Evaluating Therapeutic Efficacy of Intravesical Xenogeneic Urothelial Cell Treatment Alone and in Combination with Chemotherapy or Immune Checkpoint Inhibition in a Mouse Non-Muscle-Invasive Bladder Cancer Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell
2.2. Mice
2.3. Immunohistochemistry (IHC)
2.4. Terminal Deoxynucleotidyl Transferase (TdT)-Mediated dUTP Nick End Labeling (TUNEL) Assay
2.5. Immune Cell Proliferation Assay
2.6. Cytokine Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Immune Cell Cytotoxic Activity
2.8. Quantification of Immune Effector-Target Cell Conjugate Formation by Using Imaging Flow Cytometry
3. Results
3.1. Antitumor Activity of Intravesical Xenogeneic Urothelial Cell (XUC) Treatment as Monotherapy and in Combination with Chemotherapy and Immune Checkpoint Inhibition in the Orthotopic NMIBC Bladder Tumor-Bearing Mice
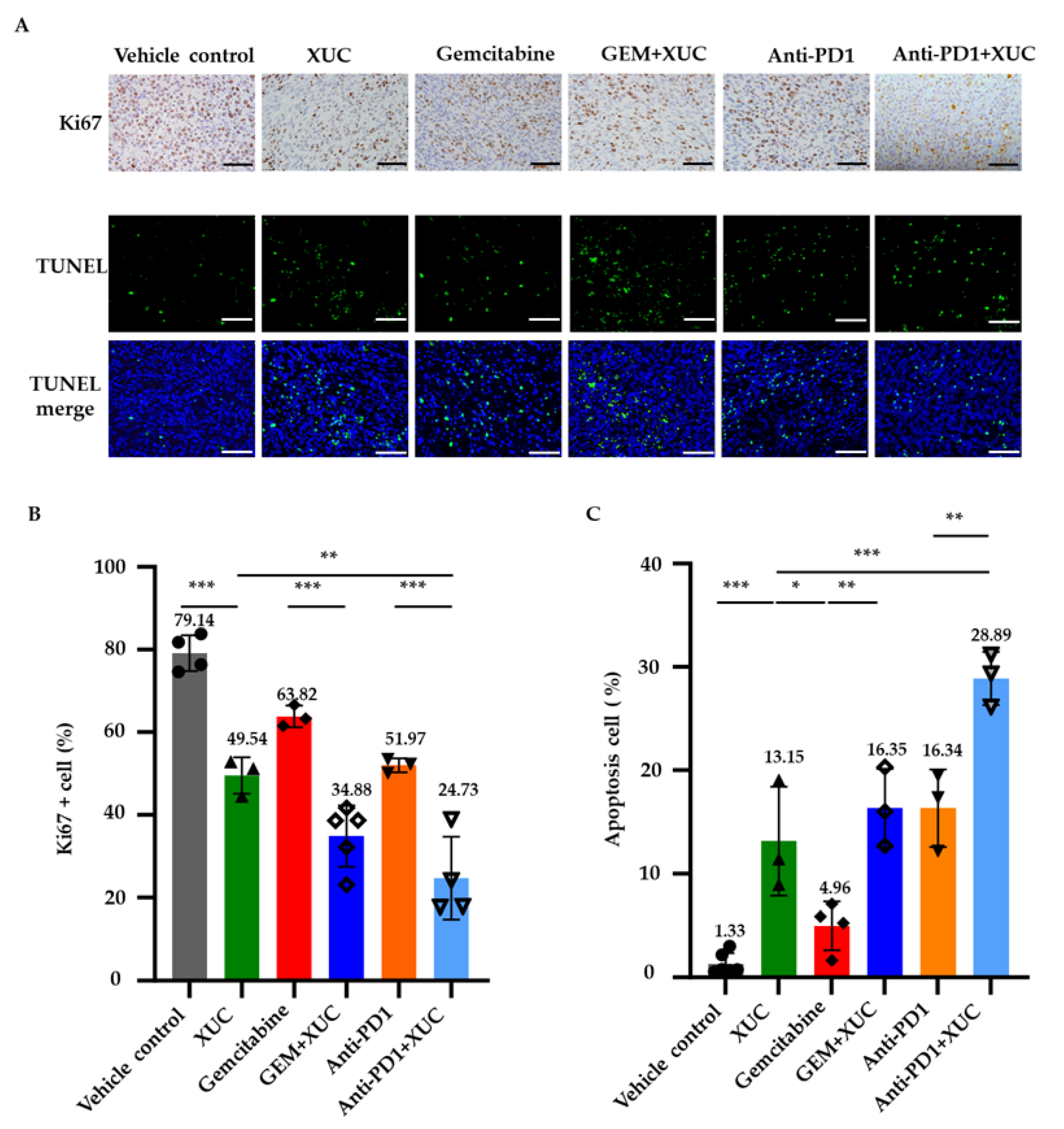
3.2. Intravesical Xenogeneic Urothelial Cell Immunotherapy Treatment Inhibits Tumor Cell Proliferation and Promotes Cell Apoptosis
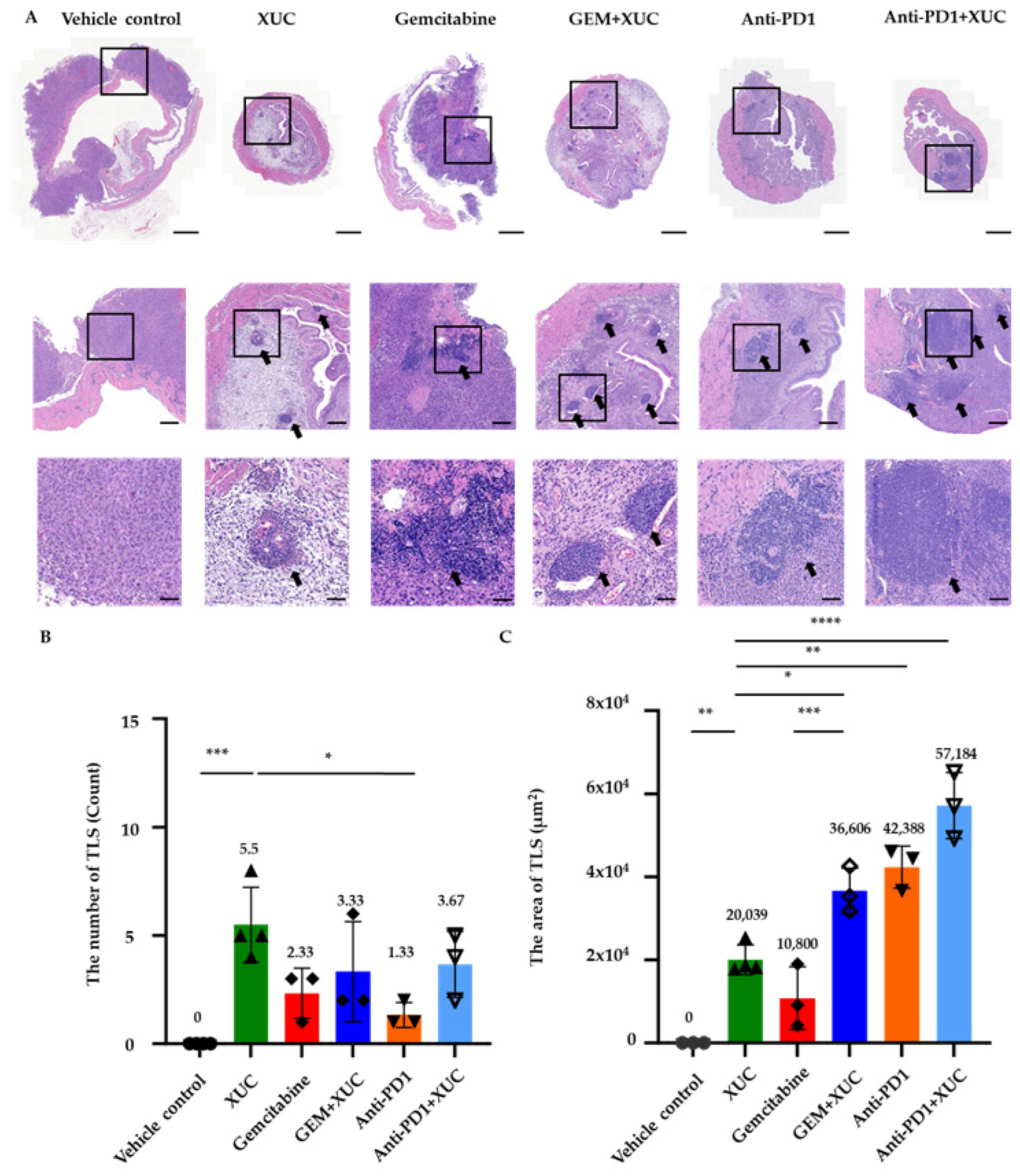
3.3. Intravesical Xenogeneic Urothelial Cell Treatment Alters the Immune Tumor Microenvironment in NMIBC Bladder Tumors
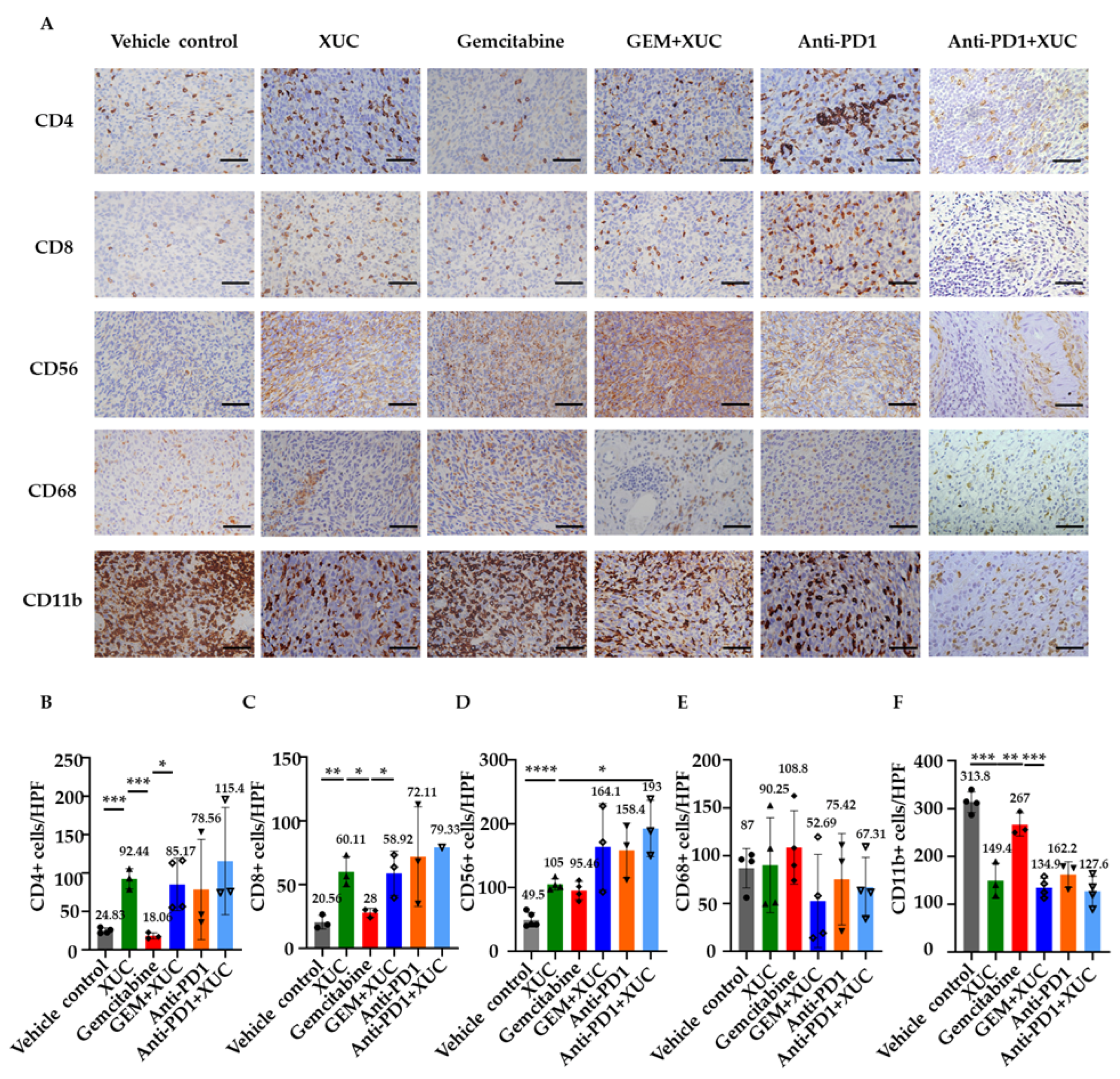
3.4. Intravesical Xenogeneic Urothelial Cell Treatment Promotes Immune Cell Infiltration
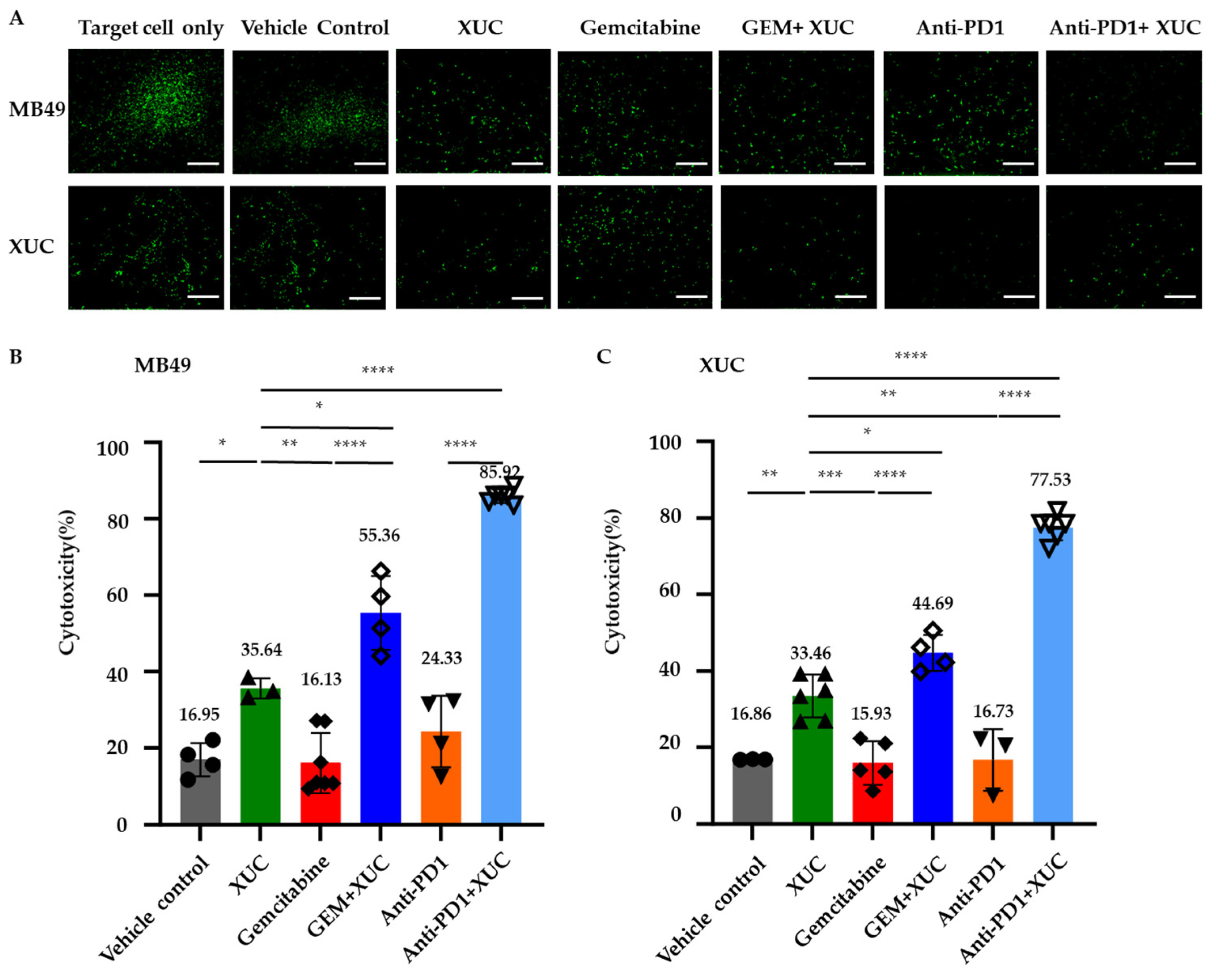
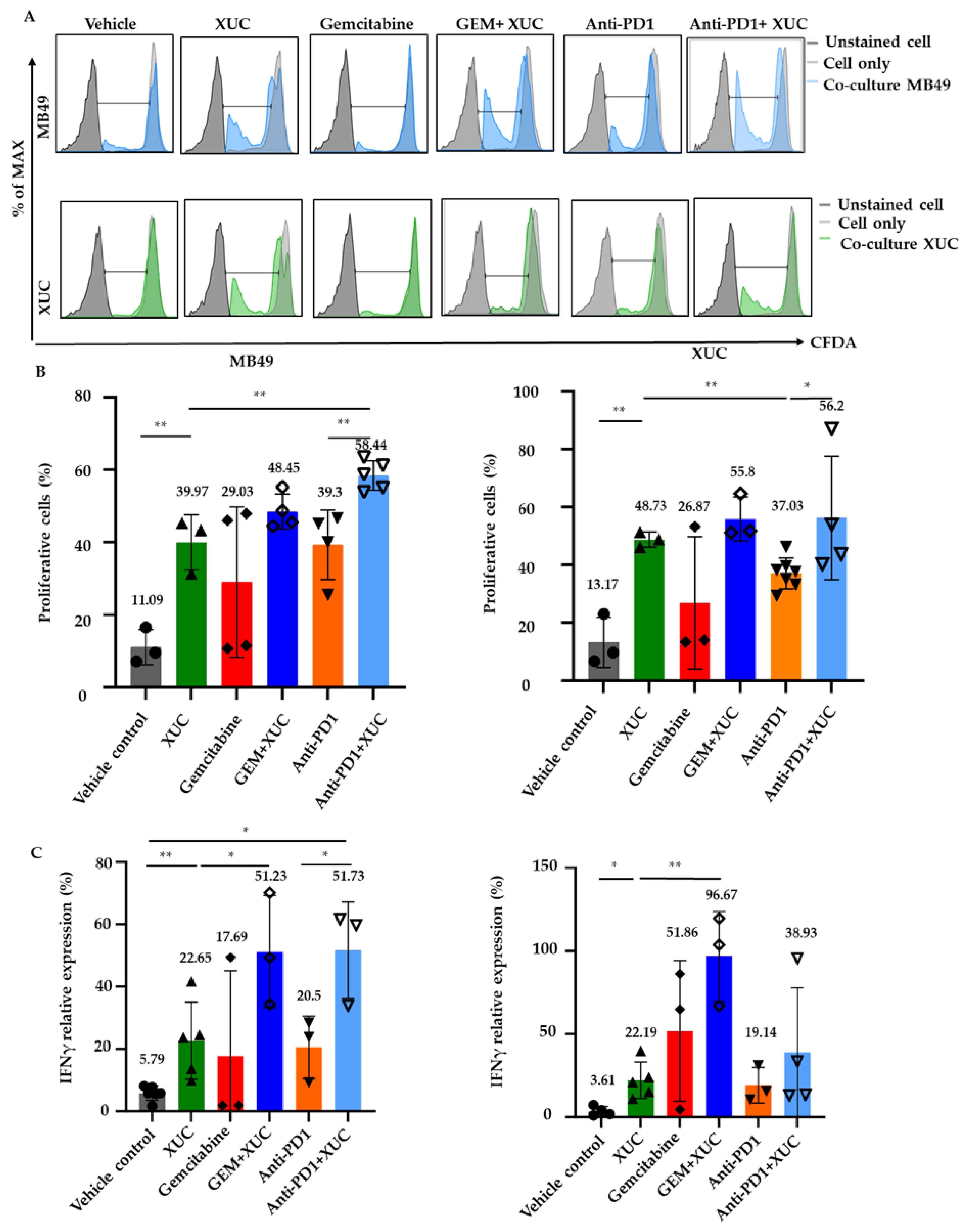
3.5. Intravesical Xenogeneic Urothelial Cell (XUC) Treatment Enhances Immune Cell Functions
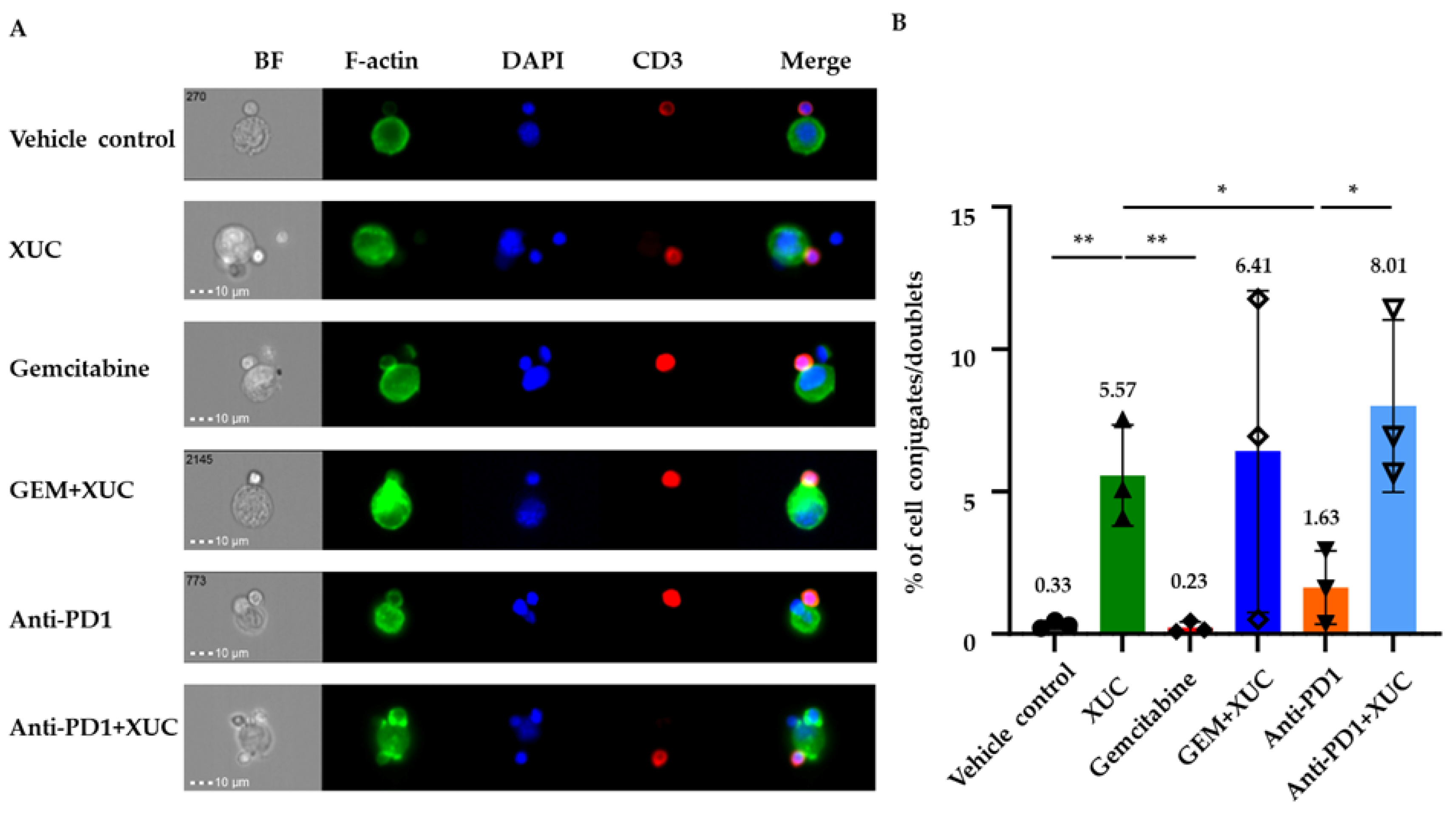
3.6. Intravesical Treatment with XUC Enhances Immune Effector-Target Cell Formation Between Immune Cells and MB49 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMIBC | Non-muscle-invasive bladder cancer |
| BCG | Bacillus Calmette–Guérin |
| XUC | Xenogeneic urothelial cells |
| PD-1 | Programmed death-ligand 1 |
| GEM | Gemcitabine |
| IHC | Immunohistochemistry |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling |
| TLS | Tertiary lymphoid structure |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Taylor, J.; Becher, E.; Steinberg, G.D. Update on the guideline of guidelines: Non-muscle-invasive bladder cancer. BJU Int. 2020, 125, 197–205. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Holzbeierlein, J.M.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Holmes, R.; James, A.C.; Kirkby, E.; McKiernan, J.M.; Schuckman, A.K. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J. Urol. 2024, 211, 533–538. [Google Scholar] [CrossRef]
- Lightfoot, A.J.; Rosevear, H.M.; O’Donnell, M.A. Recognition and treatment of BCG failure in bladder cancer. Sci. World J. 2011, 11, 602–613. [Google Scholar] [CrossRef]
- Aziz, A.; May, M.; Burger, M.; Palisaar, R.-J.; Trinh, Q.-D.; Fritsche, H.-M.; Rink, M.; Chun, F.; Martini, T.; Bolenz, C.; et al. Prediction of 90-day Mortality After Radical Cystectomy for Bladder Cancer in a Prospective European Multicenter Cohort. Eur. Urol. 2014, 66, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Maibom, S.L.; Joensen, U.N.; Poulsen, A.M.; Kehlet, H.; Brasso, K.; Røder, M.A. Short-term morbidity and mortality following radical cystectomy: A systematic review. BMJ Open 2021, 11, e043266. [Google Scholar] [CrossRef]
- Jarow, J.; Maher, V.E.; Tang, S.; Ibrahim, A.; Kim, G.; Sridhara, R.; Pazdur, R. Development of Systemic and Topical Drugs to Treat Non-muscle Invasive Bladder Cancer. Bladder Cancer 2015, 1, 133–136. [Google Scholar] [CrossRef]
- Steinberg, G.; Bahnson, R.; Brosman, S.; Middleton, R.; Wajsman, Z.; Wehle, M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J. Urol. 2000, 163, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.; Mourey, L.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Kamat, A.M.; Grivas, P.; et al. Keynote 057: Phase II trial of Pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guérin (BCG). J. Clin. Oncol. 2019, 37, 350. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; Busby, J.E.; et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021, 22, 107–117. [Google Scholar] [CrossRef]
- Chevuru, P.T.; McElree, I.M.; Mott, S.L.; Steinberg, R.L.; O’Donnell, M.A.; Packiam, V.T. Long-term follow-up of sequential intravesical gemcitabine and docetaxel salvage therapy for non-muscle invasive bladder cancer. Urol. Oncol. 2023, 41, 148.e141–148.e147. [Google Scholar] [CrossRef]
- Chamie, K.; Chang, S.S.; Kramolowsky, E.; Gonzalgo, M.L.; Agarwal, P.K.; Bassett, J.C.; Bjurlin, M.; Cher, M.L.; Clark, W.; Cowan, B.E.; et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid. 2023, 2, EVIDoa2200167. [Google Scholar] [CrossRef]
- Shyr, C.R.; Liu, L.C.; Chien, H.S.; Huang, C.P. Immunotherapeutic Agents for Intratumoral Immunotherapy. Vaccines 2023, 11, 1717. [Google Scholar] [CrossRef]
- Huang, C.P.; Chen, C.C.; Shyr, C.R. Xenogeneic cell therapy provides a novel potential therapeutic option for cancers by restoring tissue function, repairing cancer wound and reviving anti-tumor immune responses. Cancer Cell Int. 2018, 18, 9. [Google Scholar] [CrossRef]
- Huang, C.P.; Yang, C.Y.; Shyr, C.R. Utilizing Xenogeneic Cells As a Therapeutic Agent for Treating Diseases. Cell Transpl. 2021, 30, 9636897211011995. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Huang, C.P.; Wu, C.C.; Shyr, C.R. Combination of novel intravesical xenogeneic urothelial cell immunotherapy and chemotherapy enhances anti-tumor efficacy in preclinical murine bladder tumor models. Cancer Immunol. Immunother. 2021, 70, 1419–1433. [Google Scholar] [CrossRef]
- Huang, C.P.; Lu, H.L.; Shyr, C.R. Anti-tumor activity of intratumoral xenogeneic urothelial cell monotherapy or in combination with chemotherapy in syngeneic murine models of bladder cancer. Am. J. Cancer Res. 2023, 13, 2285–2306. [Google Scholar]
- Gunther, J.H.; Jurczok, A.; Wulf, T.; Brandau, S.; Deinert, I.; Jocham, D.; Bohle, A. Optimizing syngeneic orthotopic murine bladder cancer (MB49). Cancer Res. 1999, 59, 2834–2837. [Google Scholar]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Addeo, R.; Caraglia, M.; Bellini, S.; Abbruzzese, A.; Vincenzi, B.; Montella, L.; Miragliuolo, A.; Guarrasi, R.; Lanna, M.; Cennamo, G.; et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: Evaluation of efficacy and tolerance. J. Clin. Oncol. 2010, 28, 543–548. [Google Scholar] [CrossRef]
- Jacquelot, N.; Tellier, J. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 2021, 10, 1900508. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Bhat, P.; Leggatt, G.; Waterhouse, N.; Frazer, I.H. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017, 8, e2836. [Google Scholar] [CrossRef]
- Kim, J.; Akbani, R.; Creighton, C.J.; Lerner, S.P.; Weinstein, J.N.; Getz, G.; Kwiatkowski, D.J. Invasive Bladder Cancer: Genomic Insights and Therapeutic Promise. Clin. Cancer Res. 2015, 21, 4514–4524. [Google Scholar] [CrossRef]
- Bevers, R.F.; Kurth, K.H.; Schamhart, D.H. Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br. J. Cancer 2004, 91, 607–612. [Google Scholar] [CrossRef]
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L., 3rd; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Vanderlugt, C.J.; Miller, S.D. Epitope spreading. Curr. Opin. Immunol. 1996, 8, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, R.S.; von Herrath, M.G.; Christen, U.; Whitton, J.L. Molecular mimicry, bystander activation, or viral persistence: Infections and autoimmune disease. Clin. Microbiol. Rev. 2006, 19, 80–94. [Google Scholar] [CrossRef]
- Brossart, P. The Role of Antigen Spreading in the Efficacy of Immunotherapies. Clin. Cancer Res. 2020, 26, 4442–4447. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Thomas Iii, M.L.; Clark, T.; Lewis, B.G.; Hoyt, R.F.; Eckhaus, M.; Pierson Iii, R.N.; Belli, A.J.; et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat. Commun. 2016, 7, 11138. [Google Scholar] [CrossRef]
- Iwase, H.; Ekser, B.; Satyananda, V.; Bhama, J.; Hara, H.; Ezzelarab, M.; Klein, E.; Wagner, R.; Long, C.; Thacker, J.; et al. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 2015, 22, 211–220. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Corcoran, P.C.; Singh, A.K.; Azimzadeh, A.; Hoyt, R.F., Jr.; Thomas, M.L.; Eckhaus, M.A.; Seavey, C.; Ayares, D.; Pierson, R.N., 3rd; et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am. J. Transpl. 2012, 12, 763–771. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Ritchey, J.K.; Yuan, J.J.; Andriole, G.L.; Catalona, W.J. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J. Urol. 1993, 150, 1018–1023. [Google Scholar] [CrossRef]
- Bohle, A.; Jocham, D.; Bock, P.R. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J. Urol. 2003, 169, 90–95. [Google Scholar] [CrossRef]
- Alexandroff, A.B.; Jackson, A.M.; O’Donnell, M.A.; James, K. BCG immunotherapy of bladder cancer: 20 years on. Lancet 1999, 353, 1689–1694. [Google Scholar] [CrossRef]
- Huang, C.P.; Chen, C.C.; Tsai, Y.T.; Wu, C.C.; Shyr, C.R. Intravesical Administration of Xenogeneic Porcine Urothelial Cells Attenuates Cyclophosphamide-Induced Cystitis in Mice. Cell Transplant 2019, 28, 296–305. [Google Scholar] [CrossRef]
- Ourfali, S.; Ohannessian, R.; Fassi-Fehri, H.; Pages, A.; Badet, L.; Colombel, M. Recurrence Rate and Cost Consequence of the Shortage of Bacillus Calmette-Guérin Connaught Strain for Bladder Cancer Patients. Eur. Urol. Focus 2021, 7, 111–116. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyr, C.-R.; Wu, C.-F.; Yang, K.-C.; Ma, W.-L.; Huang, C.-P. Evaluating Therapeutic Efficacy of Intravesical Xenogeneic Urothelial Cell Treatment Alone and in Combination with Chemotherapy or Immune Checkpoint Inhibition in a Mouse Non-Muscle-Invasive Bladder Cancer Model. Cancers 2025, 17, 2448. https://doi.org/10.3390/cancers17152448
Shyr C-R, Wu C-F, Yang K-C, Ma W-L, Huang C-P. Evaluating Therapeutic Efficacy of Intravesical Xenogeneic Urothelial Cell Treatment Alone and in Combination with Chemotherapy or Immune Checkpoint Inhibition in a Mouse Non-Muscle-Invasive Bladder Cancer Model. Cancers. 2025; 17(15):2448. https://doi.org/10.3390/cancers17152448
Chicago/Turabian StyleShyr, Chih-Rong, Ching-Feng Wu, Kai-Cheng Yang, Wen-Lung Ma, and Chi-Ping Huang. 2025. "Evaluating Therapeutic Efficacy of Intravesical Xenogeneic Urothelial Cell Treatment Alone and in Combination with Chemotherapy or Immune Checkpoint Inhibition in a Mouse Non-Muscle-Invasive Bladder Cancer Model" Cancers 17, no. 15: 2448. https://doi.org/10.3390/cancers17152448
APA StyleShyr, C.-R., Wu, C.-F., Yang, K.-C., Ma, W.-L., & Huang, C.-P. (2025). Evaluating Therapeutic Efficacy of Intravesical Xenogeneic Urothelial Cell Treatment Alone and in Combination with Chemotherapy or Immune Checkpoint Inhibition in a Mouse Non-Muscle-Invasive Bladder Cancer Model. Cancers, 17(15), 2448. https://doi.org/10.3390/cancers17152448







