Bispecific Antibodies and Antibody–Drug Conjugates in Relapsed/Refractory Aggressive Non-Hodgkin Lymphoma, Focusing on Diffuse Large B-Cell Lymphoma
Simple Summary
Abstract
1. General Considerations Regarding Bispecific Antibodies and Antibody–Drug Conjugates
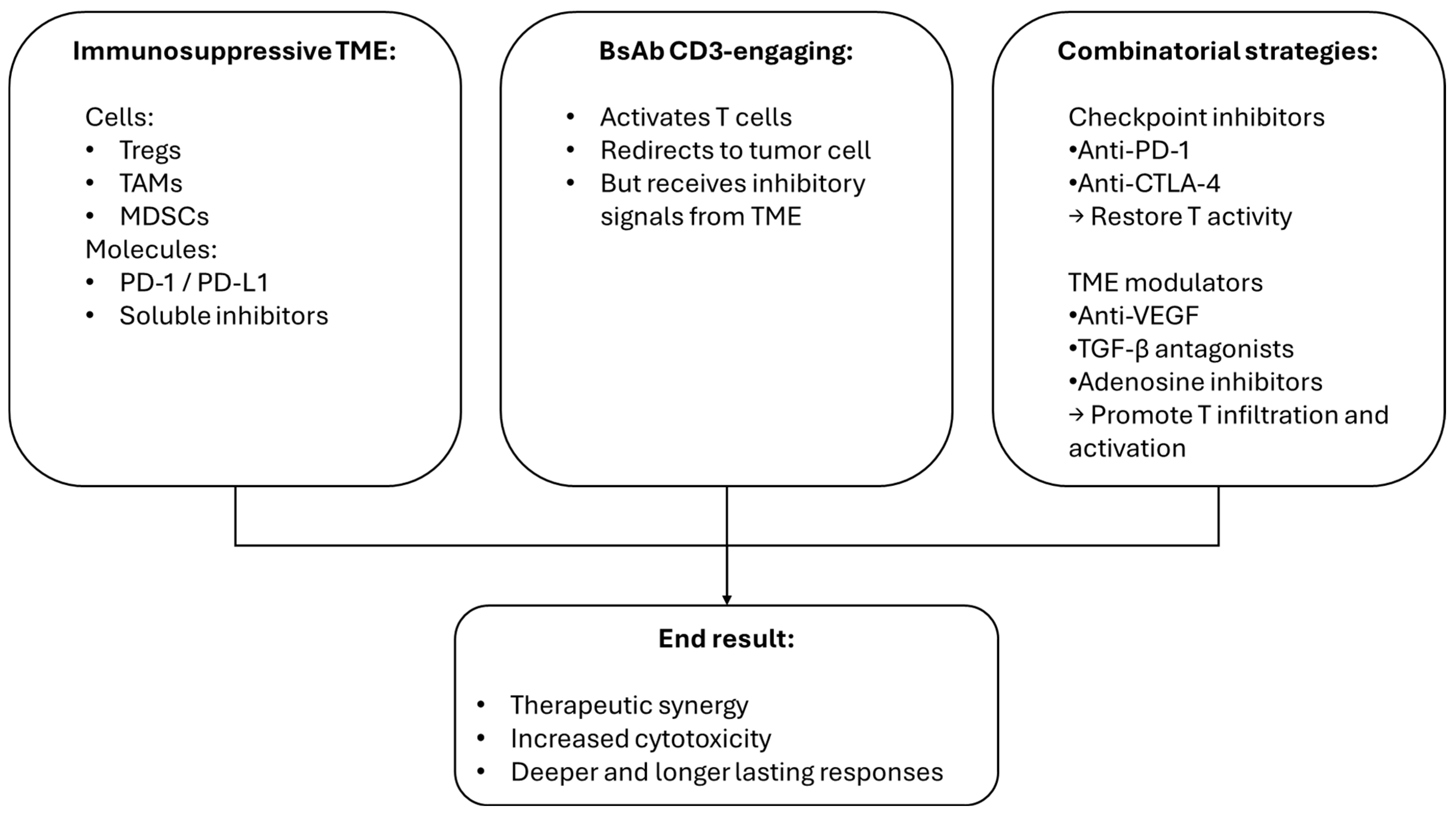
1.1. Bispecific Antibodies
1.2. Antibody–Drug Conjugates
2. What Is Relapsed/Refractory Diffuse Large B-Cell Lymphoma?
2.1. Epcoritamab
2.2. Glofitamab
2.3. Polatuzumab Vedotin
2.4. Loncastuximab
3. Future Perspectives
3.1. Odronextamab in DLBCL
3.2. Glofitamab in Mantle-Cell Lymphoma
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Tada, M.; Aoyama, M.; Ishii-Watabe, A. Target-independent Immune-cell Activation by Aggregates of T Cell-redirecting Bispecific Antibodies. J. Pharm. Sci. 2023, 112, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.; Köse, M.C.; Duray, E.; Einsele, H.; Beguin, Y.; Caers, J. Bispecific, T-cell-recruiting antibodies in B-cell malignancies. Front. Immunol. 2020, 11, 762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martínez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: A phase I trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlechter, B.L.; Ng, K. Colorectal cancer: Advancing science, improving access, and refining therapy. Hematol. Oncol. Clin. N. Am. 2022, 36, xiii–xiv. [Google Scholar] [CrossRef] [PubMed]
- Bannerji, R.; Arnason, J.E.; Advani, R.H.; Brown, J.R.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; O’Brien, S.M.; Chávez, J.C.; Duell, J.; et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): Results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022, 9, e327–e339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sehn, L.H.; Bartlett, N.L.; Matasar, M.J.; Schuster, S.J.; Assouline, S.E.; Giri, P.; Kuruvilla, J.; Shadman, M.; Cheah, C.Y.; Dietrich, S.; et al. Long-term 3-year follow-up of mosunetuzumab in relapsed or refractory follicular lymphoma after ≥2 prior therapies. Blood 2025, 145, 708–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, S.; Rau, R.E.; Kairalla, J.A.; Rabin, K.R.; Wang, C.; Angiolillo, A.L.; Alexander, S.; Carroll, A.J.; Conway, S.; Gore, L.; et al. Blinatumomab in standard-risk B-cell acute lymphoblastic leukemia in children. N. Engl. J. Med. 2025, 392, 875–891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Locatelli, F.; Eckert, C.; Hrusak, O.; Buldini, B.; Sartor, M.; Zugmaier, G.; Zeng, Y.; Pilankar, D.; Morris, J.; von Stackelberg, A. Blinatumomab overcomes poor prognostic impact of measurable residual disease in pediatric high-risk first relapse B-cell precursor acute lymphoblastic leukemia. Pediatr. Blood Cancer 2022, 69, e29715. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Kuruvilla, J.; Vassilakopoulos, T.P.; Di Maio, D.; Wei, M.C.; Blanchet Zumofen, M.-H.; Nastoupil, L.J. Indirect treatment comparisons of mosunetuzumab with third- and later-line treatments for relapsed/refractory follicular lymphoma. Clin. Lymphoma Myeloma Leuk. 2024, 24, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Salles, G. Diffuse large B-cell lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Budde, L.E.; Sehn, L.H.; Matasar, M.M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: A single-arm, multicentre, phase 2 study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Crombie, J.L.; Graff, T.; Falchi, L.; Karimi, Y.H.; Bannerji, R.; Nastoupil, L.; Thieblemont, C.; Ursu, R.; Bartlett, N.; Nachar, V.; et al. Consensus recommendations on the management of toxicity associated with CD3×CD20 bispecific antibody therapy. Blood 2024, 143, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Huw, L.Y.; Bolen, C.R.; Maximov, V.; Polson, A.G.; Hatzi, K.; Lasater, E.A.; Assouline, S.E.; Bartlett, N.L.; Budde, L.E.; et al. Loss of CD20 expression as a mechanism of resistance to mosunetuzumab in relapsed/refractory B-cell lymphomas. Blood 2024, 143, 822–832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grigg, S.; Minson, A.; Prins, E.; Dickinson, M.J. Relapse after glofitamab has a poor prognosis and rates of CD20 loss are high. Br. J. Haematol. 2024, 205, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Viardot, A.; Goebeler, M.E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goebeler, M.E.; Bargou, R. Blinatumomab: A CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk. Lymphoma 2016, 57, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 2023, 402, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gibiansky, L.; Parikh, A.; van der Linden, M.; Sanghavi, K.; Putnins, M.; Sacchi, M.; Feng, H.; Ahmadi, T.; Gupta, M.; et al. Population pharmacokinetics of epcoritamab following subcutaneous administration in relapsed or refractory B cell non-Hodgkin lymphoma. Clin. Pharmacokinet. 2025, 64, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.; Sehn, L.H. CAR T cells as a second-line therapy for large B-cell lymphoma: A paradigm shift? Blood 2022, 139, 2737–2746, Erratum in: Blood 2023, 141, 683. [Google Scholar] [CrossRef] [PubMed]
- Chari, R.V. Targeted cancer therapy: Conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2008, 41, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.J.; Hamilton, J.Z.; Jeffrey, S.C.; Hunter, J.H.; Doronina, S.O.; Okeley, N.M.; Miyamoto, J.B.; Anderson, M.E.; Stone, I.J.; Ulrich, M.L.; et al. Optimization of a PEGylated Glucuronide-Monomethylauristatin E Linker for Antibody-Drug Conjugates. Mol. Cancer Ther. 2017, 16, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Gołos, A.; Góra-Tybor, J.; Robak, T. Safety considerations for drugs newly approved for treating acute myeloid leukemia. Expert Opin Drug Saf. 2024, 23, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambert, J.M.; Berkenblit, A. Antibody-Drug Conjugates for Cancer Treatment. Annu. Rev. Med. 2018, 69, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Cilliers, C.; Nessler, I.; Christodolu, N.; Thurber, G.M. Tracking antibody distribution with near-infrared fluorescent dyes: Impact of dye structure and degree of labeling on plasma clearance. Mol. Pharm. 2017, 14, 1623–1633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polson, A.G.; Calemine-Fenaux, J.; Chan, P.; Chang, W.; Christensen, E.; Clark, S.; de Sauvage, F.J.; Eaton, D.; Elkins, K.; Elliott, J.M.; et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: Target and linker-drug selection. Cancer Res. 2009, 69, 2358–2364, Erratum in Cancer Res. 2010, 70, 1275. [Google Scholar] [CrossRef] [PubMed]
- Doronina, S.O.; Bovee, T.D.; Meyer, D.W.; Miyamoto, J.B.; Anderson, M.E.; Morris-Tilden, C.A.; Senter, P.D. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug. Chem. 2008, 19, 1960–1963. [Google Scholar] [CrossRef] [PubMed]
- Doronina, S.O.; Toki, B.E.; Torgov, M.Y.; Mendelsohn, B.A.; Cerveny, C.G.; Chace, D.F.; DeBlanc, R.L.; Gearing, R.P.; Bovee, T.D.; Siegall, C.B.; et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 2003, 21, 778–784. [Google Scholar] [CrossRef] [PubMed]
- McCombs, J.R.; Owen, S.C. Antibody drug conjugates: Design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giugliano, F.; Corti, C.; Tarantino, P.; Michelini, F.; Curigliano, G. Bystander effect of antibody-drug conjugates: Fact or fiction? Curr. Oncol. Rep. 2022, 24, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Cottin, S.; Ghani, K.; de Campos-Lima, P.O.; Caruso, M. Gemcitabine intercellular diffusion mediated by gap junctions: New implications for cancer therapy. Mol. Cancer 2010, 9, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.; Chen, B. Brentuximab vedotin for relapsed or refractory Hodgkin’s lymphoma. Drug Des. Devel. Ther. 2015, 9, 1729–1733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Pedersen, M.; Fox, S.; McCulloch, R.; Weng, W.; et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N. Engl. J. Med. 2018, 378, 878, Erratum in N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, F.; Zhang, H.; Chen, B. Brentuximab vedotin for treatment of relapsed or refractory malignant lymphoma: Results of a systematic review and meta-analysis of prospective studies. Drug Des. Devel. Ther. 2015, 9, 2277–2283. [Google Scholar] [PubMed] [PubMed Central]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sehn, L.H.; Hertzberg, M.; Opat, S.; Herrera, A.F.; Assouline, S.; Flowers, C.R.; Kim, T.M.; McMillan, A.; Ozcan, M.; Safar, V.; et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: Survival update and new extension cohort data. Blood Adv. 2022, 6, 533–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Lucero, M.; Bailey, T.; Mirams, L.; Cheung, J.; Amet, M.; Milligan, G.; Chen, L. Outcomes with loncastuximab tesirine following CAR T-cell therapy in patients with relapsed or refractory diffuse large B-cell lymphoma. Blood Cancer J. 2024, 14, 210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.M.; Jabbour, E.; Wang, T.; White, J.L.; et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019, 125, 2474–2487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, W.; Hu, S.; Wang, X. Update of antibody-drug conjugates for hematological malignancies. Curr. Opin. Oncol. 2024, 36, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Dornan, D.; Bennett, F.; Chen, Y.; Dennis, M.; Eaton, D.; Elkins, K.; French, D.; Go, M.A.; Jack, A.; Junutula, J.R.; et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 2009, 114, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M. Polatuzumab vedotin for the treatment of relapsed/refractory diffuse large B-cell lymphoma in transplant-ineligible patients. J. Adv. Pract. Oncol. 2020, 11, 521–528. [Google Scholar] [PubMed] [PubMed Central]
- Caimi, P.F.; Ai, W.Z.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma: Long-term efficacy and safety from the phase II LOTIS-2 study. Haematologica 2024, 109, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aujla, A.; Aujla, R.; Liu, D. Inotuzumab ozogamicin in clinical development for acute lymphoblastic leukemia and non-Hodgkin lymphoma. Biomark Res. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogura, M.; Tobinai, K.; Hatake, K.; Davies, A.; Crump, M.; Ananthakrishnan, R.; Ishibashi, T.; Paccagnella, M.L.; Boni, J.; Vandendries, E.; et al. Phase I study of inotuzumab ozogamicin combined with R-CVP for relapsed/refractory CD22+ B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2016, 22, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, S.; Kang, B.; Fan, S.; He, Z.; Zhou, J. CD25-targeted antibody-drug conjugate camidanlumab tesirine for relapsed or refractory classical Hodgkin lymphoma. Invest. New Drugs. 2022, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, Z.; Gao, C.; Wu, T.; Wang, L.; Li, S.; Zhang, Y.; Shi, C. Peripheral neuropathy associated with monomethyl auristatin E-based antibody-drug conjugates. iScience 2023, 26, 107778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, S.C.; Wynn, C.; Le, T.; McCandless, M.; Zhang, Y.; Patel, R.; Maihle, N.; Hillegass, W. Influence of antibody-drug conjugate cleavability, drug-to-antibody ratio, and free payload concentration on systemic toxicities: A systematic review and meta-analysis. Cancer Metastasis Rev. 2024, 44, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.; Hu, B.; Pan, Z.; Mo, C.; Zhao, X.; Liu, G.; Hou, P.; Cui, Q.; Xu, Z.; Wang, W.; et al. Antibody-Drug Conjugates (ADCs): Current and future biopharmaceuticals. J. Hematol. Oncol. 2025, 18, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kepp, O.; Zitvogel, L.; Kroemer, G. Clinical evidence that immunogenic cell death sensitizes to PD-1/PD-L1 blockade. Oncoimmunology 2019, 8, e1637188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nastoupil, L.J.; Thiruvengadam, S.K. Management of aggressive lymphoma after CAR T-cell therapy failure. Hematol. Am. Soc. Hematol. Educ. Program. 2023, 2023, 364–369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, Q.; Li, X.; Liu, C.; Jiang, Y.; He, Y.; Zhang, W.; Azevedo, H.S.; Wu, W.; Xia, Y.; He, W. Improving cancer immunotherapy via co-delivering checkpoint blockade and thrombospondin-1 downregulator. Acta Pharm. Sin. B. 2023, 13, 3503–3517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Wang, F.; Wang, L.; Liu, Z.; Liu, M.; Li, S.; Wang, Y.; Sun, X.; Jiang, J. Detection of antibody-conjugate payload in cynomolgus monkey serum by a high throughput capture LC-MS/MS bioanalysis method. J. Pharm. Biomed. Anal. 2023, 227, 115069. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.M.; Hutchings, M.; Domingo-Domenech, E.; Eichenauer, D.A.; Advani, R. Anti-CD30 antibody-drug conjugate therapy in lymphoma: Current knowledge, remaining controversies, and future perspectives. Ann. Hematol. 2023, 102, 13–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivasi, M.; Porretta Serapiglia, C.; Medici, G.; Ricchi, L. Extravasation of brentuximab vedotin, an antibody-drug conjugate, in a patient with anaplastic large cell lymphoma. Eur. J. Hosp. Pharm. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lasater, E.A.; Amin, D.N.; Bannerji, R.; Mali, R.S.; Barrett, K.; Rys, R.N.; Oeh, J.; Lin, E.; Sterne-Weiler, T.; Ingalla, E.R.; et al. Targeting MCL-1 and BCL-2 with polatuzumab vedotin and venetoclax overcomes treatment resistance in R/R non-Hodgkin lymphoma: Results from preclinical models and a Phase Ib study. Am. J. Hematol. 2023, 98, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Cancemi, G.; Campo, C.; Caserta, S.; Rizzotti, I.; Mannina, D. Single-agent and associated therapies with monoclonal antibodies: What about follicular lymphoma? Cancers 2025, 17, 1602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, L.; Cheng, W.; Luo, Q.; Huang, C.; Yu, L. Targeting epigenetic reprogramming in DLBCL and its interaction with the tumor microenvironment for novel therapeutic approaches. Int. Immunopharmacol. 2025, 161, 115012, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lian, J.; Chen, Y.; Quan, L.; Guo, X.; Zhang, J.; Liu, Z.; Liu, A. Factors affecting refractoriness or recurrence in diffuse large B-cell lymphoma: Development and validation of a novel predictive nomogram. Hematology 2025, 30, 2445395. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Cancemi, G.; Loreta, S.; Allegra, A.; Stagno, F. Hematological malignancies in older patients: Focus on the potential role of a geriatric assessment management. Diagnostics 2024, 14, 1390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izutsu, K.; Kumode, T.; Yuda, J.; Nagai, H.; Mishima, Y.; Suehiro, Y.; Yamamoto, K.; Fujisaki, T.; Ishitsuka, K.; Ishizawa, K.; et al. Efficacy and safety of epcoritamab in Japanese patients with relapsed or refractory diffuse large B-cell lymphoma: 3-year follow-up from the EPCORE NHL-3 trial. Int. J. Clin. Oncol. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fleury, I.; MacDonald, D.; Shafey, M.; Christofides, A.; Sehn, L.H. Optimal Use of Bispecific Antibodies for the Treatment of Diffuse Large B-Cell Lymphoma in Canada. Curr. Oncol. 2025, 32, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naman, J.; Shah, N.; Heyman, B.M. Antibody Therapy for Patients with Lymphoid Malignancies: Past and Present. Int. J. Mol. Sci. 2025, 26, 1711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, H.; Zou, L.; Hu, M. Cost-effectiveness analysis of glofitamab versus rituximab for relapsed or refractory diffuse large B-cell lymphoma patients in China. Int. J. Clin. Pharm. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.Q.; Zhang, T.J.; Feng, Y.; Shao, X.; Ji, Y.H.; Qian, J.; Zhou, J.D. Successful treatment of primary refractory DLBCL/HGBL—MYC/BCL2 transformed from FL using glofitamab: A case report. Front. Immunol. 2025, 16, 1566035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minson, A.G.; Dickinson, M.J. New bispecific antibodies in diffuse large B-cell lymphoma. Haematologica 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nigro, A. Reversibility of acrocyanosis and improvement of capillaroscopic pattern in a patient with polycythemia vera treated with ruxolitinib: A case report. Blood Res. 2025, 60, 8, Erratum in Blood Res. 2025, 60, 10. https://doi.org/10.1007/s44313-025-00059-5; Erratum in Blood Res. 2025, 60, 12. https://doi.org/10.1007/s44313-025-00060-y; Erratum in Blood Res. 2025, 60, 31. https://doi.org/10.1007/s44313-025-00083-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, G.; Hamadani, M.; Al-Juhaishi, T. The potential of antibody-drug conjugates for effective therapy in diffuse large B-cell lymphoma. Expert Opin. Biol. Ther. 2025, 25, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Nasta, S.D.; Barta, S.K.; Chong, E.A.; Svoboda, J.; Schuster, S.J.; Landsburg, D.J. Analysis of histologic, immunohistochemical and genomic features of large B cell lymphoma tumors may predict response to polatuzumab vedotin based therapy in patients with relapsed/refractory disease. Clin. Lymphoma Myeloma Leuk. 2025, 25, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Alshemmari, S.; Hamadah, A.; Ousia, S.; Hamed, R.A.T.; Zaky, H. Management of diffuse large B-cell lymphoma as post-transplant lymphoproliferative disorder in a kidney transplant recipient: A case report. Hematol. Rep. 2025, 17, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zelikson, V.; Gurumurthi, A.; Sawalha, Y.; Annunzio, K.; Saha, A.; Dong, N.; Qualls, D.; Amoozgar, B.; Kahl, B.; Baird, J.; et al. Loncastuximab in high-risk and heavily pretreated relapsed/refractory diffuse large B-cell lymphoma: A real-world analysis from 21 US centers. Haematologica 2025, 110, 706–714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Topp, M.S.; Matasar, M.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; Arnason, J.E.; Michot, J.M.; Goldschmidt, N.; O’Brien, S.M.; Abadi, U.; et al. Odronextamab monotherapy in R/R DLBCL after progression with CAR T-cell therapy: Primary analysis of the ELM-1 study. Blood. 2025, 145, 1498–1509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, W.S.; Kim, T.M.; Cho, S.G.; Jarque, I.; Iskierka-Jażdżewska, E.; Poon, L.M.; Prince, H.M.; Zhang, H.; Cao, J.; Zhang, M.; et al. Author Correction: Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: Primary efficacy and safety analysis in phase 2 ELM-2 trial. Nat. Cancer. 2025, 6, 528–539, Erratum in Nat. Cancer 2025, 6, 907. https://doi.org/10.1038/s43018-025-00967-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, J.; Montalvo-Ortiz, W.; Yu, L.; Krasco, A.; Olson, K.; Rizvi, S.; Fiaschi, N.; Coetzee, S.; Wang, F.; Ullman, E.; et al. CD22-targeted CD28 bispecific antibody enhances antitumor efficacy of odronextamab in refractory diffuse large B cell lymphoma models. Sci. Transl. Med. 2022, 14, eabn1082. [Google Scholar] [CrossRef] [PubMed]


| Trial Name/NCT Number | Phase | Therapeutic Combination | Indication | Objective |
|---|---|---|---|---|
| NCT06492837 | Phase I | Mosunetuzumab + Zanubrutinib | B-NHL | Assess early safety/efficacy data |
| NCT05410418 | Phase II | Mosunetuzumab + Polatuzumab Vedotin | B-NHL | Expand indications for combination therapies |
| NCT06453044 | Phase I | Mosunetuzumab + Polatuzumab Vedotin | B-NHL | Evaluate efficacy in early-line or refractory cases |
| EPCORE NHL-1 | Phase I/II | Epcoritamab monotherapy | R/R LBCL | Evaluate efficacy/safety in heavily pretreated patients |
| EPCORE DLBCL-1 | Phase III | Epcoritamab + R-CHOP vs. R-CHOP | Previously untreated DLBCL | Compare efficacy with standard chemoimmunotherapy |
| NCT05660967 | Phase II | Epcoritamab + Lenalidomide | B-NHL | Assess combination in relapsed/refractory settings |
| NCT05283720 | Phase I/II | Epcoritamab + Polatuzumab Vedotin | B-NHL | Explore safety/efficacy in combination regimens |
| NCT06071871 | Phase I/II | Glofitamab + Polatuzumab Vedotin ± Chemotherapy | Aggressive B-NHL | Assess efficacy in earlier lines or in combination regimens |
| LOTIS-3 | Phase Ib/II | Loncastuximab + Ibrutinib | R/R DLBCL | Evaluate combination therapy efficacy and safety |
| LOTIS-5 | Phase III | Loncastuximab + Rituximab vs. standard immunochemotherapy | Transplant-ineligible R/R DLBCL | Compare combination therapy to standard immunochemotherapy |
| Trial Name/NCT Number | Phase | Therapeutic Combination | Indication | Objective |
|---|---|---|---|---|
| NCT02995617 | Phase I/II | Odronextamab monotherapy | R/R DLBCL | Evaluate efficacy in pretreated patients |
| NCT06054776 | Phase I/II | Glofitamab + acalabrutinib+ Obinutuzumab | R/R MCL | Evaluate safety and MRD negativity, DoR, PFS, and OS in pretreated patients |
| NCT07003295 | Phase I/II | Glofitamab + obinutuzumab | R/R MCL | Evaluate ORR, CR rates, PFS, OS at 24 months, and the incidence of grade 3–4 CRS in post-CAR-T patients |
| NCT06252675 | Phase II | Glofitamab + pirtobrutinib | R/R MCL | Evaluate efficacy/safety and tolerability in pretreated patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caserta, S.; Campo, C.; Cancemi, G.; Neri, S.; Stagno, F.; Mannina, D.; Allegra, A. Bispecific Antibodies and Antibody–Drug Conjugates in Relapsed/Refractory Aggressive Non-Hodgkin Lymphoma, Focusing on Diffuse Large B-Cell Lymphoma. Cancers 2025, 17, 2479. https://doi.org/10.3390/cancers17152479
Caserta S, Campo C, Cancemi G, Neri S, Stagno F, Mannina D, Allegra A. Bispecific Antibodies and Antibody–Drug Conjugates in Relapsed/Refractory Aggressive Non-Hodgkin Lymphoma, Focusing on Diffuse Large B-Cell Lymphoma. Cancers. 2025; 17(15):2479. https://doi.org/10.3390/cancers17152479
Chicago/Turabian StyleCaserta, Santino, Chiara Campo, Gabriella Cancemi, Santo Neri, Fabio Stagno, Donato Mannina, and Alessandro Allegra. 2025. "Bispecific Antibodies and Antibody–Drug Conjugates in Relapsed/Refractory Aggressive Non-Hodgkin Lymphoma, Focusing on Diffuse Large B-Cell Lymphoma" Cancers 17, no. 15: 2479. https://doi.org/10.3390/cancers17152479
APA StyleCaserta, S., Campo, C., Cancemi, G., Neri, S., Stagno, F., Mannina, D., & Allegra, A. (2025). Bispecific Antibodies and Antibody–Drug Conjugates in Relapsed/Refractory Aggressive Non-Hodgkin Lymphoma, Focusing on Diffuse Large B-Cell Lymphoma. Cancers, 17(15), 2479. https://doi.org/10.3390/cancers17152479






