRI-guided radiotherapy: An in silico comparative analysis. Phys. Med. Biol. 2019, 64, 185013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Nelson, G.; Sarkar, V.; Oare, C.; Szegedi, M.; James, S.S.; Kunz, J.; Price, R.; Huang, Y.J. Comprehensive Image Quality Evaluation and Motion Phantom Studies of an Ultra-Fast (6-Second) Cone-Beam Computed Tomography Imaging System on a Ring Gantry Linear Accelerator. Adv. Radiat. Oncol. 2025, 10, 101681. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.W.; Kan, M.W.; Lee, L.K.; Wong, K.C.; Tong, M.; Chan, A.T. The effect of the magnetic fields from three different configurations of the MRIgRT systems on the dose deposition from lateral opposing photon beams in a laryngeal geometry–A Monte Carlo study. Radiat. Med. Prot. 2021, 2, 103–111. [Google Scholar] [CrossRef]
- Tseng, W.; Liu, H.; Yang, Y.; Liu, C.; Furutani, K.; Beltran, C.; Lu, B. Performance assessment of variant UNet-based deep-learning dose engines for MR-Linac-based prostate IMRT plans. Phys. Med. Biol. 2023, 68, 175004. [Google Scholar] [CrossRef]
- Cusumano, D.; Lenkowicz, J.; Votta, C.; Boldrini, L.; Placidi, L.; Catucci, F.; Dinapoli, N.; Antonelli, M.V.; Romano, A.; De Luca, V.; et al. A deep learning approach to generate synthetic CT in low field MR-guided adaptive radiotherapy for abdominal and pelvic cases. Radiother. Oncol. 2020, 153, 205–212. [Google Scholar] [CrossRef]
- Kim, J.; Garbarino, K.; Schultz, L.; Levin, K.; Movsas, B.; Siddiqui, M.S.; Chetty, I.J.; Glide-Hurst, C. Dosimetric evaluation of synthetic CT relative to bulk density assignment-based magnetic resonance-only approaches for prostate radiotherapy. Radiat. Oncol. 2015, 10, 239. [Google Scholar] [CrossRef]
- Sijtsema, N.D.; Penninkhof, J.J.; van de Schoot, A.; Kunnen, B.; Sluijter, J.H.; van de Pol, M.; Froklage, F.E.; Dirkx, M.L.P.; Petit, S.F. Dose calculation accuracy of a new high-performance ring-gantry CBCT imaging system for prostate and lung cancer patients. Radiother. Oncol. 2025, 202, 110596. [Google Scholar] [CrossRef]
- de Muinck Keizer, D.M.; Kerkmeijer, L.G.W.; Willigenburg, T.; van Lier, A.; Hartogh, M.D.D.; van der Voort van Zyp, J.R.N.; de Groot-van Breugel, E.N.; Raaymakers, B.W.; Lagendijk, J.J.W.; de Boer, J.C.J. Prostate intrafraction motion during the preparation and delivery of MR-guided radiotherapy sessions on a 1.5T MR-Linac. Radiother. Oncol. 2020, 151, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Nachbar, M.; Lo Russo, M.; Gani, C.; Boeke, S.; Wegener, D.; Paulsen, F.; Zips, D.; Roque, T.; Paragios, N.; Thorwarth, D. Automatic AI-based contouring of prostate MRI for online adaptive radiotherapy. Z. Für Med. Phys. 2024, 34, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tetar, S.U.; Bruynzeel, A.M.E.; Lagerwaard, F.J.; Slotman, B.J.; Bohoudi, O.; Palacios, M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys. Imaging Radiat. Oncol. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- Finnegan, R.N.; Quinn, A.; Horsley, P.; Chan, J.; Stewart, M.; Bromley, R.; Booth, J. Geometric and dosimetric evaluation of a commercial AI auto-contouring tool on multiple anatomical sites in CT scans. J. Appl. Clin. Med. Phys. 2025, 26, e70067. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W.; Li, B.; Zhu, J.; Peng, Y.; Li, C.; Zhu, J.; Zhou, Q.; Yin, Y. Patient-specific daily updated deep learning auto-segmentation for MRI-guided adaptive radiotherapy. Radiother. Oncol. 2022, 177, 222–230. [Google Scholar] [CrossRef]
- Zhong, H.; Pursley, J.M.; Rong, Y. Deformable dose accumulation is required for adaptive radiotherapy practice. J. Appl. Clin. Med. Phys. 2024, 25, e14457. [Google Scholar] [CrossRef]
- Brock, K.K.; Mutic, S.; McNutt, T.R.; Li, H.; Kessler, M.L. Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132. Med. Phys. 2017, 44, e43–e76. [Google Scholar] [CrossRef]
- Chetty, I.J.; Rosu-Bubulac, M. Deformable Registration for Dose Accumulation. Semin. Radiat. Oncol. 2019, 29, 198–208. [Google Scholar] [CrossRef]
- Murr, M.; Brock, K.K.; Fusella, M.; Hardcastle, N.; Hussein, M.; Jameson, M.G.; Wahlstedt, I.; Yuen, J.; McClelland, J.R.; Vasquez Osorio, E. Applicability and usage of dose mapping/accumulation in radiotherapy. Radiother. Oncol. 2023, 182, 109527. [Google Scholar] [CrossRef]
- Chen, X.; Ahunbay, E.; Paulson, E.S.; Chen, G.; Li, X.A. A daily end-to-end quality assurance workflow for MR-guided online adaptive radiation therapy on MR-Linac. J. Appl. Clin. Med. Phys. 2020, 21, 205–212. [Google Scholar] [CrossRef]
- Kim, J.P. MRgRT Quality Assurance for a Low-Field MR-Linac. Semin. Radiat. Oncol. 2024, 34, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; Lam, D.; Cai, B.; Hugo, G.D. Robustness and reproducibility of an artificial intelligence-assisted online segmentation and adaptive planning process for online adaptive radiation therapy. J. Appl. Clin. Med. Phys. 2022, 23, e13702. [Google Scholar] [CrossRef]
- Elter, A.; Dorsch, S.; Mann, P.; Runz, A.; Johnen, W.; Spindeldreier, C.K.; Kluter, S.; Karger, C.P. End-to-end test of an online adaptive treatment procedure in MR-guided radiotherapy using a phantom with anthropomorphic structures. Phys. Med. Biol. 2019, 64, 225003. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, L.; Zhong, X.; Gonzalez, Y.; Visak, J.; Meng, B.; Inam, E.; Parsons, D.; Godley, A.; Jiang, S.; et al. Clinical experience on patient-specific quality assurance for CBCT-based online adaptive treatment plan. J. Appl. Clin. Med. Phys. 2023, 24, e13918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Stanley, D.N.; Cardenas, C.E.; Harms, J.; Popple, R.A. Do we need patient-specific QA for adaptively generated plans? Retrospective evaluation of delivered online adaptive treatment plans on Varian Ethos. J. Appl. Clin. Med. Phys. 2023, 24, e13876. [Google Scholar] [CrossRef]
- Green, O.L.; Henke, L.E.; Hugo, G.D. Practical Clinical Workflows for Online and Offline Adaptive Radiation Therapy. Semin. Radiat. Oncol. 2019, 29, 219–227. [Google Scholar] [CrossRef]
- Astrom, L.M.; Behrens, C.P.; Storm, K.S.; Sibolt, P.; Serup-Hansen, E. Online adaptive radiotherapy of anal cancer: Normal tissue sparing, target propagation methods, and first clinical experience. Radiother. Oncol. 2022, 176, 92–98. [Google Scholar] [CrossRef]
- Intven, M.P.W.; de Mol van Otterloo, S.R.; Mook, S.; Doornaert, P.A.H.; de Groot-van Breugel, E.N.; Sikkes, G.G.; Willemsen-Bosman, M.E.; van Zijp, H.M.; Tijssen, R.H.N. Online adaptive MR-guided radiotherapy for rectal cancer; feasibility of the workflow on a 1.5T MR-linac: Clinical implementation and initial experience. Radiother. Oncol. 2021, 154, 172–178. [Google Scholar] [CrossRef]
- Wegener, S.; Exner, F.; Weick, S.; Stark, S.; Hutzel, H.; Lutyj, P.; Tamihardja, J.; Razinskas, G. Prospective risk analysis of the online-adaptive artificial intelligence-driven workflow using the Ethos treatment system. Z. Für Med. Phys. 2024, 34, 384–396. [Google Scholar] [CrossRef]
- Otazo, R.; Lambin, P.; Pignol, J.P.; Ladd, M.E.; Schlemmer, H.P.; Baumann, M.; Hricak, H. MRI-guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology. Radiology 2021, 298, 248–260. [Google Scholar] [CrossRef]
- Bai, T.; Ray, X.; Parsons, D.; Lin, M.H. Cone Beam Computed Tomography-Guided Online Adaptive Radiation Therapy: Clinical Insights From a Nationwide Staffing Survey. Int. J. Radiat. Oncol. Biol. Phys. 2025, 122, 884–892. [Google Scholar] [CrossRef]
- Viscariello, N.N.; McConnell, K.; Harms, J.; Pogue, J.A.; Ray, X.; Laugeman, E.; Popple, R.A.; Stanley, D.N.; Cardenas, C.E. Quantitative Assessment of Full-Time Equivalent Effort for Kilovoltage-Cone Beam Computed Tomography Guided Online Adaptive Radiation Therapy for Medical Physicists. Pract. Radiat. Oncol. 2025, 15, e72–e81. [Google Scholar] [CrossRef]
- Shepherd, M.; Joyce, E.; Williams, B.; Graham, S.; Li, W.; Booth, J.; McNair, H.A. Training for tomorrow: Establishing a worldwide curriculum in online adaptive radiation therapy. Tech. Innov. Patient Support Radiat. Oncol. 2025, 33, 100304. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.; Hafeez, S.; Lewis, R.; McNair, H.; Syndikus, I.; Henry, A.; Staffurth, J.; Dewan, M.; Vassallo-Bonner, C.; Moinuddin, S.A.; et al. Clinical Outcomes of a Randomized Trial of Adaptive Plan-of-the-Day Treatment in Patients Receiving Ultra-hypofractionated Weekly Radiation Therapy for Bladder Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 412–424. [Google Scholar] [CrossRef]
- Kong, F.S.; Hu, C.; Pryma, D.A.; Duan, F.; Matuszak, M.; Xiao, Y.; Ten Haken, R.; Siegel, M.J.; Hanna, L.; Curran, W.J.; et al. Primary Results of NRG-RTOG1106/ECOG-ACRIN 6697: A Randomized Phase II Trial of Individualized Adaptive (chemo)Radiotherapy Using Midtreatment (18)F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Stage III Non-Small Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 3935–3946. [Google Scholar] [CrossRef] [PubMed]
- Jagt, T.Z.; Janssen, T.M.; Betgen, A.; Wiersema, L.; Verhage, R.; Garritsen, S.; Vijlbrief-Bosman, T.; de Ruiter, P.; Remeijer, P.; Marijnen, C.A.M.; et al. Benchmarking daily adaptation using fully automated radiotherapy treatment plan optimization for rectal cancer. Phys. Imaging Radiat. Oncol. 2022, 24, 7–13. [Google Scholar] [CrossRef]
- Archambault, Y.; Boylan, C.; Bullock, D.; Morgas, T.; Peltola, J.; Ruokokosi, E.; Genghi, A.; Haas, B.; Suhonen, P.; Thompson, S. Making on-line adaptive radiotherapy possible using artificial intelligence and machine learning for efficient daily re-planning. Med. Phys. Int. J. 2020, 8, 77–86. [Google Scholar]
- Sibolt, P.; Andersson, L.M.; Calmels, L.; Sjostrom, D.; Bjelkengren, U.; Geertsen, P.; Behrens, C.F. Clinical implementation of artificial intelligence-driven cone-beam computed tomography-guided online adaptive radiotherapy in the pelvic region. Phys. Imaging Radiat. Oncol. 2021, 17, 1–7. [Google Scholar] [CrossRef]
- Lee, A.; Pasetsky, J.; Wang, Y.F.; Elliston, C.; Price, M.; Kachnic, L.A.; Horowitz, D.P. CT-Guided Online Adaptive Radiotherapy for Rectal Cancer: Initial Dosimetric and Clinical Experience. Available online: https://poster.astro.org/astro/2024/eposters/4139008/albert.lee.cbct-guided.online.adaptive.radiotherapy.for.rectal.cancer.initial.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D2%2Atopic%3D18164 (accessed on 25 July 2025).
- Chiloiro, G.; Gani, C.; Boldrini, L. Rectal Cancer MRI Guided Radiotherapy: A Practical Review for the Physician. Semin. Radiat. Oncol. 2024, 34, 64–68. [Google Scholar] [CrossRef]
- Han, K.; Cummings, B.J.; Lindsay, P.; Skliarenko, J.; Craig, T.; Le, L.W.; Brierley, J.; Wong, R.; Dinniwell, R.; Bayley, A.J.; et al. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 587–594. [Google Scholar] [CrossRef]
- Chuong, M.D.; Lee, P.; Low, D.A.; Kim, J.; Mittauer, K.E.; Bassetti, M.F.; Glide-Hurst, C.K.; Raldow, A.C.; Yang, Y.; Portelance, L.; et al. Stereotactic MR-guided on-table adaptive radiation therapy (SMART) for borderline resectable and locally advanced pancreatic cancer: A multi-center, open-label phase 2 study. Radiother. Oncol. 2024, 191, 110064. [Google Scholar] [CrossRef] [PubMed]
| Approach | Timing | Use Case | Pros | Cons |
|---|---|---|---|---|
| Offline ART | Replanning between fractions (e.g., mid-treatment or weekly) | Account for systematic changes over time (tumor shrinkage, weight loss) | Utilizes existing workflow; less resource-intensive than daily adaptation; can selectively adapt for major changes | Cannot respond to random day-to-day variations; optimal timing/thresholds for replanning are unclear |
| Online ART | Replanning immediately before each fraction (patient on table) | Account for unpredictable interfraction changes (daily organ motion, positioning) | Maximizes precision each fraction; ensures consistent target coverage and OAR sparing despite anatomy changes | Resource and time intensive; requires specialized technology and rapid workflow for imaging, contouring, and QA |
| Real-Time ART | Adaptation during beam delivery (e.g., gated or beam-tracked radiotherapy) | Account for intrafraction motion (respiration, organ drift during treatment) | Enables margin reduction for moving targets; can “track” tumor motion continuously | Highly technically complex |
| TNM Stage | Primary Tumor Dose | Involved Node Dose | Elective Nodal Dose |
|---|---|---|---|
| T1–2, N0 With T < 4 cm | 5040 cGy in 28 fractions | N/A | 4200 cGy in 28 fractions |
| T2 ≥ 4 cm T3–4, N0 | 5400 cGy in 30 fractions | N/A | 4500 cGy in 30 fractions |
| Any T with N+ (<3 cm) | 5400 cGy in 30 fractions | 5040 cGy in 30 fractions | 4500 cGy in 30 fractions |
| Any T with N+ (>3 cm) | 5400 cGy in 30 fractions | 5400 cGy in 30 fractions | 4500 cGy in 30 fractions |
| OAR | Dose Constraints |
|---|---|
| Bowel | D0.03 cc < 5000–5600 cGy |
| V4500 cGy < 20–60 cc | |
| V3500 cGy < 40–150 cc | |
| Bladder | D50% < 3300–4500 cGy |
| D5% < 5000–5600 cGy | |
| Femoral head | D50% < 3000–4500 cGy |
| D5% < 4400–5500 cGy | |
| Genitalia | D50% < 2000–3500 cGy |
| Bone Marrow | Mean < 2000–3000 cGy |
| V1000 cGy < 70–90% | |
| Skin | D50% < 2000–3500 cGy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horowitz, D.P.; Wang, Y.-F.; Lee, A.; Kachnic, L.A. Optimizing Treatment Precision: Role of Adaptive Radiotherapy in Modern Anal Cancer Management. Cancers 2025, 17, 2478. https://doi.org/10.3390/cancers17152478
Horowitz DP, Wang Y-F, Lee A, Kachnic LA. Optimizing Treatment Precision: Role of Adaptive Radiotherapy in Modern Anal Cancer Management. Cancers. 2025; 17(15):2478. https://doi.org/10.3390/cancers17152478
Chicago/Turabian StyleHorowitz, David P., Yi-Fang Wang, Albert Lee, and Lisa A. Kachnic. 2025. "Optimizing Treatment Precision: Role of Adaptive Radiotherapy in Modern Anal Cancer Management" Cancers 17, no. 15: 2478. https://doi.org/10.3390/cancers17152478
APA StyleHorowitz, D. P., Wang, Y.-F., Lee, A., & Kachnic, L. A. (2025). Optimizing Treatment Precision: Role of Adaptive Radiotherapy in Modern Anal Cancer Management. Cancers, 17(15), 2478. https://doi.org/10.3390/cancers17152478





