Combined Minimal Residual Disease Evaluation in Bone Marrow and Apheresis Samples in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation Improves Outcome Prediction
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Flow Cytometry Assessment of MRD Status in Bone Marrow and Apheresis Sample
2.2. Statistical Analysis
3. Results
3.1. Patients
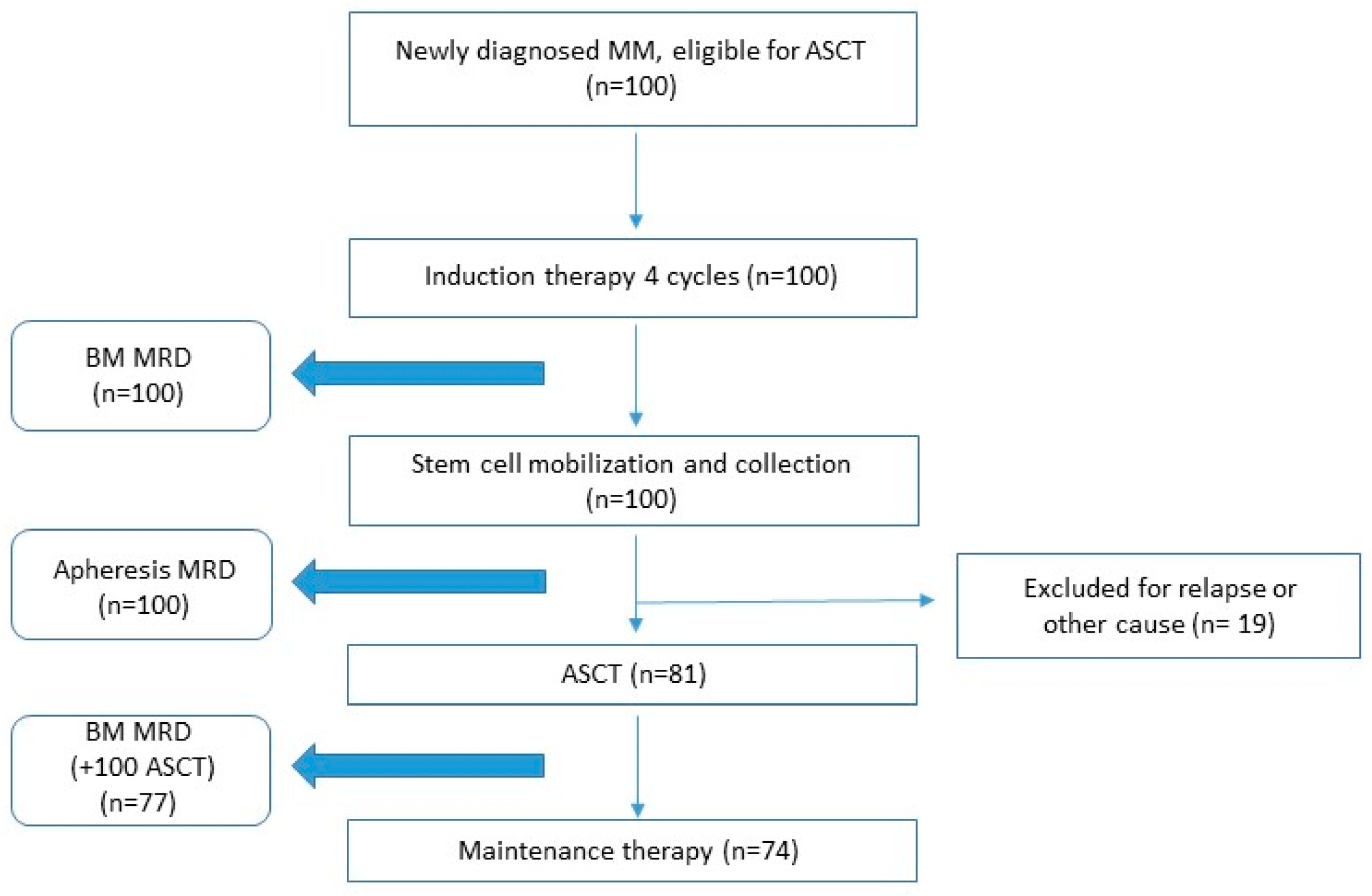
3.2. Bone Marrow and Apheresis Sample MRD Results and Phenotypic Features of APCs
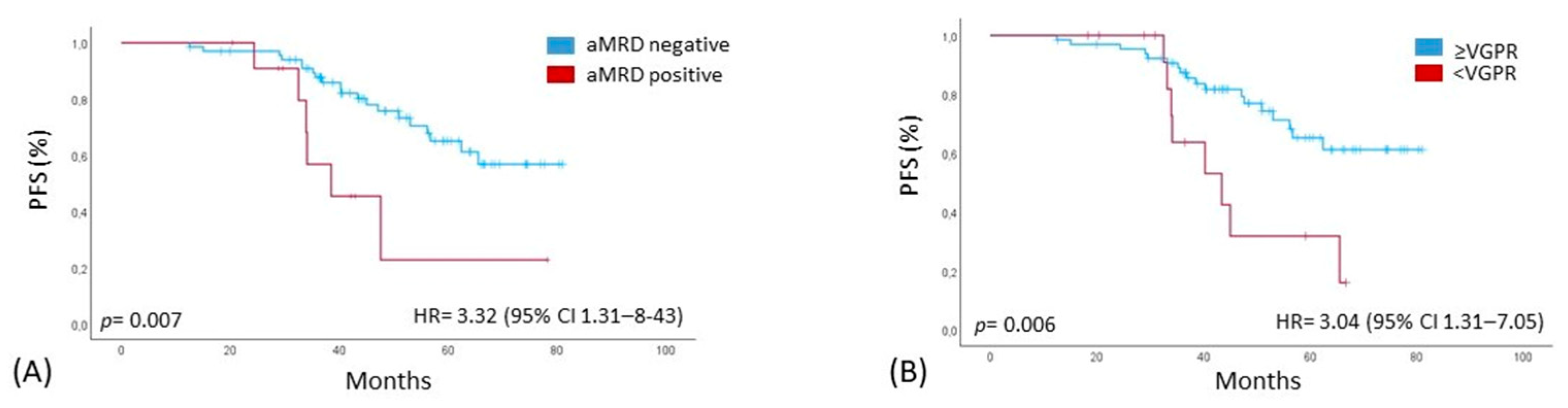
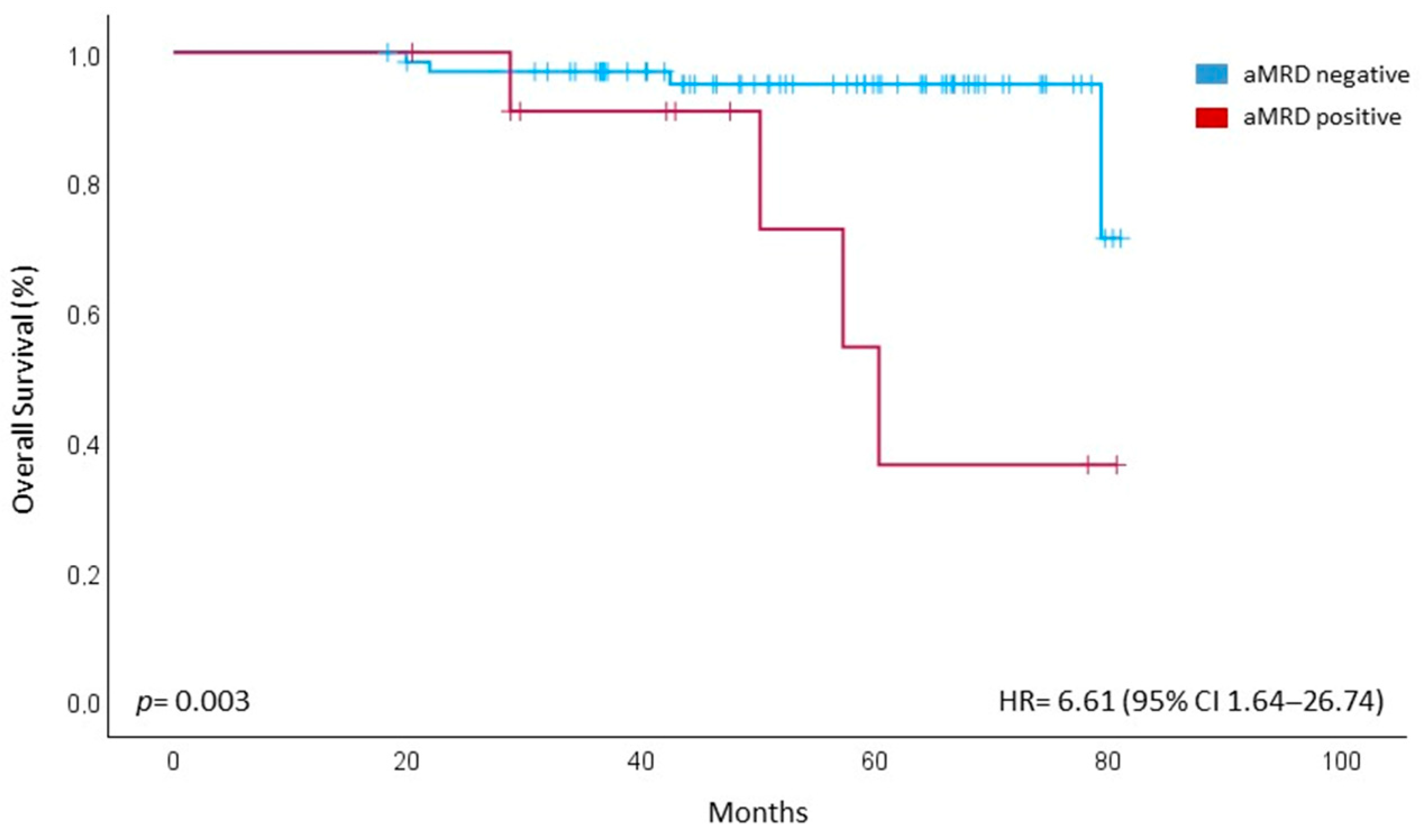
3.3. Outcome and Correlation with PFS and OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma. JAMA 2022, 327, 464. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Miguel, J.S.; Sonneveld, P.; Mateos, M.V.; Zamagni, E.; Avet-Loiseau, H.; Hajek, R.; Dimopoulos, M.A.; Ludwig, H.; Einsele, H.; et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv52–iv61. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, J.; Ismaila, N.; Cheung, M.C.; Costello, C.; Dhodapkar, M.V.; Kumar, S.; Lacy, M.; Lipe, B.; Little, R.F.; Nikonova, A.; et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. 2019, 37, 1228–1263. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous haematopoietic stem-cell transplantation versus bortezomib–melphalan–prednisone, with or without bortezomib–lenalidomide–dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [CrossRef]
- Sonneveld, P.; Goldschmidt, H.; Rosiñol, L.; Bladé, J.; Lahuerta, J.J.; Cavo, M.; Tacchetti, P.; Zamagni, E.; Attal, M.; Lokhorst, H.M.; et al. Bortezomib-Based Versus Nonbortezomib-Based Induction Treatment Before Autologous Stem-Cell Transplantation in Patients with Previously Untreated Multiple Myeloma: A Meta-Analysis of Phase III Randomized, Controlled Trials. J. Clin. Oncol. 2013, 31, 3279–3287. [Google Scholar] [CrossRef]
- Vogel, W.; Kopp, H.-G.; Kanz, L.; Einsele, H. Myeloma cell contamination of peripheral blood stem-cell grafts can predict the outcome in multiple myeloma patients after high-dose chemotherapy and autologous stem-cell transplantation. J. Cancer Res. Clin. Oncol. 2005, 131, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kopp, H.G.; Yildirim, S.; Weisel, K.C.; Kanz, L.; Vogel, W. Contamination of autologous peripheral blood progenitor cell grafts predicts overall survival after high-dose chemotherapy in multiple myeloma. J. Cancer Res. Clin. Oncol. 2009, 135, 637–642. [Google Scholar] [CrossRef]
- Wuillème, S.; Lok, A.; Robillard, N.; Dupuis, P.; Stocco, V.; Migné, H.; Dusquesne, A.; Touzeau, C.; Tiab, M.; Béné, M.C.; et al. Assessment of tumoral plasma cells in apheresis products for autologous stem cell transplantation in multiple myeloma. Bone Marrow Transpl. 2016, 51, 1143–1145. [Google Scholar] [CrossRef]
- Pasvolsky, O.; Milton, D.R.; Rauf, M.; Ghanem, S.; Masood, A.; Mohamedi, A.H.; Tanner, M.R.; Bashir, Q.; Srour, S.; Saini, N.; et al. Impact of clonal plasma cells in autografts on outcomes in high-risk multiple myeloma patients. Blood Cancer J. 2023, 13, 68. [Google Scholar] [CrossRef]
- Kostopoulos, I.V.; Eleutherakis-Papaiakovou, E.; Rousakis, P.; Ntanasis-Stathopoulos, I.; Panteli, C.; Orologas-Stavrou, N.; Kanellias, N.; Malandrakis, P.; Liacos, C.I.; Papaioannou, N.E.; et al. Aberrant Plasma Cell Contamination of Peripheral Blood Stem Cell Autografts, Assessed by Next-Generation Flow Cytometry, Is a Negative Predictor for Deep Response Post Autologous Transplantation in Multiple Myeloma; A Prospective Study in 199 Patients. Cancers 2021, 13, 4047. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, S.; Morabito, F.; Guerrini, F.; Palumbo, G.A.; Azzará, A.; Martino, M.; Benedetti, E.; Di Raimondo, F.; Petrini, M. Peripheral blood stem cell contamination evaluated by a highly sensitive molecular method fails to predict outcome of autotransplanted multiple myeloma patients. Br. J. Haematol. 2003, 120, 405–412. [Google Scholar] [CrossRef]
- Seval, G.C.; Beksac, M. Is Quantification of Measurable Clonal Plasma Cells in Stem Cell Grafts (gMRD) Clinically Meaningful? Front. Oncol. 2022, 12, 800711. [Google Scholar] [CrossRef]
- Gertz, M.; Witzig, T.; Pineda, A.; Greipp, P.; Kyle, R.; Litzow, M. Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transpl. 1997, 19, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengas, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Ross, F.M.; Avet-Loiseau, H.; Ameye, G.; Gutiérrez, N.C.; Liebisch, P.; O’Connor, S.; Dalva, K.; Fabris, S.; Testi, A.M.; Jarosova, M.; et al. Report from the European myeloma network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012, 97, 1272–1277. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International MyelomaWorking Group consensus criteria for response and minimalresidual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Soh, K.T.; Came, N.; Otteson, G.E.; Jevremovic, D.; Shi, M.; Olteanu, H.; Natoni, A.; Lagoo, A.; Theakston, E.; Þórir Óskarsson, J.; et al. Evaluation of multiple myeloma measurable residual disease by high sensitivity flow cytometry: An international harmonized approach for data analysis. Cytom. B Clin. Cytom. 2022, 102, 88–106. [Google Scholar] [CrossRef]
- Flores-Montero, J.; de Tute, R.; Paiva, B.; Perez, J.J.; Böttcher, S.; Wind, H.; Sanoja, L.; Puig, N.; Lecrevisse, Q.; Vidriales, M.B.; et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytom. Part B 2016, 90, 61–72. [Google Scholar] [CrossRef]
- Arro, M.; Came, N.; Lin, P.; Chen, W.; Yuan, C.; Lagoo, A.; Monreal, M.; de Tute, R.; Vergilio, J.; Rawstron, A.C.; et al. Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytom. B Clin. Cytom. 2016, 90, 31–39. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: A meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Paiva, B.; Zherniakova, A.; Nunez-Cordoba, J.M.; Rodriguez-Otero, P.; Shi, Q.; Munshi, N.C.; Durie, B.G.M.; San-Miguel, J. Impact of treatment effect on MRD and PFS: An aggregate data analysis from randomized clinical trials in multiple myeloma. Am. Soc. Hematol. 2024, 8, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Devlin, S.; Boulad, M.; Mailankody, S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: A meta-analysis. Bone Marrow Transpl. 2016, 51, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Avet-Loiseau, H.; Ludwig, H.; Landgren, O.; Paiva, B.; Morris, C.; Yang, H.; Zhou, K.; Ro, S.; Mateos, M.V. Minimal Residual Disease Status as a Surrogate Endpoint for Progression-free Survival in Newly Diagnosed Multiple Myeloma Studies: A Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e30–e37. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Anderson, K.C.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef]
- Perrot, A.; Lauwers-Cances, V.; Corre, J.; Robillard, N.; Hulin, C.; Chretien, M.L.; Dejoie, T.; Maheo, S.; Stoppa, A.M.; Pegourie, B.; et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 2018, 132, 2456–2464. [Google Scholar] [CrossRef]
- Paiva, B.; Puig, N.; Cedena, M.T.; Rosiñol, L.; Cordón, L.; Vidriales, M.B.; Burgos, L.; Flores-Montero, J.; Sanoja-Flores, L.; Lopez-Anglada, L.; et al. Measurable Residual Disease by Next-Generation Flow Cytometry in Multiple Myeloma. J. Clin. Oncol. 2019, 38, 784–792. [Google Scholar] [CrossRef]
- Oliva, S.; Genuardi, E.; Parisc, L.; D’Agostino, M.; Rogers, J.; Rota-Scalabrini, D.; Jacob, A.P.; Patriarca, F.; Luppi, M.; Bertazzoni, P.; et al. Prospective evaluation of minimal residual disease in the phase II FORTE trial: A head-to-head comparison between multiparameter flow cytometry and next-generation sequencing. eClinicalMedicine 2023, 60, 102016. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients (No. = 100) |
|---|---|
| Sex—no. of pts | |
| Male | 51 (51%) |
| Female | 49 (49%) |
| Median age at diagnosis (range) | 59 (37–70) |
| Cytogenetic risk—no. of pts (%) | |
| Standard risk | 68 (68%) |
| High risk | 26 (26%) |
| - del(17p) | 4 |
| - t(4;14) | 13 |
| - t(14;16) | 1 |
| - gain(1q)/amp(1q) | 15 |
| NA | 6 (6%) |
| International Staging System (ISS)—no. of pts (%) | |
| -I | 31 (31%) |
| -II | 23 (23%) |
| -III | 20 (20%) |
| NA | 26 (26%) |
| Revised ISS (R-ISS)—no. of pts (%) | |
| -I | 26 (26%) |
| -II | 40 (40%) |
| -III | 6 (6%) |
| NA | 28 (28%) |
| Induction treatment | |
| VTD | 96 (96%) |
| PAD | 4 (4%) |
| ASCT—no. of patients (%) | |
| Performed | 81 (81%) |
| Not performed | 19 (19%) |
| Risk Factors | No. pts | Median PFS (month) | PFS, 24 month (%) | p. | |
|---|---|---|---|---|---|
| Pre-ASCT response (n° = 81) | ≥VGPR and aMRD− | 59 | N.R. | 96% | <0.001 |
| ≥VGPR and aMRD+ | 7 | 47.6 | 86% | ||
| <VGPR and aMRD− | 10 | 45.1 | 90% | ||
| <VGPR and aMRD+ | 5 | 33.9 | 90% | ||
| bmMRD pre-ASCT (n° = 81) | bmMRD− and aMRD− | 35 | N.R. | 100% | 0.025 |
| bmMRD+ and aMRD+ | 12 | 38.6 | 97% | ||
| bmMRD+ and aMRD− | 34 | N.R. | 94% | ||
| Post-ASCT response (n° = 81) | ≥CR and aMRD− | 52 | N.R. | 98% | <0.001 |
| ≥CR and aMRD+ | 8 | 47.7 | 99% | ||
| <CR and aMRD− | 17 | 62.2 | 99% | ||
| <CR and aMRD+ | 4 | 33.9 | 99% | ||
| bmMRD post-ASCT (n° = 77) | bmMRD− and aMRD− | 52 | N.R. | 98% | <0.001 |
| bmMRD− and aMRD+ | 15 | N.R. | 100% | ||
| bmMRD+ and aMRD− | 5 | N.R. | 100% | ||
| bmMRD+ and aMRD+ | 5 | 35 | 80% |
| Risk Factors | No. pts | Median OS (month) | OS, 48 month (%) | p. | |
|---|---|---|---|---|---|
| Pre-SCT response (n° = 81) | ≥VGPR and aMRD− | 59 | N.R. | 95% | <0.001 |
| ≥VGPR and aMRD+ | 7 | N.R. | 97% | ||
| <VGPR and aMRD− | 10 | N.R. | 97% | ||
| <VGPR and aMRD+ | 5 | 57 | 90% | ||
| bmMRD pre-ASCT (n° = 81) | bmMRD− and aMRD− | 35 | N.R. | 94% | 0.010 |
| bmMRD+ and aMRD+ | 12 | 60 | 90% | ||
| bmMRD+ and aMRD− | 34 | N.R. | 95% | ||
| Post-ASCT response (n° = 81) | ≥CR and aMRD− | 52 | N.R. | 92% | <0.001 |
| ≥CR and aMRD+ | 8 | N.R. | 83% | ||
| <CR and aMRD− | 17 | N.R. | 95% | ||
| <CR and aMRD+ | 4 | 58 | 95% | ||
| bmMRD post-ASCT (n° = 77) | bmMRD− and aMRD− | 52 | N.R. | 97% | 0.012 |
| bmMRD− and aMRD+ | 5 | N.R. | 99% | ||
| bmMRD+ and aMRD− | 15 | N.R. | 99% | ||
| bmMRD+ and aMRD+ | 5 | 60 | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attucci, I.; Peruzzi, B.; Nozzoli, C.; Guerrieri, S.; Pilerci, S.; Boncompagni, R.; Urbani, S.; Orazzini, C.; Bencini, S.; Capone, M.; et al. Combined Minimal Residual Disease Evaluation in Bone Marrow and Apheresis Samples in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation Improves Outcome Prediction. Cancers 2025, 17, 2439. https://doi.org/10.3390/cancers17152439
Attucci I, Peruzzi B, Nozzoli C, Guerrieri S, Pilerci S, Boncompagni R, Urbani S, Orazzini C, Bencini S, Capone M, et al. Combined Minimal Residual Disease Evaluation in Bone Marrow and Apheresis Samples in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation Improves Outcome Prediction. Cancers. 2025; 17(15):2439. https://doi.org/10.3390/cancers17152439
Chicago/Turabian StyleAttucci, Irene, Benedetta Peruzzi, Chiara Nozzoli, Serena Guerrieri, Sofia Pilerci, Riccardo Boncompagni, Serena Urbani, Chiara Orazzini, Sara Bencini, Manuela Capone, and et al. 2025. "Combined Minimal Residual Disease Evaluation in Bone Marrow and Apheresis Samples in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation Improves Outcome Prediction" Cancers 17, no. 15: 2439. https://doi.org/10.3390/cancers17152439
APA StyleAttucci, I., Peruzzi, B., Nozzoli, C., Guerrieri, S., Pilerci, S., Boncompagni, R., Urbani, S., Orazzini, C., Bencini, S., Capone, M., Messeri, M., Caporale, R., Annunziato, F., Vannucchi, A. M., & Antonioli, E. (2025). Combined Minimal Residual Disease Evaluation in Bone Marrow and Apheresis Samples in Multiple Myeloma Patients Undergoing Autologous Stem Cell Transplantation Improves Outcome Prediction. Cancers, 17(15), 2439. https://doi.org/10.3390/cancers17152439







