Diagnosis and Management of Upper Tract Urothelial Carcinoma: A Review
Simple Summary
Abstract
1. Introduction
2. Diagnosis and Guideline Overview
3. Neoadjuvant Therapy
4. Surgical Management
4.1. Endoscopic (Kidney-Sparing) Management (EM)
4.2. Radical Surgery
4.3. Surgical Decision Making
5. Adjuvant Intraluminal Therapy
6. Adjuvant Systemic Therapy
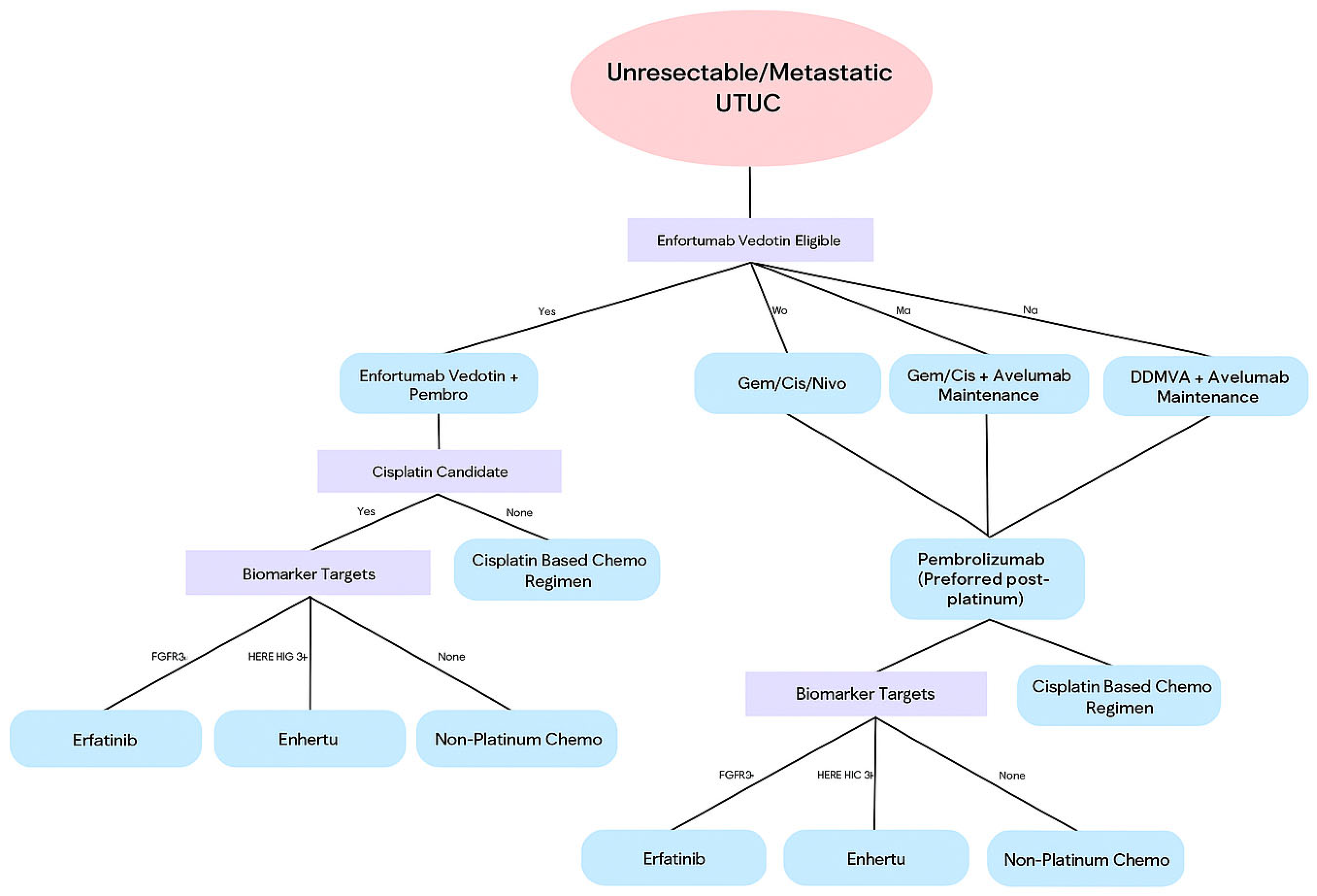
7. Systemic Therapy in Metastatic Disease
8. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Ng, K.L. The Etiology of Renal Cell Carcinoma and Upper Tract Urothelial Carcinoma. In Urologic Cancers; Barber, N., Ali, A., Eds.; Exon Publications: Brisbane, Australia, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK585973/ (accessed on 21 May 2025).
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Andersson, I.G.; Liedberg, F.; Mariappan, P.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Eva, C.; Johannes, K.; Shahrokh, S.; Gabriel, W. Updates on Urothelial Carcinoma of the Upper Urinary Tract with a Focus on Molecular Findings. Surg. Pathol. Clin. 2025, 18, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.M.; Lee, H.Y.; Singla, N.; Margulis, V.; Lotan, Y.; Woldu, S.L.; Huang, C.-N.; Li, C.-C.; Ke, H.-L.; Li, W.-M.; et al. Prognostic Factors for Contralateral Recurrence of Upper Tract Urothelial Carcinoma after Nephroureterectomy: A Large Multiregional Study. Cancers 2021, 13, 5935. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Kikuchi, E.; Ogihara, K.; Niwa, N.; Shigeta, K.; Masuda, T.; Baba, Y.; Mizuno, R.; Oya, M. Urinary pH is an independent predictor of upper tract recurrence in non-muscle-invasive bladder cancer patients with a smoking history. Sci. Rep. 2021, 11, 20675. [Google Scholar] [CrossRef] [PubMed]
- Golabesk, T.; Palou, J.; Rodriguez, O.; Parada, R.; Skrobot, S.; Peña, J.A.; Villavicencio, H. Long-term Bladder and Upper Urinary Tract Follow-up Recurrence and Progression Rates of G1-2 Non-muscle-invasive Urothelial Carcinoma of the Bladder. Urology 2017, 100, 145–150. [Google Scholar] [CrossRef]
- Coleman, J.A.; Clark, P.E.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Chou, R.; Hoffman-Censits, J.; Kulkarni, G.S.; Matin, S.F.; Pierorazio, P.M.; et al. Diagnosis and Management of Non-Metastatic Upper Tract Urothelial Carcinoma: AUA/SUO Guideline. J. Urol. 2023, 209, 1071–1081. [Google Scholar] [CrossRef]
- Oswald, D.; Pallauf, M.; Deininger, S.; Törzsök, P.; Sieberer, M.; Eiben, C. Neoadjuvant Chemotherapy before Nephroureterectomy in High-Risk Upper Tract Urothelial Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4841. [Google Scholar] [CrossRef]
- Leow, J.J.; Chong, Y.L.; Chang, S.L.; Valderrama, B.P.; Powles, T.; Bellmunt, J. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-analysis, and Future Perspectives on Systemic Therapy. Eur. Urol. 2021, 79, 635–654. [Google Scholar] [CrossRef]
- D’Andrea, D.; Matin, S.; Black, P.C.; Petros, F.G.; Zargar, H.; Dinney, C.P.; Cookson, M.S.; Kassouf, W.; Dall’ERa, M.A.; McGrath, J.S.; et al. Comparative effectiveness of neoadjuvant chemotherapy in bladder and upper urinary tract urothelial carcinoma. BJU Int. 2021, 127, 528–537. [Google Scholar] [CrossRef]
- Coleman, J.A.; Yip, W.; Wong, N.C.; Sjoberg, D.D.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Herr, H.W.; Cha, E.K.; Donahue, T.F.; et al. Multicenter Phase II Clinical Trial of Gemcitabine and Cisplatin as Neoadjuvant Chemotherapy for Patients with High-Grade Upper Tract Urothelial Carcinoma. J. Clin. Oncol. 2023, 41, 1618–1625. [Google Scholar] [CrossRef]
- Adibi, M.; McCormick, B.; Economides, M.P.; Petros, F.; Xiao, L.; Guo, C.; Shah, A.; Kamat, A.M.; Dinney, C.; Navai, N.; et al. Five and Ten-Year Outcomes of Neoadjuvant Chemotherapy and Surgery for High-Risk Upper Tract Urothelial Carcinoma. Clin. Genitourin. Cancer 2022, 20, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Margulis, V.; Puligandla, M.; Trabulsi, E.J.; Plimack, E.R.; Kessler, E.R.; Matin, S.F.; Godoy, G.; Alva, A.; Hahn, N.M.; Carducci, M.A.; et al. Phase II Trial of Neoadjuvant Systemic Chemotherapy Followed by Extirpative Surgery in Patients with High Grade Upper Tract Urothelial Carcinoma. J. Urol. 2020, 203, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Gravis, G.; Flechon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulie, M.; Allory, Y.; et al. Perioperative dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer (VESPER): Survival endpoints at 5 years in an open-label, randomised, phase 3 study. Lancet Oncol. 2024, 25, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Martini, A.; Raggi, D.; Cucchiara, V.; Colecchia, M.; Lucianò, R.; Villa, L.; Mazzone, E.; Basile, G.; Scuderi, S.; et al. A feasibility study of preoperative pembrolizumab before radical nephroureterectomy in patients with high-risk, upper tract urothelial carcinoma: PURE-02. Urol. Oncol. 2022, 40, e1–e10. [Google Scholar] [CrossRef]
- Houédé, N.; Chevallier, T.; Audenet, F.; Thibault, C.; Neuzillet, Y.; Abraham, C.; Masson-Lecomte, A.; Gauthier, H.; Gravis, G.; Pignot, G.; et al. Safety and Efficacy of Neoadjuvant Durvalumab Plus Gemcitabine/Cisplatin or Carboplatin in Patients with Operable High-Risk Upper Tract Urothelial Carcinoma: The iNDUCT-GETUG V08 Trial. J. Clin. Oncol. 2025, 43, 1578–1586. [Google Scholar] [CrossRef]
- Myers, A.A.; Pak, R.W. Novel laser therapies and new technologies in the endoscopic management of upper tract urothelial carcinoma: A narrative review. Transl. Androl. Urol. 2023, 12, 1723–1731. [Google Scholar] [CrossRef]
- Gallioli, A.; Uleri, A.; Verri, P.; Tedde, A.; Mertens, L.S.; Moschini, M.; Del Giudice, F.; Soria, F.; Laukhtina, E.; Subiela, J.D.; et al. Oncologic Outcomes of Endoscopic Management of Upper Tract Urothelial Carcinoma: A Systematic Review and Pooled Analysis from the EAU-YAU Urothelial Working Group. Eur. Urol. Focus 2025. [Google Scholar] [CrossRef]
- Grasso, M.; Fishman, A.I.; Cohen, J.; Alexander, B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: A 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012, 110, 1618–1626. [Google Scholar] [CrossRef]
- Scotland, K.B.; Kleinmann, N.; Cason, D.; Hubbard, L.; Tanimoto, R.; Healy, K.A.; Hubosky, S.G.; Bagley, D.H. Ureteroscopic Management of Large ≥2 cm Upper Tract Urothelial Carcinoma: A Comprehensive 23-Year Experience. Urology 2018, 121, 66–73. [Google Scholar] [CrossRef]
- Gallioli, A.; Boissier, R.; Territo, A.; Reyes, H.V.; Sanguedolce, F.; Gaya, J.M.; Regis, F.; Subiela, J.D.; Palou, J.; Breda, A. Adjuvant Single-Dose Upper Urinary Tract Instillation of Mitomycin C After Therapeutic Ureteroscopy for Upper Tract Urothelial Carcinoma: A Single-Centre Prospective Non-Randomized Trial. J. Endourol. 2020, 34, 573–580. [Google Scholar] [CrossRef]
- Cutress, M.L.; Stewart, G.D.; Wells-Cole, S.; Phipps, S.; Thomas, B.G.; Tolley, D.A. Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int. 2012, 110, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Shenhar, C.; Veredgorn, Y.; Bulis, S.; Aviv, T.; Darawsha, A.E.; Gilad, R.; Baniel, J.; Ehrlich, Y.; Lifshitz, D. Endoscopic Management of Low-Grade Upper Tract Urothelial Carcinoma: Characterizing the Long-term Burden of Care in Comparison to Radical Nephroureterectomy. Urology 2022, 159, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Thacker, K.; Raman, J.D.; McLean, T.; Said, J.; Oliver, L.; Gore, J.L. Understanding the Economic Burden of Treating Low-Grade Upper Tract Urothelial Cancer in the United States. Urol. Pract. 2021, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. The characteristics of recurrent upper tract urothelial carcinoma after radical nephroureterectomy without bladder cuff excision. Yonsei Med. J. 2015, 56, 375–381. [Google Scholar] [CrossRef]
- Lughezzani, G.; Sun, M.; Perrotte, P.; Shariat, S.F.; Jeldres, C.; Budaus, L.; Alasker, A.; Duclos, A.; Widmer, H.; Latour, M.; et al. Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur. Urol. 2010, 57, 956–962. [Google Scholar] [CrossRef]
- Guo, R.; Zhu, Y.; Xiong, G.; Li, X.; Zhang, K.; Zhou, L. Role of lymph node dissection in the management of upper tract urothelial carcinomas: A meta-analysis. BMC Urol. 2018, 18, 24. [Google Scholar] [CrossRef]
- Chan, V.W.S.; Wong, C.H.M.; Yuan, Y.; Teoh, J.Y.C. Lymph node dissection for upper tract urothelial carcinoma: A systematic review. Arab. J. Urol. 2020, 19, 37–45. [Google Scholar] [CrossRef]
- O’Brien, T.; Ray, E.; Singh, R.; Coker, B.; Beard, R.; British Association of Urological Surgeons Section of Oncology. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: A prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur. Urol. 2011, 60, 703–710. [Google Scholar] [CrossRef]
- Ito, A.; Shintaku, I.; Satoh, M.; Ioritani, N.; Aizawa, M.; Tochigi, T.; Kawamura, S.; Aoki, H.; Numata, I.; Takeda, A.; et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: The THP Monotherapy Study Group Trial. J. Clin. Oncol. 2013, 31, 1422–1427. [Google Scholar] [CrossRef]
- Freifeld, Y.; Ghandour, R.; Singla, N.; Woldu, S.; Bagrodia, A.; Lotan, Y.; Rapoport, L.M.; Gazimiev, M.; Delafuente, K.; Kulangara, R.; et al. Intraoperative prophylactic intravesical chemotherapy to reduce bladder recurrence following radical nephroureterectomy. Urol. Oncol. 2020, 38, e11–e737. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Guaglianone, S.; Ferriero, M.; Leonardo, C.; Forastiere, E.; Gallucci, M. Laparoscopic versus open nephroureterectomy: Perioperative and oncologic outcomes from a randomised prospective study. Eur. Urol. 2009, 56, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, C.; Sun, S.; Ning, K.; Tang, S. Kidney sparing surgery versus radical nephroureterectomy in upper tract urothelial carcinoma: A meta-analysis and systematic review. Front. Oncol. 2025, 15, 1448079. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Laukhtina, E.; Quhal, F.; Yanagisawa, T.; Rajwa, P.; Pallauf, M.; von Deimling, M.; Bianchi, A.; Pradere, B.; Fajkovic, H.; et al. Oncologic and Safety Outcomes for Endoscopic Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An Updated Systematic Review and Meta-analysis. Eur. Urol. Focus 2023, 9, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Kleinmann, N.; Matin, S.F.; Pierorazio, P.M.; Gore, J.L.; Shabsigh, A.; Hu, B.; Chamie, K.; Godoy, G.; Hubosky, S.; Rivera, M.; et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): An open-label, single-arm, phase 3 trial. Lancet Oncol. 2020, 21, 776–785. [Google Scholar] [CrossRef]
- Labbate, C.; Woldu, S.; Murray, K.; Rose, K.; Sexton, W.; Tachibana, I.; Kaimakliotis, H.; Jacob, J.; Dickstein, R.; Linehan, J.; et al. Efficacy and Safety of Mitomycin Gel (UGN-101) as an Adjuvant Therapy After Complete Endoscopic Management of Upper Tract Urothelial Carcinoma. J. Urol. 2023, 209, 872–881. [Google Scholar] [CrossRef]
- Territo, A.; Fontanet, S.; Meneghetti, I.; Gallioli, A.; Sanguedolce, F.; Rodriguez-Faba, Ó.; Gaya, J.M.; Palou, J.; Huguet, J.; Breda, A. Management of primary upper urinary tract carcinoma in situ diagnosed by ureteroscopic biopsy: Is bacillus Calmette-Guerin an alternative to nephroureterectomy? Actas Urológicas Españolas (Engl. Ed.) 2023, 47, 221–228. [Google Scholar] [CrossRef]
- Redrow, G.P.; Guo, C.C.; Brausi, M.A.; Coleman, J.A.; Fernandez, M.I.; Kassouf, W.; Keeley, F.X.; Margulis, V.; Raman, J.D.; Roupret, M.; et al. Upper Urinary Tract Carcinoma In Situ: Current Knowledge, Future Direction. J. Urol. 2017, 197, 287–295. [Google Scholar] [CrossRef]
- Fontanet, S.; Gallioli, A.; Baboudjian, M.; Huguet, J.; Territo, A.; Gaya, J.M.; Gavrilov, P.; Izquierdo, P.; Verri, P.; Algaba, F.; et al. Topical instillation of BCG immunotherapy for biopsy-proven primary upper urinary tract carcinoma in situ: A single institution series and systematic review. Urol. Oncol. 2023, 41, 274–283. [Google Scholar] [CrossRef]
- Katims, A.B.; Tam, A.W.; Rosen, D.C.; Zampini, A.M.; Atallah, W.; Mehrazin, R.; Gupta, M. Novel treatment of upper tract urothelial carcinoma in situ with docetaxel in BCG refractory patients. Urol. Oncol. 2021, 39, e9–e234. [Google Scholar] [CrossRef]
- Foerster, B.; D’Andrea, D.; Abufaraj, M.; Broenimann, S.; Karakiewicz, P.I.; Rouprêt, M.; Gontero, P.; Lerner, S.P.; Shariat, S.F.; Soria, F. Endocavitary treatment for upper tract urothelial carcinoma: A meta-analysis of the current literature. Urol. Oncol. 2019, 37, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Birtle, A.J.; Jones, R.; Chester, J.; Lewis, R.; Biscombe, K.; Johnson, M.; Blacker, A.; Bryan, R.T.; Catto, J.W.; Choudhury, A.; et al. Improved Disease-Free Survival with Adjuvant Chemotherapy After Nephroureterectomy for Upper Tract Urothelial Cancer: Final Results of the POUT Trial. J. Clin. Oncol. 2024, 42, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Ballman, K.V.; Sonpavde, G.; Berg, S.; Kim, W.Y.; Parikh, R.; Teo, M.Y.; Sweis, R.F.; Geynisman, D.M.; Grivas, P.; et al. Adjuvant Pembrolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2025, 392, 45–55. [Google Scholar] [CrossRef]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Milowsky, M.I.; Petrylak, D.P.; Hoimes, C.J.; Flaig, T.W.; Mar, N.; Moon, H.H.; Friedlander, T.W.; McKay, R.R.; Bilen, M.A.; et al. Enfortumab Vedotin with or without Pembrolizumab in Cisplatin-Ineligible Patients with Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2023, 41, 4107–4117. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- Scher, H.I. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J. Urol. 1992, 148, 1625–1626. [Google Scholar]
- Sternberg, C.N.; de Mulder, P.H.; Schornagel, J.H.; Théodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, F.; Spina, M.; van Groeningen, C.J.; de Balincourt, C.; et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J. Clin. Oncol. 2001, 19, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; de Mulder, P.; Schornagel, J.H.; Theodore, C.; Fossa, S.; van Oosterom, A.; Witjes, J.; Spina, M.; van Groeningen, C.; Duclos, B.; et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur. J. Cancer 2006, 42, 50–54. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Vuky, J.; Balar, A.V.; Castellano, D.; O’dOnnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.; Hahn, N.M.; Savage, M.J.; et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients with Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2020, 38, 2658–2666. [Google Scholar] [CrossRef]
- Grivas, P.; Daneshmand, S.; Makarov, V.; Bellmunt, J.; Sridhar, S.S.; Sonpavde, G.P.; Cole, S.; Tripathi, A.; Faltas, B.M.; Lerner, S.P.; et al. Fibroblast growth factor receptor 3 (FGFR3) alterations in PROOF 302: A phase III trial of infigratinib (BGJ398) as adjuvant therapy in patients (pts) with invasive urothelial carcinoma (UC). J. Clin. Oncol. 2023, 41 (Suppl. 16), 4511. [Google Scholar] [CrossRef]
- Sfakianos, J.P.; Cha, E.K.; Iyer, G.; Scott, S.N.; Zabor, E.C.; Shah, R.H.; Ren, Q.; Bagrodia, A.; Kim, P.H.; Hakimi, A.A.; et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur. Urol. 2015, 68, 970–977. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J.H.; Valderrama, B.P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1961–1971. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Subiela, J.D.; Territo, A.; Mercadé, A.; Balañà, J.; Aumatell, J.; Calderon, J.; Gallioli, A.; González-Padilla, D.A.; Gaya, J.M.; Palou, J.; et al. Diagnostic accuracy of ureteroscopic biopsy in predicting stage and grade at final pathology in upper tract urothelial carcinoma: Systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Huelster, H.L.; Gould, B.; Schiftan, E.A.; Camperlengo, L.; Davaro, F.; Rose, K.M.; Soupir, A.C.; Jia, S.; Zheng, T.; Sexton, W.J.; et al. Novel Use of Circulating Tumor DNA to Identify Muscle-invasive and Non-organ-confined Upper Tract Urothelial Carcinoma. Eur. Urol. 2024, 85, 283–292. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Ji, X.; Wang, Y.; Niu, H. Integrated multiomics analysis and machine learning refine molecular subtypes and prognosis for muscle-invasive urothelial cancer. Mol. Ther. Nucleic Acids 2023, 33, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Shu, T.; Al Shaarani, M.; Cen, P. Complete Pathologic Response with Pembrolizumab and Enfortumab Vedotin in Urothelial Carcinoma of the Upper Urinary Tract. J. Investig. Med. High Impact Case Rep. 2024, 12, 23247096241257333. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Deng, Y.; Guo, H.; Wu, H. Postoperative adjuvant treatment of HER2 overexpression in upper urinary tract urothelial carcinoma with the combination of disitamab vedotin and toripalimab: A real-world retrospective study. J. Clin. Oncol. 2025, 43 (Suppl. 5), 673. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2023-04595 (accessed on 8 June 2025).
- Pembrolizumab and Enfortumab Vedotin with Pembrolizumab Prior to and After Radical Nephroureterectomy for High-Risk Upper Tract Urothelial Cancer. ClinicalTrials.Gov IDNCT05775471. Available online: https://www.clinicaltrials.gov/study/NCT05775471 (accessed on 8 June 2025).
- O’Donnell, P.H.; Hoimes, C.J.; Rosenberg, J.E.; Petrylak, D.P.; Mar, N.; Barata, P.C.; Srinivas, S.; Gourdin, T.S.; Henry, E.; Bilen, M.A.; et al. Study EV-103: Neoadjuvant treatment with enfortumab vedotin monotherapy in cisplatin-ineligible patients with muscle invasive bladder cancer (MIBC)—2-year event-free survival and safety data for Cohort H. J. Clin. Oncol. 2024, 42 (Suppl. 16), 4564. [Google Scholar] [CrossRef]
- Necchi, A.; Bedke, J.; Galsky, M.D.; Shore, N.D.; Plimack, E.R.; Xylinas, E.; Jia, C.; Hennika, T.; Moreno, B.H.; Witjes, A.A. Phase 3 KEYNOTE-905/EV-303: Perioperative pembrolizumab (pembro) or pembro + enfortumab vedotin (EV) for muscle-invasive bladder cancer (MIBC). J. Clin. Oncol. 2023, 41 (Suppl. 6), TPS585. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Loriot, Y.; Bedke, J.; Nishiyama, H.; Kataria, R.S.; Moreno, B.H.; Galsky, M.D. Perioperative enfortumab vedotin (EV) plus pembrolizumab (pembro) versus chemotherapy in cisplatin-eligible patients (pts) with muscle-invasive bladder cancer (MIBC): Phase 3 KEYNOTE-B15/EV-304. J. Clin. Oncol. 2023, 41 (Suppl. 6), TPS588. [Google Scholar] [CrossRef]
| Feature | Low Risk | High Risk | ||
| Histologic Grade | Low grade | High grade | ||
| Sub-stratification | Favorable | Unfavorable | Favorable | Unfavorable |
| Cytology | Negative | No HGUC | Any | HGUC |
| Imaging | No invasion, obstruction, or LAD | No invasion or LAD; Yes, obstruction | No invasion, obstruction, or LAD | Yes, invasion, obstruction, suspicious LAD |
| Focality | Unifocal | Multifocal | Unifocal | Multifocal |
| Appearance | Papillary | Papillary | Papillary | Sessile or flat |
| Lower Tract Involvement | No | Yes | No | Yes |
| Indications | Surgical Approach | Intravesical Chemotherapy | Oncologic Outcomes | Surveillance | |
| Endoscopic Management (EM) |
|
|
|
|
|
| Radical Surgery (RNU or SU) |
|
|
|
|
|
| Instillation Approach | Agents Studied | Evidence |
|---|---|---|
|
|
|
| Study | Adjuvant Therapy | Population | Disease Free Survival |
|---|---|---|---|
| POUT | Platinum-Based Chemo | 261 patients w/non-metastatic UTUC | DFS at 5 years: 66% vs. 57% |
| CheckMate 274 | Nivolumab | 709 patients with MIBC, including 149 with UTUC | No DFS benefit in UTUC subgroup |
| AMBASSADOR | Pembrolizumab | 702 patients with MIBC, including 154 with UTUC | No DFS benefit in UTUC subgroup |
| IMvigor010 | Atezolizumab | 809 patients with MIBC, including 54 with UTUC | No DFS benefit in UTUC subgroup |
| Study | Systemic Therapy | Median OS | Key Outcomes |
|---|---|---|---|
| EV-302 | Enfortumab Vedotin vs. Platinum-Based Chemo | 31.5 months vs. 16.1 months | Median OS: not estimable vs. 18.4 months in UTUC subgroup |
| CheckMate 901 | Gem/Cis + Nivolumab vs. Gem/Cis | 21.7 months vs. 18.9 months | UTUC comprised 10.9% of nivolumab arm and 14.5% of the chemo arm |
| von der Maase H et al. | Gem/Cis vs. ddMVAC | 13.8 months vs. 14.8 months | Gem/Cis has comparable OS to ddMVAC w/ better safety profile |
| JAVELIN Bladder 100 | Maintenance Avelumab post Platinum Chemo vs. Observation | 21.4 months vs. 14.3 months | UTUC comprised 26% of study population |
| KEYNOTE 052 | Pembrolizumab | 11.3 months | Approved for patients ineligible for any platinum-containing therapy |
| THOR | Erdafitinib vs. Chemo | 12.1 months vs. 7.8 months | Approved for patients with FGFR2/3 mutations |
| DESTINY-PanTumor02 | Trastuzumab deruxtecan (T-DXd) | 12.8 months | Approved for HER2-positive IHC3+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, D.; Wang, C.; Suboc, N.; D’Souza, A.; Tulpule, V. Diagnosis and Management of Upper Tract Urothelial Carcinoma: A Review. Cancers 2025, 17, 2467. https://doi.org/10.3390/cancers17152467
Escobar D, Wang C, Suboc N, D’Souza A, Tulpule V. Diagnosis and Management of Upper Tract Urothelial Carcinoma: A Review. Cancers. 2025; 17(15):2467. https://doi.org/10.3390/cancers17152467
Chicago/Turabian StyleEscobar, Domenique, Christopher Wang, Noah Suboc, Anishka D’Souza, and Varsha Tulpule. 2025. "Diagnosis and Management of Upper Tract Urothelial Carcinoma: A Review" Cancers 17, no. 15: 2467. https://doi.org/10.3390/cancers17152467
APA StyleEscobar, D., Wang, C., Suboc, N., D’Souza, A., & Tulpule, V. (2025). Diagnosis and Management of Upper Tract Urothelial Carcinoma: A Review. Cancers, 17(15), 2467. https://doi.org/10.3390/cancers17152467






