The Impact of DDR Gene Mutations on the Efficacy of Etoposide Plus Cisplatin in Grade 3 Metastatic Gastroenteropancreatic (GEP)—Neuroendocrine Carcinoma (NEC)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Distribution of DDR Pathway Mutations
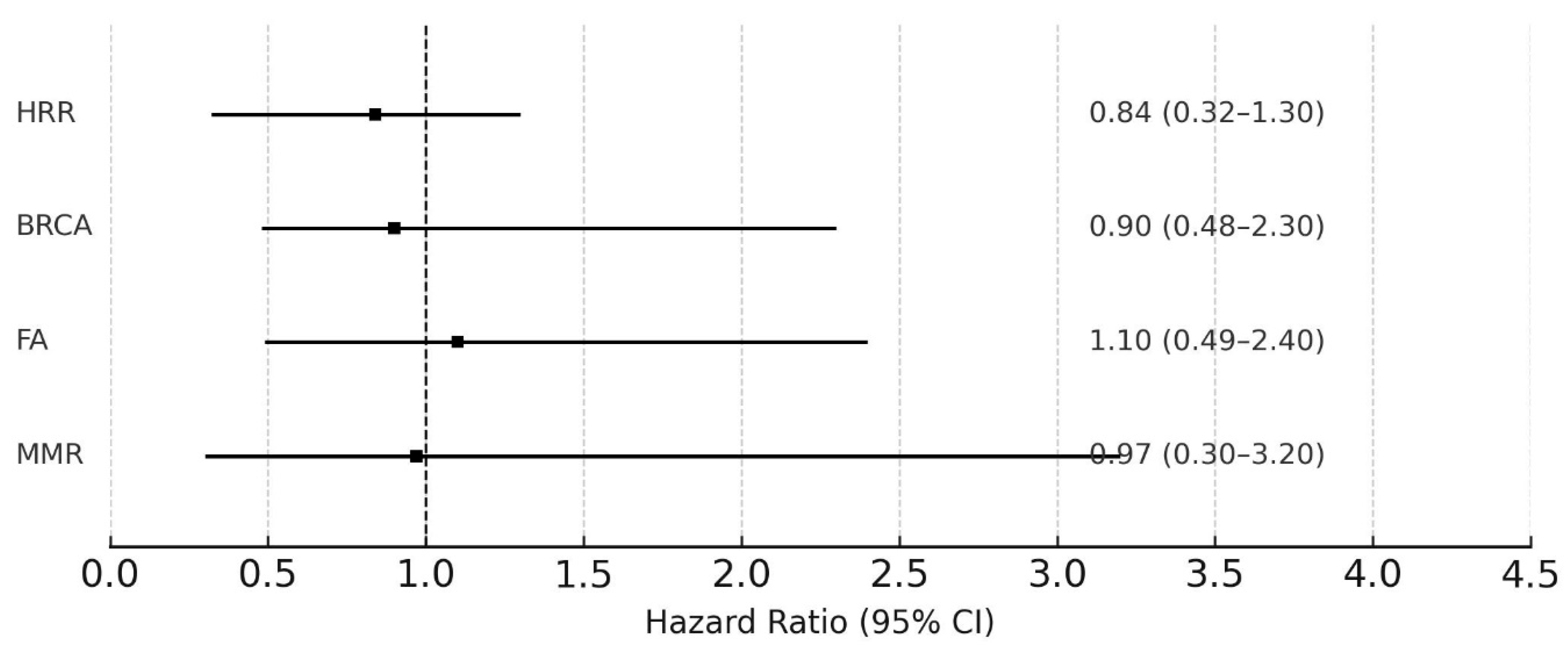
3.3. Efficacy of Cisplatin According to DDR Mutations
3.3.1. Objective Response Rate (ORR)
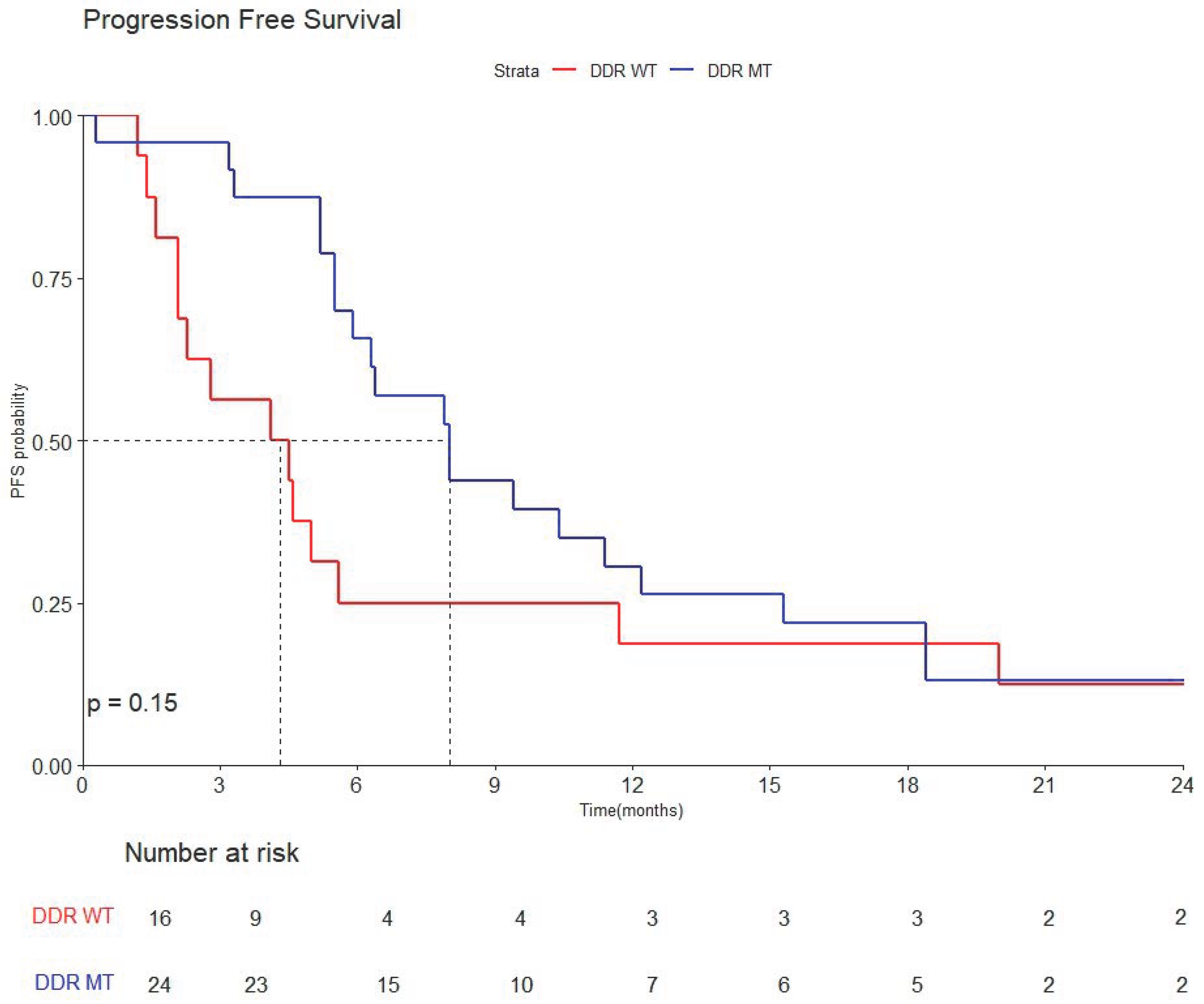
3.3.2. Progression-Free Survival (PFS)
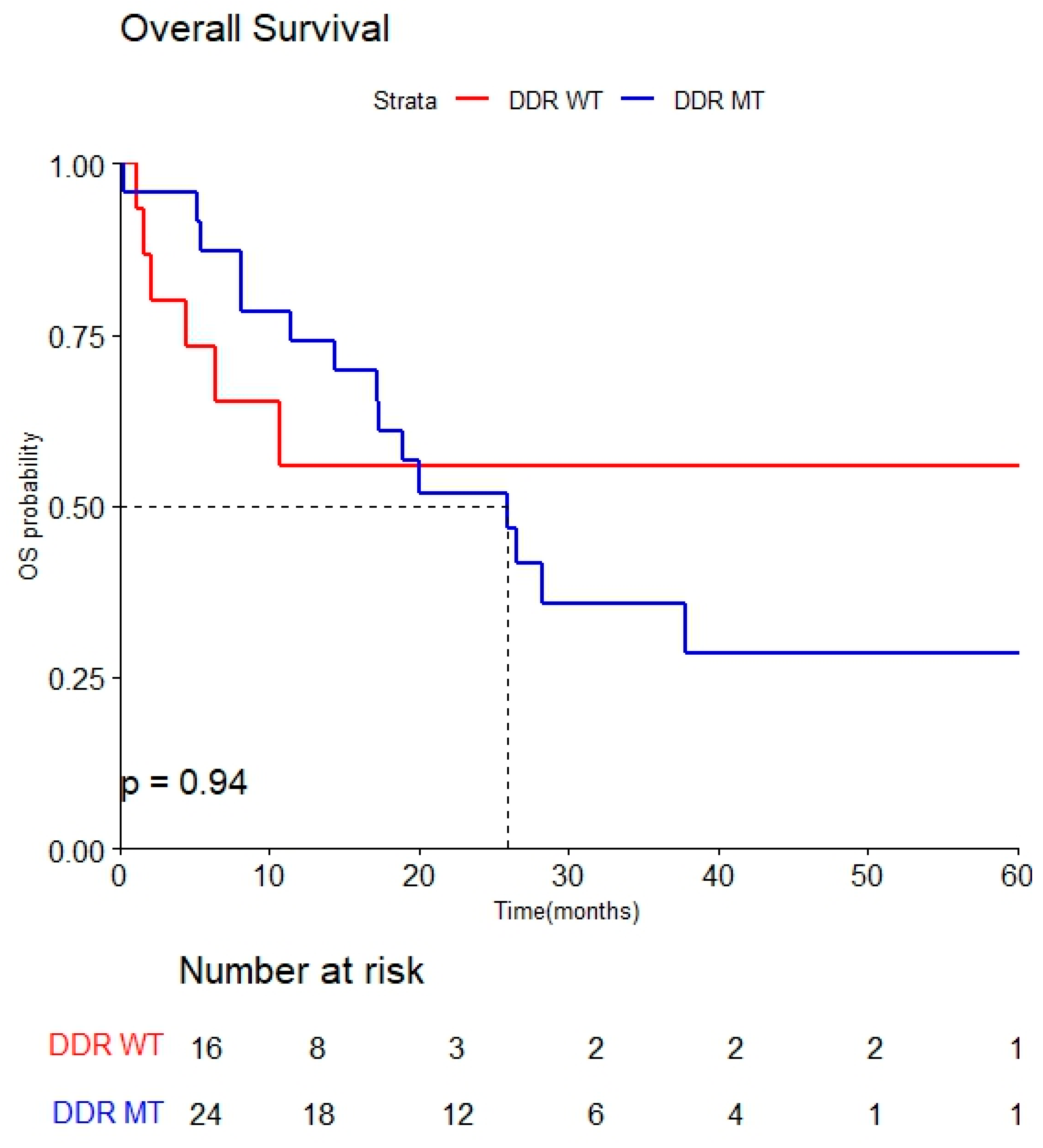
3.3.3. Overall Survival (OS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, S.; Morizane, C.; Okusaka, T.; Ueno, H.; Ikeda, M.; Kondo, S.; Tanaka, T.; Nakachi, K.; Mitsunaga, S.; Kojima, Y.; et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn. J. Clin. Oncol 2010, 40, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Strosberg, J.; Baudin, E.; Klimstra, D.S.; Yao, J.C. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014, 120, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Aouida, M.; Alhaj Sulaiman, A.; Madhusudan, S.; Ramotar, D. Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted. Int. J. Mol. Sci. 2022, 23, 7241. [Google Scholar] [CrossRef] [PubMed]
- Fjallskog, M.L.; Granberg, D.P.; Welin, S.L.; Eriksson, C.; Oberg, K.E.; Janson, E.T.; Eriksson, B.K. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer 2001, 92, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Elledge, S.J. The DNA damage response: Ten years after. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Bambury, R.M.; Zabor, E.C.; Jordan, E.; Al-Ahmadie, H.; Boyd, M.E.; Bouvier, N.; Mullane, S.A.; Cha, E.K.; Roper, N.; et al. DNA Damage Response and Repair Gene Alterations Are Associated with Improved Survival in Patients with Platinum-Treated Advanced Urothelial Carcinoma. Clin. Cancer Res. 2017, 23, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.D. The Fanconi Anemia/BRCA signaling pathway: Disruption in cisplatin-sensitive ovarian cancers. Cell Cycle 2003, 2, 290–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mota, J.M.; Barnett, E.; Nauseef, J.T.; Nguyen, B.; Stopsack, K.H.; Wibmer, A.; Flynn, J.R.; Heller, G.; Danila, D.C.; Rathkopf, D.; et al. Platinum-Based Chemotherapy in Metastatic Prostate Cancer With DNA Repair Gene Alterations. JCO Precis. Oncol. 2020, 4, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Nombela, P.; Lozano, R.; Aytes, A.; Mateo, J.; Olmos, D.; Castro, E. BRCA2 and Other DDR Genes in Prostate Cancer. Cancers 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Sun, L.; Zheng, Y.; Chen, H.; Zheng, X.; Luo, J.; Gu, C.; Lin, R.; Huang, M.; Bai, Y.; et al. DNA damage repair gene mutations predict the efficacy of platinum-based chemotherapy and immunotherapy plus platinum-based chemotherapy in advanced non-small cell lung cancer: A retrospective Chinese cohort study. Transl. Lung Cancer Res. 2022, 11, 2539–2566. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.Y.; Guner, G.; Guven, D.C.; Arslan, C.; Dizdar, O. Exploiting DNA repair defects in breast cancer: From chemotherapy to immunotherapy. Expert Rev. Anticancer Ther. 2019, 19, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Shu, T.; Zhu, S.; Zhang, C.; Gao, M.; Zhang, N.; Wang, H.; Yuan, J.; Tai, Z.; Xia, X.; et al. Construction and Validation of a Platinum Sensitivity Predictive Model With Multiple Genomic Variations for Epithelial Ovarian Cancer. Front. Oncol. 2021, 11, 725264. [Google Scholar] [CrossRef] [PubMed]
- Sternschuss, M.; Paynter, A.; Xu, W.; Nichols, C.; Hoppe, J.; Davis, N.B.; Jiang, D.M.; Shah, N.J. DNA damage repair alterations as predictive biomarker for platinum-based chemotherapy in metastatic urothelial cancer. J. Clin. Oncol. 2025, 43, 853. [Google Scholar] [CrossRef]
- Lozano, R.; Castro, E.; Aragon, I.M.; Cendon, Y.; Cattrini, C.; Lopez-Casas, P.P.; Olmos, D. Genetic aberrations in DNA repair pathways: A cornerstone of precision oncology in prostate cancer. Br. J. Cancer 2021, 124, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pabla, N.; Tang, C.; He, L.; Dong, Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch. Toxicol. 2015, 89, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Tangen, C.M.; Sarfaty, M.; Regazzi, A.M.; Lee, I.L.; Fong, M.; Choi, W.; Dinney, C.P.N.; Flaig, T.W.; Thompson, I.M., Jr.; et al. DNA Damage Response Alterations Predict for Neoadjuvant Chemotherapy Sensitivity in Muscle-Invasive Bladder Cancer: A Correlative Analysis of the SWOG S1314 Trial. JCO Precis. Oncol. 2024, 8, e2400287. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.R.; Cooney, S.A.; Chin-Sinex, H.; Mendonca, M.S.; Turchi, J.J. DNA damage response (DDR) pathway engagement in cisplatin radiosensitization of non-small cell lung cancer. DNA Repair 2016, 40, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Lerksuthirat, T.; Prasopporn, S.; Wikiniyadhanee, R.; Chitphuk, S.; Stitchantrakul, W.; Owneium, P.; Jirawatnotai, S.; Dejsuphong, D. DNA damage response mutations enhance the antitumor efficacy of ATR and PARP inhibitors in cholangiocarcinoma cell lines. Oncol. Lett. 2025, 29, 128. [Google Scholar] [CrossRef] [PubMed]
- Psyrri, A.; Gkotzamanidou, M.; Papaxoinis, G.; Krikoni, L.; Economopoulou, P.; Kotsantis, I.; Anastasiou, M.; Souliotis, V.L. The DNA damage response network in the treatment of head and neck squamous cell carcinoma. ESMO Open 2021, 6, 100075. [Google Scholar] [CrossRef] [PubMed]
- Elvebakken, H.; Venizelos, A.; Perren, A.; Couvelard, A.; Lothe, I.M.B.; Hjortland, G.O.; Myklebust, T.A.; Svensson, J.; Garresori, H.; Kersten, C.; et al. Treatment outcome according to genetic tumour alterations and clinical characteristics in digestive high-grade neuroendocrine neoplasms. Br. J. Cancer 2024, 131, 676–684. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Total (n = 40) | DDR WT (n = 16) | DDR MT (n = 24) | p-Value |
|---|---|---|---|---|
| Age (years), median (range) | 64.7 ± 9.2 | 66.2 ± 11.0 | 63.7 ± 7.9 | 0.418 |
| Sex | 0.543 | |||
| Male | 26 (65.0%) | 9 (56.2%) | 17 (70.8%) | |
| Female | 14 (35.0%) | 7 (43.8%) | 7 (29.2%) | |
| ECOG performance status | 0.686 | |||
| 0 | 2 (5.0%) | 1 (6.2%) | 1 (4.2%) | |
| 1 | 37 (92.5%) | 15 (93.8%) | 22 (91.7%) | |
| 2 | 1 (2.5%) | 0 (0.0%) | 1 (4.2%) | |
| Primary site | 0.225 | |||
| Esophagus | 1 (2.5%) | 1 (6.2%) | 0 (0.0%) | |
| Stomach | 8 (20.0%) | 3 (18.8%) | 5 (20.8%) | |
| Pancreas | 10 (25.0%) | 7 (43.8%) | 3 (12.5%) | |
| Small bowel | 1 (2.5%) | 0 (0.0%) | 1 (4.2%) | |
| GB/Duct | 5 (12.5%) | 2 (12.5%) | 3 (12.5%) | |
| Rectum | 4 (10.0%) | 0 (0.0%) | 4 (16.7%) | |
| Others | 3 (7.5%) | 1 (6.2%) | 2 (8.3%) | |
| Unknown | 8 (20.0%) | 2 (12.5%) | 6 (25.0%) | |
| Pathology | 0.618 | |||
| SCNEC | 5 (12.5%) | 1 (6.2%) | 4 (16.7%) | |
| LCNEC | 1 (2.5%) | 0 (0.0%) | 1 (4.2%) | |
| Other G3 NEC | 22 (55.0%) | 10 (62.5%) | 12 (50.0%) | |
| NEC NOS | 12 (30.0%) | 5 (31.2%) | 7 (29.2%) | |
| Ki-67 | 0.856 | |||
| 20–55% | 11 (27.5%) | 5 (31.3%) | 6 (25.0%) | |
| ≥55% | 17 (42.5%) | 6 (37.5%) | 11 (45.8%) | |
| Unknown | 12 (30.0%) | 5 (31.3%) | 7 (29.2%) | |
| Distant metastatic lesions | ||||
| Liver | 26 (65.0%) | 13 (81.2%) | 13 (54.2%) | 0.155 |
| Lung | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Bone | 3 (7.5%) | 0 (0.0%) | 3 (12.5%) | 0.391 |
| Peritoneum | 4 (10.0%) | 2 (12.5%) | 2 (8.3%) | 1.000 |
| LN | 22 (55.0%) | 9 (56.2%) | 13 (54.2%) | 1.000 |
| DDR Pathway | Patients with Mutation (n = 24) | Mutated Genes (Frequency) |
|---|---|---|
| HRR | 16 (66.7%) | BRCA1 (9), BRCA2 (9), PALB2 (3), ATM (4), ATR (1), RAD52 (1) |
| MMR | 4 (16.7%) | MLH1 (3), MSH6 (1) |
| FA | 8 (33.3%) | FANCA (3), FANCI (3), FANCG (3), FANCC (1), FANCF (1) |
| NEHJ/NER/BER | 0 |
| Treatment Response | DDR WT (n = 16) | DDR MT (n = 24) |
|---|---|---|
| Overall ORR, No. (%) | ||
| Complete response | 0 (0.0%) | 2 (8.3%) |
| Partial response | 2 (12.5%) | 12 (50.0%) |
| Stable disease | 6 (37.5%) | 8 (33.3%) |
| Progression disease | 4 (25.0%) | 1 (4.2%) |
| Not evaluated | 4 (25.0%) | 1 (4.2%) |
| Objective response rate, % | 12.5% | 58.3% |
| Disease control rate, % | 50.0% | 91.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.E.; Kwon, M.; Lim, S.H.; Hong, J.Y.; Kim, S.T. The Impact of DDR Gene Mutations on the Efficacy of Etoposide Plus Cisplatin in Grade 3 Metastatic Gastroenteropancreatic (GEP)—Neuroendocrine Carcinoma (NEC). Cancers 2025, 17, 2436. https://doi.org/10.3390/cancers17152436
Shin JE, Kwon M, Lim SH, Hong JY, Kim ST. The Impact of DDR Gene Mutations on the Efficacy of Etoposide Plus Cisplatin in Grade 3 Metastatic Gastroenteropancreatic (GEP)—Neuroendocrine Carcinoma (NEC). Cancers. 2025; 17(15):2436. https://doi.org/10.3390/cancers17152436
Chicago/Turabian StyleShin, Ji Eun, Minsuk Kwon, Sung Hee Lim, Jung Yong Hong, and Seung Tae Kim. 2025. "The Impact of DDR Gene Mutations on the Efficacy of Etoposide Plus Cisplatin in Grade 3 Metastatic Gastroenteropancreatic (GEP)—Neuroendocrine Carcinoma (NEC)" Cancers 17, no. 15: 2436. https://doi.org/10.3390/cancers17152436
APA StyleShin, J. E., Kwon, M., Lim, S. H., Hong, J. Y., & Kim, S. T. (2025). The Impact of DDR Gene Mutations on the Efficacy of Etoposide Plus Cisplatin in Grade 3 Metastatic Gastroenteropancreatic (GEP)—Neuroendocrine Carcinoma (NEC). Cancers, 17(15), 2436. https://doi.org/10.3390/cancers17152436







