Metformin Beyond Diabetes: A Precision Gerotherapeutic and Immunometabolic Adjuvant for Aging and Cancer
Simple Summary
Abstract
1. Introduction
2. Methodology
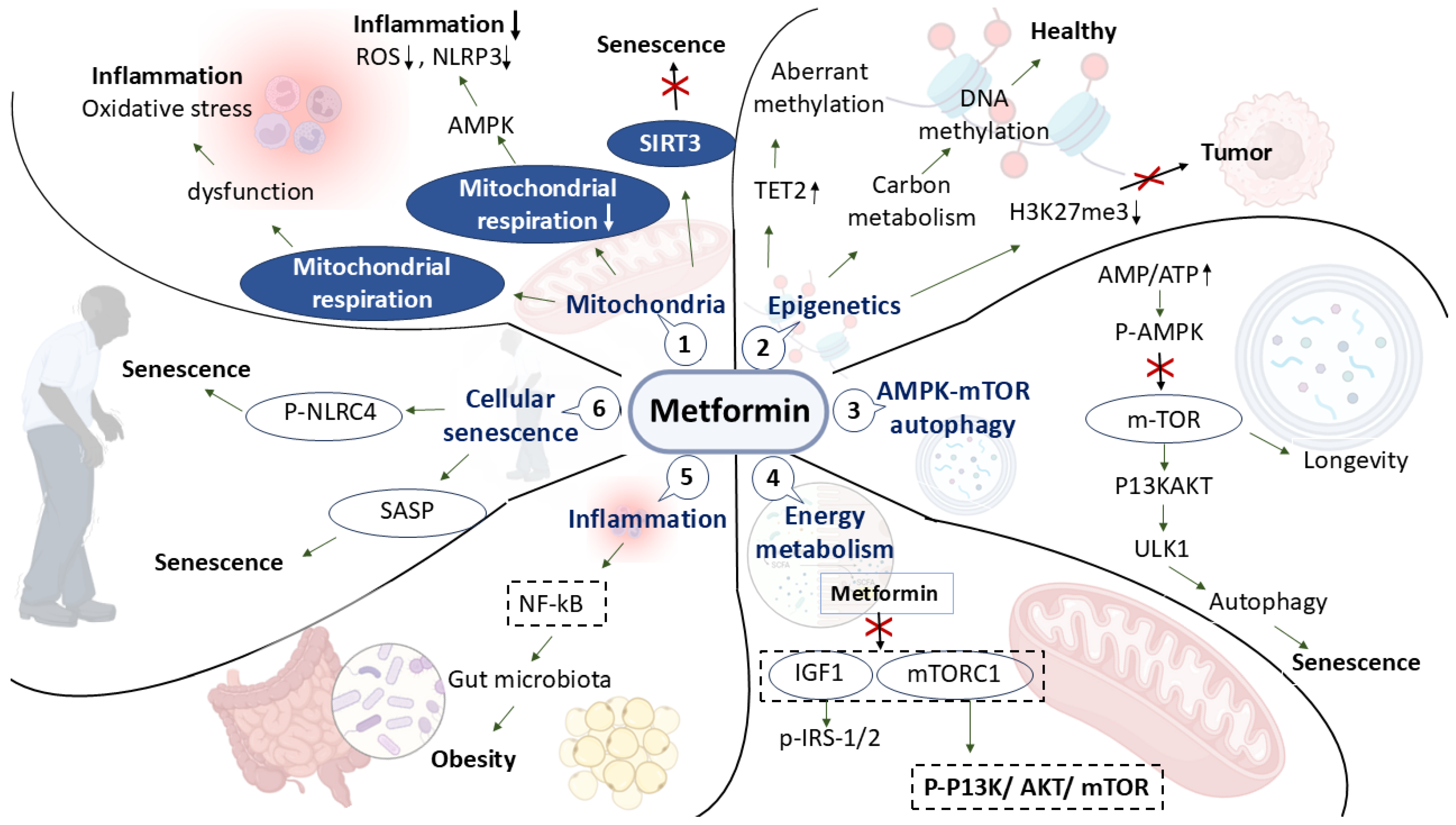
3. Molecular Pharmacology: Systems Level Mechanisms
4. Metabolic Reprogramming and Immunomodulation
5. Addressing the Hallmarks of Aging
6. Future Directions Toward Precision Gerotherapeutics and Oncology
6.1. Combination Therapies
6.2. Optimized Dosing and Delivery
6.3. Precision Biomarkers
6.4. Regulatory and Translational Pathways
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| A. muciniphila | Akkermansia muciniphila |
| AC-T | Adriamycin and Cyclophosphamide followed by Taxane |
| AKT | Protein Kinase B |
| AMPK | AMP-activated Protein Kinase |
| AMP/ATP | Adenosine Monophosphate/Adenosine Triphosphate |
| BID | Twice Daily |
| BMI | Body Mass Index |
| BCS | Breast-Conserving Surgery |
| CAR-T | Chimeric Antigen Receptor T-cell |
| CD107a | Cluster of Differentiation 107a |
| circRNA | Circular RNA |
| CR | Complete Response |
| CRPC | Castration-Resistant Prostate Cancer |
| CRTC2 | CREB-Regulated Transcription Coactivator 2 |
| CTL | Cytotoxic T Lymphocyte |
| CTLA4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| DC | Dendritic Cell |
| DFS | Disease-Free Survival |
| DLBCL | Diffuse Large B-cell Lymphoma |
| DR4/DR5 | Death Receptor 4/5 |
| ERAD | Endoplasmic Reticulum-Associated Degradation |
| ET | Endocrine Therapy |
| FAZA | Fluoroazomycin Arabinoside |
| FHV | FAZA Hypoxic Volume |
| FOXP3 | Forkhead Box Protein P3 |
| FAS | Fatty Acid Synthase |
| FXR | Farnesoid X Receptor |
| G6Pase | Glucose-6-Phosphatase |
| GI | Gastrointestinal |
| GLUT4 | Glucose Transporter Type 4 |
| H2BS36ph | Histone H2B Serine 36 Phosphorylation |
| H3K27me3 | Tri-methylation of Lysine 27 on Histone H3 |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| HR | Hazard Ratio/Hormone Receptor |
| IFN-γ | Interferon-gamma |
| IGF1 | Insulin-like Growth Factor 1 |
| IL-6 | Interleukin-6 |
| IRS-1/2 | Insulin Receptor Substrate 1/2 |
| LVEF | Left Ventricular Ejection Fraction |
| MA.32 | Metformin Atrial Trial 32 |
| MCI | Mild Cognitive Impairment |
| MDSC | Myeloid-Derived Suppressor Cells |
| MET | Metformin (as nanoparticles) |
| MQ | Macrophage |
| mCRPC | Metastatic Castration-Resistant Prostate Cancer |
| MHC-I | Major Histocompatibility Complex Class I |
| mPTP | Mitochondrial Permeability Transition Pore |
| mTOR | Mammalian Target of Rapamycin |
| mTORC1 | mTOR Complex 1 |
| NACT | Neoadjuvant Chemotherapy |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NK cell | Natural Killer Cell |
| NLRC4 | NLR Family CARD Domain-Containing Protein 4 |
| NLRP3 | NOD-like Receptor Family Pyrin Domain-Containing Protein 3 |
| NRF2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| NR | Not Reached |
| NSCLC | Non-Small Cell Lung Cancer |
| ORR | Overall Response Rate |
| OS | Overall Survival |
| P-AKT | Phosphorylated AKT |
| P-AMPK | Phosphorylated AMPK |
| P-NLRC4 | Phosphorylated NLRC4 |
| P-PD-L1 Ser195 | Phosphorylated Programmed Death-Ligand 1 at Serine 195 |
| P-PI3K | Phosphorylated Phosphoinositide 3-Kinase |
| PC | Pemetrexed + Cisplatin |
| PEPCK | Phosphoenolpyruvate Carboxykinase |
| pCR | Pathological Complete Response |
| PGC1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| PhenoAge | Phenotypic Age |
| PR | Partial Response |
| RCT | Randomized Controlled Trial |
| R-CHOP | Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| SCFA | Short-Chain Fatty Acids |
| SIRT3 | Sirtuin 3 |
| T-DM1 | Trastuzumab Emtansine |
| TAME | Targeting Aging with Metformin |
| Tcm | Central Memory T Cell |
| Tem | Effector Memory T Cell |
| TET2 | Ten-Eleven Translocation Methylcytosine Dioxygenase 2 |
| TIM-3 | T-cell immunoglobulin and mucin-domain-containing-3 |
| TGR5 | Takeda G-protein–coupled Receptor 5 |
| TME | Tumor Microenvironment |
| Treg | Regulatory T Cell |
| ULK1 | Unc-51 Like Autophagy Activating Kinase 1 |
| VEGF | Vascular Endothelial Growth Factor |
| VDAC1 | Voltage-Dependent Anion Channel 1 |
References
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Bailey, C.J. The origins of type 2 diabetes medications. Br. J. Diabetes 2022, 22, 112–120. [Google Scholar] [CrossRef]
- Al-Mosawi, A.J. The use of metformin in Non-Diabetic obesity: An educational article and Expert Opinion. J. Cancer Res. Cell. Ther. 2023, 7, 155. [Google Scholar] [CrossRef]
- Agius, L.; Ford, B.E.; Chachra, S.S. The metformin mechanism on gluconeogenesis and AMPK activation: The metabolite perspective. Int. J. Mol. Sci. 2020, 21, 3240. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Hsu, C.-C.; Wahlqvist, M.L.; Tsai, H.-N.; Chang, Y.-H.; Huang, Y.-C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-y.; Li, J.; Walcott, F.L.; Kang, J.-G.; Starost, M.F.; Talagala, S.L.; Zhuang, J.; Park, J.-H.; Huffstutler, R.D.; Bryla, C.M. Inhibiting mitochondrial respiration prevents cancer in a mouse model of Li-Fraumeni syndrome. J. Clin. Investig. 2017, 127, 132–136. [Google Scholar] [CrossRef]
- Higurashi, T.; Hosono, K.; Takahashi, H.; Komiya, Y.; Umezawa, S.; Sakai, E.; Uchiyama, T.; Taniguchi, L.; Hata, Y.; Uchiyama, S. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016, 17, 475–483. [Google Scholar] [CrossRef]
- Lord, S.R.; Harris, A.L. Is it still worth pursuing the repurposing of metformin as a cancer therapeutic? Br. J. Cancer 2023, 128, 958–966. [Google Scholar] [CrossRef]
- Klil-Drori, A.J.; Azoulay, L.; Pollak, M.N. Cancer, obesity, diabetes, and antidiabetic drugs: Is the fog clearing? Nat. Rev. Clin. Oncol. 2017, 14, 85–99. [Google Scholar] [CrossRef]
- Lord, S.R.; Cheng, W.-C.; Liu, D.; Gaude, E.; Haider, S.; Metcalf, T.; Patel, N.; Teoh, E.J.; Gleeson, F.; Bradley, K. Integrated pharmacodynamic analysis identifies two metabolic adaption pathways to metformin in breast cancer. Cell Metab. 2018, 28, 679–688.e674. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Peng, D.; Cai, Z.; Lin, H.-K. AMPK signaling and its targeting in cancer progression and treatment. Semin. Cancer Biol. 2022, 85, 52–68. [Google Scholar] [CrossRef]
- Chao, Y.; Wei, T.; Li, Q.; Liu, B.; Hao, Y.; Chen, M.; Wu, Y.; Song, F.; Chen, Q.; Liu, Z. Metformin-containing hydrogel scaffold to augment CAR-T therapy against post-surgical solid tumors. Biomaterials 2023, 295, 122052. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, S.; Espeland, M. Trials of geroscience-based therapeutics–the targeting aging with metformin (tame) example. Innov. Aging 2018, 2, 823. [Google Scholar] [CrossRef]
- Induri, S.N.R.; Kansara, P.; Thomas, S.C.; Xu, F.; Saxena, D.; Li, X. The gut microbiome, metformin, and aging. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 85–108. [Google Scholar] [CrossRef]
- Bharath, L.P.; Nikolajczyk, B.S. The intersection of metformin and inflammation. Am. J. Physiol.-Cell Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, Z.; Xu, X.; Sun, X.; Dong, X.; Luo, Y.; Sun, X. Extensive therapeutic effects, underlying molecular mechanisms and disease treatment prediction of Metformin: A systematic review. Transl. Res. 2024, 263, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B. Pro-Aging Metabolic Reprogramming: A Unified Theory of Aging. Engineering 2025, 44, 37–43. [Google Scholar] [CrossRef]
- Noll, J.H.; Levine, B.L.; June, C.H.; Fraietta, J.A. Beyond youth: Understanding CAR T cell fitness in the context of immunological aging. Semin. Immunol. 2023, 70, 101840. [Google Scholar] [CrossRef]
- Fu, T.E.; Zhou, Z. Senescent cells as a target for anti-aging interventions: From senolytics to immune therapies. J. Transl. Intern. Med. 2025, 13, 33–47. [Google Scholar] [CrossRef]
- Liu, Z.; Zuo, L.; Zhou, Z.; Liu, S.; Ba, Y.; Zuo, A.; Ren, Y.; Zhang, C.; Chen, Y.; Ma, H. Targeting immunosenescence for improved tumor immunotherapy. MedComm 2024, 5, e777. [Google Scholar] [CrossRef]
- Wrona, M.V.; Ghosh, R.; Coll, K.; Chun, C.; Yousefzadeh, M.J. The 3 I’s of immunity and aging: Immunosenescence, inflammaging, and immune resilience. Front. Aging 2024, 5, 1490302. [Google Scholar] [CrossRef]
- Ajzashokouhi, A.; Bostan, H.; Jomezadeh, V.; Hayes, A.; Karimi, G. A review on the cardioprotective mechanisms of metformin against doxorubicin. Hum. Exp. Toxicol. 2020, 39, 237–248. [Google Scholar] [CrossRef]
- Kuburas, R. The Investigation of the Cardioprotective Properties of Metformin DuZring Sunitinib-Induced Cytotoxicity. Doctoral Thesis, Coventry University, Coventry, UK, 2020. [Google Scholar]
- Haque, R.; Rajia, S. Metformin and Its Unique Molecular Pharmacology in Regulating Type 2 Diabetes. Eur. J. Biomed. 2022, 9, 92–98. [Google Scholar]
- Moonira, T.N. Mechanism of Action of Metformin on Glucose 6-Phosphate in Hepatocytes. Ph.D. Thesis, Newcastle University, Callaghan, Australia, 2019. [Google Scholar]
- Nogueira-Ferreira, R.; Oliveira, P.F.; Ferreira, R. Liver metabolism: The pathways underlying glucose utilization and production. In Glycolysis; Elsevier: Amsterdam, The Netherlands, 2024; pp. 141–156. [Google Scholar]
- Xu, J.; Wang, N.; Yang, L.; Zhong, J.; Chen, M. Intestinal flora and bile acid interactions impact the progression of diabetic kidney disease. Front. Endocrinol. 2024, 15, 1441415. [Google Scholar] [CrossRef]
- Gao, R.; Meng, X.; Xue, Y.; Mao, M.; Liu, Y.; Tian, X.; Sui, B.; Li, X.; Zhang, P. Bile acids-gut microbiota crosstalk contributes to the improvement of type 2 diabetes mellitus. Front. Pharmacol. 2022, 13, 1027212. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, M.; Liu, Y.; Zhou, Y.; Liu, H.; Wang, S.; Ji, Y. Role of Akkermansia muciniphila in insulin resistance. J. Gastroenterol. Hepatol. 2025, 40, 19–32. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Shetty, P.; Sainath, P.; Bharati, S.; Ahmed, A.Z.; Singh, V.K.; Ashwal, A. Metformin and dapagliflozin attenuate doxorubicin-induced acute cardiotoxicity in Wistar rats: An electrocardiographic, biochemical, and histopathological approach. Cardiovasc. Toxicol. 2023, 23, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and cancer hallmarks: Shedding new lights on therapeutic repurposing. J. Transl. Med. 2023, 21, 403. [Google Scholar] [CrossRef]
- Suwa, M.; Egashira, T.; Nakano, H.; Sasaki, H.; Kumagai, S. Metformin increases the PGC-1α protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J. Appl. Physiol. 2006, 101, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Hieber, C.; Grabbe, S.; Bros, M. Counteracting Immunosenescence—Which Therapeutic Strategies Are Promising? Biomolecules 2023, 13, 1085. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.-C.; Zhang, J.-Q.; Zou, J.-Y.; Zhang, X.; Chen, W.-M.; Gu, Y.; Hong, H. The effect of metformin on senescence of T lymphocytes. Immun. Ageing 2023, 20, 73. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Ye, X.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol. Res. 2022, 177, 106114. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.; Jurado-Aguilar, J.; Wahli, W.; Palomer, X.; Vázquez-Carrera, M. Increased hepatic gluconeogenesis and type 2 diabetes mellitus. Trends Endocrinol. Metab. 2024, 35, 1062–1077. [Google Scholar] [CrossRef]
- Lee, J.O.; Lee, S.K.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J. Biol. Chem. 2012, 287, 44121–44129. [Google Scholar] [CrossRef]
- Ko, M.; Kim, J.; Lazim, R.; Lee, J.Y.; Kim, J.Y.; Gosu, V.; Lee, Y.; Choi, S.; Kwon, H.J. The anticancer effect of metformin targets VDAC1 via ER-mitochondria interactions-mediated autophagy in HCC. Exp. Mol. Med. 2024, 56, 2714–2725. [Google Scholar] [CrossRef]
- De La Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Liu, Z.; Yang, K.; Lin, H.; Xiong, K. Non-coding RNAs as potential targets in metformin therapy for cancer. Cancer Cell Int. 2024, 24, 333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Yang, S.-F.; Yang, C.-K.; Tsai, H.-D.; Chen, T.-H.; Chou, M.-C.; Hsiao, Y.-H. Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells. Mol. Med. Rep. 2021, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Pescador, N.; Francisco, V.; Vázquez, P.; Esquinas, E.M.; González-Páramos, C.; Valdecantos, M.P.; García-Martínez, I.; Urrutia, A.A.; Ruiz, L.; Escalona-Garrido, C. Metformin reduces macrophage HIF1α-dependent proinflammatory signaling to restore brown adipocyte function in vitro. Redox Biol. 2021, 48, 102171. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Najafi, M. Targeting of the tumor immune microenvironment by metformin. J. Cell Commun. Signal. 2022, 16, 333–348. [Google Scholar] [CrossRef]
- Liu, X.; Yu, P.; Xu, Y.; Wang, Y.; Chen, J.; Tang, F.; Hu, Z.; Zhou, J.; Liu, L.; Qiu, W. Metformin induces tolerogenicity of dendritic cells by promoting metabolic reprogramming. Cell. Mol. Life Sci. 2023, 80, 283. [Google Scholar] [CrossRef]
- Abd El-Fattah, E.E.; Zakaria, A.Y. Metformin modulate immune fitness in hepatocellular carcinoma: Molecular and cellular approach. Int. Immunopharmacol. 2022, 109, 108889. [Google Scholar] [CrossRef] [PubMed]
- Hart, T. Metformin Action on Nuclear Transport. Doctoral Dissertation, Harvard University, Cambridge, MA, USA, 2023. [Google Scholar]
- Tao, H.; Zhu, P.; Xia, W.; Chu, M.; Chen, K.; Wang, Q.; Gu, Y.; Lu, X.; Bai, J.; Geng, D. The emerging role of the mitochondrial respiratory chain in skeletal aging. Aging Dis. 2024, 15, 1784. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, F. From bud scars to molecular insights: Investigating V-ATPase function and assembly in yeast replicative aging. bioRxiv 2024. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and insulin resistance: A review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Udono, H.; Nishida, M. Metformin-ROS-Nrf2 connection in the host defense mechanism against oxidative stress, apoptosis, cancers, and ageing. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2022, 1866, 130171. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Anand, U.; Nahon-Crystal, E.; Di Carlo, M.; Shteinfer-Kuzmine, A. Adverse effects of metformin from diabetes to COVID-19, cancer, neurodegenerative diseases, and aging: Is VDAC1 a common target? Front. Physiol. 2021, 12, 730048. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Kozłowska, M.; Krekora, J.; Śliwińska, A. The Gut Microbiota-Related Antihyperglycemic Effect of Metformin. Pharmaceuticals 2025, 18, 55. [Google Scholar] [CrossRef]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, D.; Avvakumov, N.; Côté, J. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef]
- Giordo, R.; Posadino, A.M.; Mangoni, A.A.; Pintus, G. Metformin-mediated epigenetic modifications in diabetes and associated conditions: Biological and clinical relevance. Biochem. Pharmacol. 2023, 215, 115732. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Verdura, S.; Martin-Castillo, B.; Menendez, J.A. Metformin: Targeting the metabolo-epigenetic link in cancer biology. Front. Oncol. 2021, 10, 620641. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Aquilano, K.; Punziano, C.; Minopoli, G.; Faraonio, R. MicroRNAs, long non-coding RNAs, and circular RNAs in the redox control of cell senescence. Antioxidants 2022, 11, 480. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, L.; Makarczyk, M.J.; Feng, P.; Zhang, J. The Anti-Aging Mechanism of Metformin: From Molecular Insights to Clinical Applications. Molecules 2025, 30, 816. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Ashayeri Ahmadabad, H.; Mohammadi Panah, S.; Ghasemnejad-Berenji, H.; Ghojavand, S.; Ghasemnejad-Berenji, M.; Khezri, M.R. Metformin and the PI3K/AKT signaling pathway: Implications for cancer, cardiovascular, and central nervous system diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 1035–1055. [Google Scholar] [CrossRef]
- Andrzejewski, S.; Gravel, S.-P.; Pollak, M.; St-Pierre, J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Algire, C.; Moiseeva, O.; Deschênes-Simard, X.; Amrein, L.; Petruccelli, L.; Birman, E.; Viollet, B.; Ferbeyre, G.; Pollak, M.N. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev. Res. 2012, 5, 536–543. [Google Scholar] [CrossRef]
- Du, F.; Liu, M.; Wang, J.; Hu, L.; Zeng, D.; Zhou, S.; Zhang, L.; Wang, M.; Xu, X.; Li, C. Metformin coordinates with mesenchymal cells to promote VEGF-mediated angiogenesis in diabetic wound healing through Akt/mTOR activation. Metabolism 2023, 140, 155398. [Google Scholar] [CrossRef]
- Hsu, S.-K.; Cheng, K.-C.; Mgbeahuruike, M.O.; Lin, Y.-H.; Wu, C.-Y.; Wang, H.-M.D.; Yen, C.-H.; Chiu, C.-C.; Sheu, S.-J. New insight into the effects of metformin on diabetic retinopathy, aging and cancer: Nonapoptotic cell death, immunosuppression, and effects beyond the AMPK pathway. Int. J. Mol. Sci. 2021, 22, 9453. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.; Park, S.J.; Park, J.J.; Cheon, J.H.; Kim, W.H.; Kim, T.I. Metformin suppresses cancer stem cells through AMPK activation and inhibition of protein prenylation of the mevalonate pathway in colorectal cancer. Cancers 2020, 12, 2554. [Google Scholar] [CrossRef]
- Khosravi, G.R.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneim, M.; Aboalela, M.A.; Naoe, Y.; Matsumura, S.; Eissa, I.R.; Bustos-Villalobos, I.; Sibal, P.A.; Takido, Y.; Kodera, Y.; Kasuya, H. The impact of metformin on tumor-infiltrated immune cells: Preclinical and clinical studies. Int. J. Mol. Sci. 2023, 24, 13353. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Jalil, A.T.; Abd Alzahraa, Z.H.; Aminov, Z.; Alsaikhan, F.; Ramírez-Coronel, A.A.; Ramaiah, P.; Najafi, M. The Metformin Immunoregulatory Actions in Tumor Suppression and Normal Tissues Protection. Curr. Med. Chem. 2024, 31, 5370–5396. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Li, Y.; Gong, Q.; Luo, K. Metformin-based nanomedicines for reprogramming tumor immune microenvironment. Theranostics 2025, 15, 993. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Zheng, B.; Zhou, S. Metabolic hallmarks of natural killer cells in the tumor microenvironment and implications in cancer immunotherapy. Oncogene 2023, 42, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Xu, W. CD147-mediated reprogrammed glycolytic metabolism potentially induces immune escape in the tumor microenvironment. Oncol. Rep. 2019, 41, 2945–2956. [Google Scholar] [CrossRef]
- Ganjoo, S.; Gupta, P.; Corbali, H.I.; Nanez, S.; Riad, T.S.; Duong, L.K.; Barsoumian, H.B.; Masrorpour, F.; Jiang, H.; Welsh, J.W. The role of tumor metabolism in modulating T-Cell activity and in optimizing immunotherapy. Front. Immunol. 2023, 14, 1172931. [Google Scholar] [CrossRef]
- Cha, J.-H.; Yang, W.-H.; Xia, W.; Wei, Y.; Chan, L.-C.; Lim, S.-O.; Li, C.-W.; Kim, T.; Chang, S.-S.; Lee, H.-H. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol. Cell 2018, 71, 606–620.E7. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Lu, C.; Zhang, B.; Li, H.; Zhao, X.; Chen, H.; Liu, Y.; Cui, X. Advances in metabolic reprogramming of NK cells in the tumor microenvironment on the impact of NK therapy. J. Transl. Med. 2024, 22, 229. [Google Scholar] [CrossRef]
- Mostafavi, S.; Zalpoor, H.; Hassan, Z.M. The promising therapeutic effects of metformin on metabolic reprogramming of cancer-associated fibroblasts in solid tumors. Cell. Mol. Biol. Lett. 2022, 27, 58. [Google Scholar] [CrossRef]
- Borde, S.; Matosevic, S. Metabolic adaptation of NK cell activity and behavior in tumors: Challenges and therapeutic opportunities. Trends Pharmacol. Sci. 2023, 44, 832–848. [Google Scholar] [CrossRef]
- Singh, L.; Nair, L.; Kumar, D.; Arora, M.K.; Bajaj, S.; Gadewar, M.; Mishra, S.S.; Rath, S.K.; Dubey, A.K.; Kaithwas, G. Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival. Front. Oncol. 2023, 13, 1034205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, R.; Sun, S.; Wu, C.; Yu, Q.; Awadasseid, A.; Wang, J.; Zhang, W. Recent advances and mechanisms of action of PD-L1 degraders as potential therapeutic agents. Eur. J. Med. Chem. 2024, 268, 116267. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, T.; Zeng, F.; Zhang, J. New insights into T cell metabolism in liver cancer: From mechanism to therapy. Cell Death Discov. 2025, 11, 118. [Google Scholar] [CrossRef]
- Panaampon, J.; Zhou, Y.; Saengboonmee, C. Metformin as a booster of cancer immunotherapy. Int. Immunopharmacol. 2023, 121, 110528. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Peng, L.; Wang, H. Metformin facilitates anti-PD-L1 efficacy through the regulation of intestinal microbiota. Genes Immun. 2024, 25, 7–13. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Pleiotropic effects of metformin on the antitumor efficiency of immune checkpoint inhibitors. Front. Immunol. 2021, 11, 586760. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ye, X.; Hou, H.; Wang, Y. Clinical evidence for the prognostic impact of metformin in cancer patients treated with immune checkpoint inhibitors. Int. Immunopharmacol. 2024, 134, 112243. [Google Scholar] [CrossRef] [PubMed]
- Amengual-Cladera, E.; Morla-Barcelo, P.M.; Morán-Costoya, A.; Sastre-Serra, J.; Pons, D.G.; Valle, A.; Roca, P.; Nadal-Serrano, M. Metformin: From diabetes to Cancer—Unveiling Molecular mechanisms and therapeutic strategies. Biology 2024, 13, 302. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Yumashev, A.; Hjazi, A.; Toama, M.A.; AbdRabou, M.A.; Gehlot, A.; Alwaily, E.R.; Shirsalimi, N.; Yadav, P.K. Effects of metformin on cancers in experimental and clinical studies: Focusing on autophagy and AMPK/mTOR signaling pathways. Cell Biochem. Funct. 2024, 42, e4071. [Google Scholar] [CrossRef] [PubMed]
- Koritzinsky, M. Metformin: A novel biological modifier of tumor response to radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 454–464. [Google Scholar] [CrossRef]
- Drzał, A.; Dziurman, G.; Hoła, P.; Lechowski, J.; Delalande, A.; Swakoń, J.; Pichon, C.; Elas, M. Murine Breast Cancer Radiosensitization Using Oxygen Microbubbles and Metformin: Vessels Are the Key. Int. J. Mol. Sci. 2023, 24, 12156. [Google Scholar] [CrossRef]
- Mitra, A.; Kumar, A.; Amdare, N.P.; Pathak, R. Current Landscape of Cancer Immunotherapy: Harnessing the Immune Arsenal to Overcome Immune Evasion. Biology 2024, 13, 307. [Google Scholar] [CrossRef]
- Xiao, Y.-L.; Gong, Y.; Qi, Y.-J.; Shao, Z.-M.; Jiang, Y.-Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- Lavudi, K.; Nuguri, S.M.; Pandey, P.; Kokkanti, R.R.; Wang, Q.-E. ALDH and cancer stem cells: Pathways, challenges, and future directions in targeted therapy. Life Sci. 2024, 356, 123033. [Google Scholar] [CrossRef]
- Samuel, S.M.; Varghese, E.; Koklesová, L.; Líšková, A.; Kubatka, P.; Büsselberg, D. Counteracting chemoresistance with metformin in breast cancers: Targeting cancer stem cells. Cancers 2020, 12, 2482. [Google Scholar] [CrossRef]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Della Vedova, L.; Baron, G.; Morazzoni, P.; Aldini, G.; Gado, F. The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action. Pharmaceuticals 2025, 18, 138. [Google Scholar] [CrossRef]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular senescence: Molecular targets, biomarkers, and senolytic drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef]
- Abdelgawad, I.Y.; Agostinucci, K.; Sadaf, B.; Grant, M.K.; Zordoky, B.N. Metformin mitigates SASP secretion and LPS-triggered hyper-inflammation in Doxorubicin-induced senescent endothelial cells. Front. Aging 2023, 4, 1170434. [Google Scholar] [CrossRef] [PubMed]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663. [Google Scholar] [CrossRef]
- Fei, Q.; Ma, H.; Zou, J.; Wang, W.; Zhu, L.; Deng, H.; Meng, M.; Tan, S.; Zhang, H.; Xiao, X. Metformin protects against ischaemic myocardial injury by alleviating autophagy-ROS-NLRP3-mediated inflammatory response in macrophages. J. Mol. Cell. Cardiol. 2020, 145, 1–13. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K. Neuronutraceuticals combating Neuroinflammaging: Molecular insights and translational challenges—A systematic review. Nutrients 2022, 14, 3029. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.M.; Bairy, L.K.; Pirasanthan, R.; Vaihnav, R. Grape seed extract and zinc containing nutritional food supplement decreases the oxidative stress induced by carbon tetrachloride in rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 626–631. [Google Scholar]
- Satyam, S.M.; Bairy, L.K.; Rehman, A.; Attia, M.; Ahmed, L.; Emad, K.; Jaafer, Y.; Bahaaeldin, A. Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats. Biology 2024, 13, 473. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Pirasanthan, R. Influence of grape seed extract and zinc containing multivitamin-mineral nutritional food supplement on lipid profile in normal and diet-induced hypercholesterolemic rats. J. Clin. Diagn. Res. JCDR 2014, 8, HC12–HC15. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 2020, 32, 44–55.e46. [Google Scholar] [CrossRef]
- Bridgeman, S.C.; Ellison, G.C.; Melton, P.E.; Newsholme, P.; Mamotte, C.D.S. Epigenetic effects of metformin: From molecular mechanisms to clinical implications. Diabetes Obes. Metab. 2018, 20, 1553–1562. [Google Scholar] [CrossRef]
- Menendez, J.A. Metformin: Sentinel of the epigenetic landscapes that underlie cell fate and identity. Biomolecules 2020, 10, 780. [Google Scholar] [CrossRef]
- Sorrenti, V.; Benedetti, F.; Buriani, A.; Fortinguerra, S.; Caudullo, G.; Davinelli, S.; Zella, D.; Scapagnini, G. Immunomodulatory and antiaging mechanisms of resveratrol, rapamycin, and metformin: Focus on mTOR and AMPK signaling networks. Pharmaceuticals 2022, 15, 912. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Q.; Nie, L.; Zhu, R.; Zhou, X.; Zhao, P.; Ji, N.; Liang, X.; Ding, Y.; Yuan, Q. Hyperglycemia-induced inflamm-aging accelerates gingival senescence via NLRC4 phosphorylation. J. Biol. Chem. 2019, 294, 18807–18819. [Google Scholar] [CrossRef] [PubMed]
- Alzokaky, A.A.; Al-Karmalawy, A.A.; Saleh, M.A.; Abdo, W.; Farage, A.E.; Belal, A.; Abourehab, M.A.; Antar, S.A. Metformin ameliorates doxorubicin-induced cardiotoxicity targeting HMGB1/TLR4/NLRP3 signaling pathway in mice. Life Sci. 2023, 316, 121390. [Google Scholar] [CrossRef]
- Guarente, L.; Sinclair, D.A.; Kroemer, G. Human trials exploring anti-aging medicines. Cell Metab. 2024, 36, 354–376. [Google Scholar] [CrossRef]
- Garay, R.P. Clinical studies with drugs and biologics aimed at slowing or reversing normal aging processes—Emerging results and future perspectives. Explor. Drug Sci. 2024, 2, 144–153. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Preiss, D.; Lloyd, S.M.; Ford, I.; McMurray, J.J.; Holman, R.R.; Welsh, P.; Fisher, M.; Packard, C.J.; Sattar, N. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): A randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a tool to target aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J. Effect of metformin vs placebo on invasive disease–free survival in patients with breast cancer: The MA. 32 randomized clinical trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef]
- Martin-Castillo, B.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; Amillano, K.; Domínguez, S. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: The METTEN study. Oncotarget 2018, 9, 35687. [Google Scholar] [CrossRef]
- Alghandour, R.; Ebrahim, M.A.; Elshal, A.M.; Ghobrial, F.; Elzaafarany, M.; ELbaiomy, M.A. Repurposing metformin as anticancer drug: Randomized controlled trial in advanced prostate cancer (MANSMED). Urol. Oncol. Semin. Orig. Investig. 2021, 39, 831.e1–831.e10. [Google Scholar] [CrossRef]
- Pimentel, I.; Lohmann, A.E.; Ennis, M.; Dowling, R.J.; Cescon, D.; Elser, C.; Potvin, K.; Haq, R.; Hamm, C.; Chang, M.C. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast 2019, 48, 17–23. [Google Scholar] [CrossRef]
- Barakat, H.E.; Hussein, R.R.; Elberry, A.A.; Zaki, M.A.; Ramadan, M.E. The impact of metformin use on the outcomes of locally advanced breast cancer patients receiving neoadjuvant chemotherapy: An open-labelled randomized controlled trial. Sci. Rep. 2022, 12, 7656. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J. Effect of metformin versus placebo on new primary cancers in Canadian cancer trials group MA. 32: A secondary analysis of a phase III randomized double-blind trial in early breast cancer. J. Clin. Oncol. 2023, 41, 5356–5362. [Google Scholar] [CrossRef]
- Strømland, P.P.; Bertelsen, B.-E.; Viste, K.; Chatziioannou, A.C.; Bellerba, F.; Robinot, N.; Trolat, A.; Flågeng, M.H.; Scalbert, A.; Keski-Rahkonen, P. Effects of metformin on transcriptomic and metabolomic profiles in breast cancer survivors enrolled in the randomized placebo-controlled MetBreCS trial. Sci. Rep. 2025, 15, 16897. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Chen, B.E.; Sathe, C.; Parulekar, W.R.; Lemieux, J.; Ligibel, J.A.; Gelmon, K.A.; Whelan, T.J.; Goodwin, P.J. Metformin, placebo, and endocrine therapy discontinuation among participants in a randomized double-blind trial of metformin vs placebo in hormone receptor-positive early-stage breast cancer (CCTG MA32). Breast Cancer Res. Treat. 2023, 200, 93–102. [Google Scholar] [CrossRef]
- Brown, J.R.; Chan, D.K.; Shank, J.J.; Griffith, K.A.; Fan, H.; Szulawski, R.; Yang, K.; Reynolds, R.K.; Johnston, C.; McLean, K. Phase II clinical trial of metformin as a cancer stem cell–targeting agent in ovarian cancer. JCI Insight 2020, 5, e133247. [Google Scholar] [CrossRef] [PubMed]
- Serageldin, M.A.; El-Bassiouny, N.A.; El-Kerm, Y.; Aly, R.G.; Helmy, M.W.; El-Mas, M.M.; Kassem, A.B. A randomized controlled study of neoadjuvant metformin with chemotherapy in nondiabetic breast cancer women: The METNEO study. Br. J. Clin. Pharmacol. 2024, 90, 3160–3175. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.L.; Lengyel, E.; Hendrickson, A.E.W.; Rodriguez, G.C.; Leath III, C.A.; Rocconi, R.P.; Goodheart, M.J.; Dewdney, S.; Karrison, T.; Fleming, G.F. Metformin for patients with advanced stage ovarian cancer: A randomized phase II placebo-controlled trial. Gynecol. Oncol. 2025, 194, 18–24. [Google Scholar] [CrossRef]
- Han, K.; Fyles, A.; Shek, T.; Croke, J.; Dhani, N.; D’Souza, D.; Lee, T.-Y.; Chaudary, N.; Bruce, J.; Pintilie, M. A phase II randomized trial of chemoradiation with or without metformin in locally advanced cervical cancer. Clin. Cancer Res. 2022, 28, 5263–5271. [Google Scholar] [CrossRef]
- Hegazy, A.; Ali, M.S.; Mubarak, N.A.; Alagizy, H.A.M.; Sohaib, A. Metformin in Combination with Standard Therapy in Patients with Diffuse Large B-Cell Lymphoma: A Randomized Phase II Clinical Trial. Asian Pac. J. Cancer Prev. APJCP 2024, 25, 2351. [Google Scholar] [CrossRef]
- Martin, M.P.; Borchiellini, D.; Thamphya, B.; Guillot, A.; Paoli, J.-B.; Besson, D.; Hilgers, W.; Priou, F.; El Kouri, C.; Hoch, B. TAXOMET: A French prospective multicentric randomized phase II study of docetaxel plus metformin versus docetaxel plus placebo in metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 2021, 19, 501–509. [Google Scholar] [CrossRef]
- Padki, M.M.; Stambler, I. Targeting aging with metformin (TAME). In Encyclopedia of Gerontology and Population Aging; Springer: Berlin/Heidelberg, Germany, 2022; pp. 4908–4910. [Google Scholar]
- Kulkarni, A.S.; Brutsaert, E.F.; Anghel, V.; Zhang, K.; Bloomgarden, N.; Pollak, M.; Mar, J.C.; Hawkins, M.; Crandall, J.P.; Barzilai, N. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 2018, 17, e12723. [Google Scholar] [CrossRef]
- Kirkland, J.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Turgut, Ş.; Atasever, E.; Cebe, T.; Andican, G.; Çakatay, U. Senotherapeutic repurposing of metformin for age-related diseases and their signaling pathways. Mol. Biol. Rep. 2025, 52, 410. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Altamimi, A.S.A.; Almalki, W.H.; Kazmi, I.; Al-Abbasi, F.A.; Alzarea, S.I.; Makeen, H.A.; Albratty, M. A study of the molecular mechanism of quercetin and dasatinib combination as senolytic in alleviating age-related and kidney diseases. J. Food Biochem. 2022, 46, e14471. [Google Scholar] [CrossRef]
- Zheng, L.; He, S.; Wang, H.; Li, J.; Liu, Y.; Liu, S. Targeting cellular senescence in aging and age-related diseases: Challenges, considerations, and the emerging role of senolytic and senomorphic therapies. Aging Dis. 2024, 15, 2554. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, P.; Ling, X.; Zhou, L. Advancements in therapeutic drugs targeting of senescence. Ther. Adv. Chronic Dis. 2020, 11, 2040622320964125. [Google Scholar] [CrossRef]
- Hunt, N.J.; Lockwood, G.P.; Kang, S.W.; Westwood, L.J.; Limantoro, C.; Chrzanowski, W.; McCourt, P.A.; Kuncic, Z.; Le Couteur, D.G.; Cogger, V.C. Quantum dot nanomedicine formulations dramatically improve pharmacological properties and alter uptake pathways of metformin and nicotinamide mononucleotide in aging mice. ACS Nano 2021, 15, 4710–4727. [Google Scholar] [CrossRef]
- Imai, S.-i.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Fan, L.; Cacicedo, J.M.; Ido, Y. Impaired nicotinamide adenine dinucleotide (NAD+) metabolism in diabetes and diabetic tissues: Implications for nicotinamide-related compound treatment. J. Diabetes Investig. 2020, 11, 1403–1419. [Google Scholar] [CrossRef]
- Cacciatore, S.; Calvani, R.; Esposito, I.; Massaro, C.; Gava, G.; Picca, A.; Tosato, M.; Marzetti, E.; Landi, F. Emerging targets and treatments for sarcopenia: A narrative review. Nutrients 2024, 16, 3271. [Google Scholar] [CrossRef]
- Braileanu, A.L. Teaching an Old Dog New Tricks: Introducing a Hypothetical Mechanism of Synergy Between Metformin and New Generation mTOR Inhibitors in the Fight Against Cancer; The University of Arizona: Tucson, AZ, USA, 2019. [Google Scholar]
- Blagosklonny, M.V. Rapamycin-induced glucose intolerance: Hunger or starvation diabetes. Cell Cycle 2011, 10, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. From rapalogs to anti-aging formula. Oncotarget 2017, 8, 35492. [Google Scholar] [CrossRef]
- Javed, S.A.; Najmi, A.; Ahsan, W.; Zoghebi, K. Targeting PD-1/PD-L-1 immune checkpoint inhibition for cancer immunotherapy: Success and challenges. Front. Immunol. 2024, 15, 1383456. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-J.; Wang, L.; Li, Z.; Ku, C.-L.; Ho, P.-C. Metabolic challenges and interventions in CAR T cell therapy. Sci. Immunol. 2023, 8, eabq3016. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, S.; Siegel, P.M.; St-Pierre, J. Metabolic profiles associated with metformin efficacy in cancer. Front. Endocrinol. 2018, 9, 372. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, Y.-S.; Wang, L.-C.; Huang, J.-B. Advances in metformin-based metabolic therapy for non-small cell lung cancer. Oncol. Rep. 2022, 47, 55. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, K.; Ren, Z.; Yin, D.; Zhou, Y. Metformin as anticancer agent and adjuvant in cancer combination therapy: Current progress and future prospect. Transl. Oncol. 2024, 44, 101945. [Google Scholar] [CrossRef]
- Ningrum, V.D.; Ikawati, Z.; Sadewa, A.H.; Ikhsan, M.R. Patient-factors associated with metformin steady-state levels in type 2 diabetes mellitus with therapeutic dosage. J. Clin. Transl. Endocrinol. 2018, 12, 42–47. [Google Scholar] [CrossRef]
- Singh, S.V.; Chaube, B.; Mayengbam, S.S.; Singh, A.; Malvi, P.; Mohammad, N.; Deb, A.; Bhat, M.K. Metformin induced lactic acidosis impaired response of cancer cells towards paclitaxel and doxorubicin: Role of monocarboxylate transporter. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166011. [Google Scholar] [CrossRef]
- Scheen, A.J. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 1996, 30, 359–371. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Verma, R.K.; Dhingra, D.; Dilbaghi, N.; Kim, K.-H. Metformin-loaded alginate nanoparticles as an effective antidiabetic agent for controlled drug release. J. Pharm. Pharmacol. 2017, 69, 143–150. [Google Scholar] [CrossRef]
- Zi, Y.; Yang, K.; He, J.; Wu, Z.; Liu, J.; Zhang, W. Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv. Drug Deliv. Rev. 2022, 188, 114449. [Google Scholar] [CrossRef]
- Olusanya, T.O.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Kulesza, B.; Jonak, K.; Baj, J.; Grochowski, C. Metformin as potential therapy for high-grade glioma. Cancers 2020, 12, 210. [Google Scholar] [CrossRef]
- Subramaniam, K.; Joseph, M.P.; Babu, L.A. A common drug causing a common side effect at an uncommon time: Metformin-induced chronic diarrhea and weight loss after years of treatment. Clin. Diabetes 2021, 39, 237–240. [Google Scholar] [CrossRef]
- Dhiman, S.; Philip, N.; Gurjeet Singh, T.; Babbar, R.; Garg, N.; Diwan, V.; Singh, P. An insight on novel approaches & perspectives for gastro-retentive drug delivery systems. Curr. Drug Deliv. 2023, 20, 708–729. [Google Scholar] [CrossRef]
- Drenth-van Maanen, A.C.; Wilting, I.; Jansen, P.A. Prescribing medicines to older people—How to consider the impact of ageing on human organ and body functions. Br. J. Clin. Pharmacol. 2020, 86, 1921–1930. [Google Scholar] [CrossRef]
- Pollak, M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia 2017, 60, 1662–1667. [Google Scholar] [CrossRef]
- Türk, D.; Scherer, N.; Selzer, D.; Dings, C.; Hanke, N.; Dallmann, R.; Schwab, M.; Timmins, P.; Nock, V.; Lehr, T. Significant impact of time-of-day variation on metformin pharmacokinetics. Diabetologia 2023, 66, 1024–1034. [Google Scholar] [CrossRef]
- Barnea, M.; Haviv, L.; Gutman, R.; Chapnik, N.; Madar, Z.; Froy, O. Metformin affects the circadian clock and metabolic rhythms in a tissue-specific manner. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1796–1806. [Google Scholar] [CrossRef]
- Li, C.; Chen, T.; Li, Y.; Zhou, C.; Du, J.; Li, X.; Tang, C.; Ma, C.; Deng, N.; Cui, H. Impact of diabetes and metformin on cuproptosis and ferroptosis in breast cancer patients: An immunohistochemical analysis. Discov. Oncol. 2025, 16, 634. [Google Scholar] [CrossRef]
- Capitanio, S.; Marini, C.; Sambuceti, G.; Morbelli, S. Metformin and cancer: Technical and clinical implications for FDG-PET imaging. World J. Radiol. 2015, 7, 57. [Google Scholar] [CrossRef]
- Wang, S.; Prizment, A.; Thyagarajan, B.; Blaes, A. Cancer treatment-induced accelerated aging in cancer survivors: Biology and assessment. Cancers 2021, 13, 427. [Google Scholar] [CrossRef]


| System/Target | Primary Mechanisms | Functional Outcome |
|---|---|---|
| Mitochondria [36] | Partial inhibition of Complex I via ND3 subunit binding; increased AMP/ATP ratio; mild ROS elevation (1.8×); mPTP stabilization via VDAC1 | AMPK activation, tumor cell metabolic stress induction, cardiomyocyte protection under chemotherapy |
| Liver [37] | CRTC2 inactivation; decreased PEPCK (−72%) and G6Pase (−68%) expression; gluconeogenesis inhibition | Improved insulin sensitivity and hepatic glucose output reduction |
| Skeletal Muscle [38] | AMPK-mediated GLUT4 translocation (+58%); increased PGC1α-driven mitochondrial biogenesis (+35–40%) | Enhanced glucose uptake and mitochondrial function, improved insulin responsiveness |
| Cardiomyocytes [39] | Mitochondrial membrane potential stabilization via VDAC1 binding | Reduction in doxorubicin-induced cardiotoxicity and maintenance of cardiac function |
| Gut Microbiome [40] | Akkermansia muciniphila expansion (+12-fold); FXR inhibition; TGR5 activation; SCFA modulation | Improved gut barrier integrity, systemic inflammation reduction, and anti-endotoxemic effects |
| Epigenetic Remodeling [41,42] | AMPK-mediated phosphorylation; chromatin opening at tumor suppressor loci (p21, p53); circRNA_1805 downregulation | Support for anti-aging and anticancer gene expression landscapes |
| Tumor Microenvironment [43,44,45] | Lactate reduction (−65%); HIF-1α suppression (−58%); increased MHC-I expression; M2-to-M1 macrophage repolarization; enhanced dendritic cell maturation and T-cell infiltration | Conversion of immunosuppressive milieu to immunostimulatory microenvironment |
| Systemic Immunomodulation [15,46] | NRF2 activation; SASP suppression; NLRP3 inflammasome inhibition; enhanced antiviral T-cell response | Delay in immunosenescence, reduced inflammaging, improved immune surveillance and vaccine efficacy |
| Trial Name | Year | Population | Intervention | Primary Outcomes | Remarks |
|---|---|---|---|---|---|
| MANSMED Trial [119] | 2021 | 124 men with high-risk prostate cancer | Metformin + ADT vs. ADT | Increased CRPC-free survival; benefit observed in localized and low-volume metastatic disease | Improved disease control; no OS benefit |
| Metformin in Metastatic Breast Cancer [120] | 2019 | 40 non-diabetic ER/PR+ metastatic breast cancer patients | Metformin + chemotherapy vs. placebo | No difference in response rate, PFS, or OS | No survival benefit despite tolerability |
| Neoadjuvant Metformin Breast Cancer Trial [121] | 2022 | 80 non-diabetic women with locally advanced breast cancer | NACT + metformin vs. NACT | Increased ORR, cCR, pCR, and BCR; serum metformin positively correlated with outcomes | Enhanced clinical/pathological response |
| MA.32 Trial [122] | 2023 | 3649 non-diabetic high-risk breast cancer patients | Metformin 850 mg BID vs. placebo | No reduction in invasive disease-free survival or cancer incidence | Ineffective for cancer prevention |
| MetBreCS [123] | 2025 | Postmenopausal breast cancer survivors (BMI > 25) | Metformin vs. placebo | Transcriptomic and metabolic shifts observed; decreased expression of immune genes | Potential preventive effect via tissue remodeling |
| CCTG MA.32 [124] | 2023 | 2521 HR+ breast cancer patients | Metformin vs. placebo with endocrine therapy | Increased non-adherence with metformin; no difference in ET discontinuation | Emphasizes need for adherence strategies |
| Ovarian Cancer Phase II RCT [125] | 2020 | 108 advanced ovarian cancer patients | Metformin + chemo vs. placebo | No improvement in PFS or OS | Safe but ineffective as adjunct |
| The METNEO Study [126] | 2024 | 70 non-diabetic breast cancer patients randomized 1:1 | Neoadjuvant AC-T chemotherapy (Adriamycin + Cyclophosphamide followed by Paclitaxel) with or without metformin (850 mg BID) | Improved clinical response; increased breast-conserving surgery; higher pCR; DR4/DR5 mRNA upregulation; CD133+ cancer stem cell reduction | Enhances neoadjuvant efficacy Metformin modulates apoptosis and stemness by upregulating death receptors and downregulating CD133+ stem cells, acting as a molecular chemosensitizer |
| Phase II RCT [127] | 2025 | 108 patients with advanced-stage ovarian cancer (54 in each arm); majority received neoadjuvant chemotherapy (66%) or primary debulking surgery (31%); 88% had high-grade serous histology | Platinum/taxane-based chemotherapy + Metformin 850 mg orally BID (n = 54) vs. placebo (n = 54); followed by 2-year maintenance therapy (metformin or placebo) | No significant improvement in PFS or OS | Addition of metformin was safe and well tolerated but did not result in significant improvements in survival outcomes |
| FAZA-PET Hypoxia Trial [128] | 2022 | Stage IB–IVA cervical cancer with FAZA+ tumors | Metformin + chemoradiotherapy | Reduced tumor hypoxia; trend toward improved DFS | Potential radiosensitizer; improves hypoxia |
| DLBCL RCT [129] | 2024 | 100 adult patients with histologically confirmed DLBCL eligible for first-line R-CHOP, PS ≤ 2, life expectancy ≥ 6 months; randomized 1:1 (50 per arm) | R-CHOP chemotherapy ± Metformin; dosage unspecified in abstract | Higher CR; lower relapse/progression; reduced mortality; improved DFS, PFS, and OS | Metformin addition significantly improved remission, reduced relapse and mortality, and extended DFS, PFS, and OS in DLBCL patient |
| TAXOMET Trial [130] | 2019 | 99 mCRPC patients | Docetaxel + metformin vs. placebo | No significant difference in PSA response, PFS, or OS | Limited synergy with docetaxel |
| TAME Trial [131] | Ongoing | 3000 non-diabetic older adults aged 65–79 years, free from major chronic illness at baseline; multi-center, placebo-controlled trial planned across U.S. aging research centers | Metformin 1500 mg/day vs. placebo for up to 4 years | Designed to detect reduction in incidence of age-related chronic diseases (e.g., MI, stroke, cancer, dementia, mortality) | TAME is the first large-scale randomized trial to test whether a generic drug can delay multiple age-related diseases simultaneously; outcome may redefine aging as a modifiable risk factor |
| MILES Trial [132] | 2018 | Older adults (mean age ~70 years), non-diabetic, relatively healthy, recruited for short-term mechanistic aging study; designed as a randomized, placebo-controlled trial | Metformin 1500–2000 mg/day vs. placebo for 6 weeks | Significant changes in aging-related gene expression: increased mitochondrial function and decreased inflammatory signaling | Short-term metformin treatment showed transcriptional rejuvenation, including mitochondrial biogenesis, decreased inflammatory signaling, and metabolic reprogramming; supports metformin’s role in modulating key aging pathways at a molecular level |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, A.; Satyam, S.M.; El-Tanani, M.; Prabhakar, S.; Kumari, R.; Shetty, P.; Mohammed, S.S.N.; Nafees, Z.; Alomar, B. Metformin Beyond Diabetes: A Precision Gerotherapeutic and Immunometabolic Adjuvant for Aging and Cancer. Cancers 2025, 17, 2466. https://doi.org/10.3390/cancers17152466
Rehman A, Satyam SM, El-Tanani M, Prabhakar S, Kumari R, Shetty P, Mohammed SSN, Nafees Z, Alomar B. Metformin Beyond Diabetes: A Precision Gerotherapeutic and Immunometabolic Adjuvant for Aging and Cancer. Cancers. 2025; 17(15):2466. https://doi.org/10.3390/cancers17152466
Chicago/Turabian StyleRehman, Abdul, Shakta Mani Satyam, Mohamed El-Tanani, Sainath Prabhakar, Rashmi Kumari, Prakashchandra Shetty, Sara S. N. Mohammed, Zaina Nafees, and Basma Alomar. 2025. "Metformin Beyond Diabetes: A Precision Gerotherapeutic and Immunometabolic Adjuvant for Aging and Cancer" Cancers 17, no. 15: 2466. https://doi.org/10.3390/cancers17152466
APA StyleRehman, A., Satyam, S. M., El-Tanani, M., Prabhakar, S., Kumari, R., Shetty, P., Mohammed, S. S. N., Nafees, Z., & Alomar, B. (2025). Metformin Beyond Diabetes: A Precision Gerotherapeutic and Immunometabolic Adjuvant for Aging and Cancer. Cancers, 17(15), 2466. https://doi.org/10.3390/cancers17152466






