The Prognostic Value of High-Sensitive Troponin T Rise Within the Upper Reference Limit in Breast Cancer: A Prospective Pilot Study
Simple Summary
Abstract
1. Introduction
2. Material and Methods
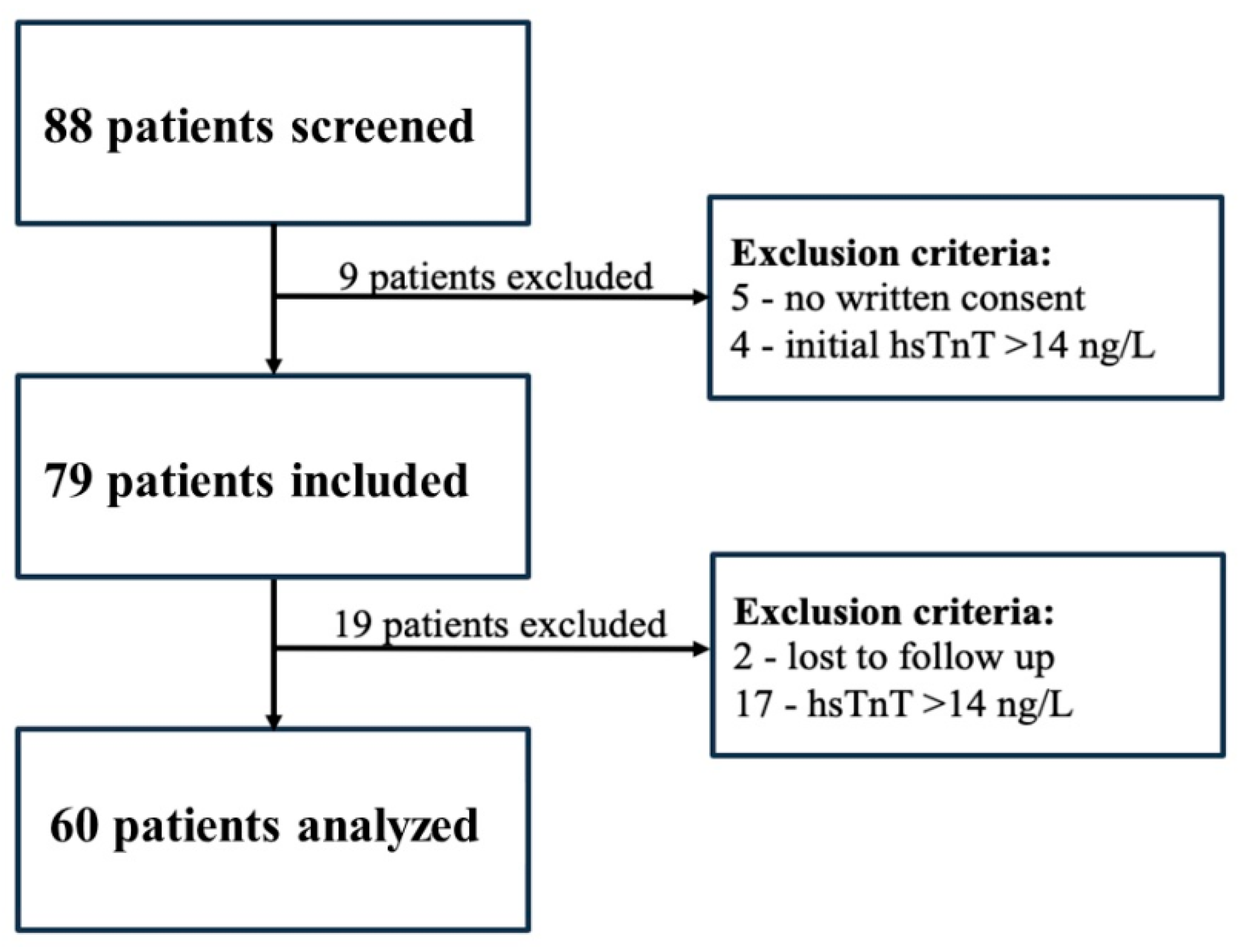
2.1. Study Design and Participants
2.2. Echocardiographic Data Acquisition
2.3. Blood Sampling and Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Population and Baseline Characteristics
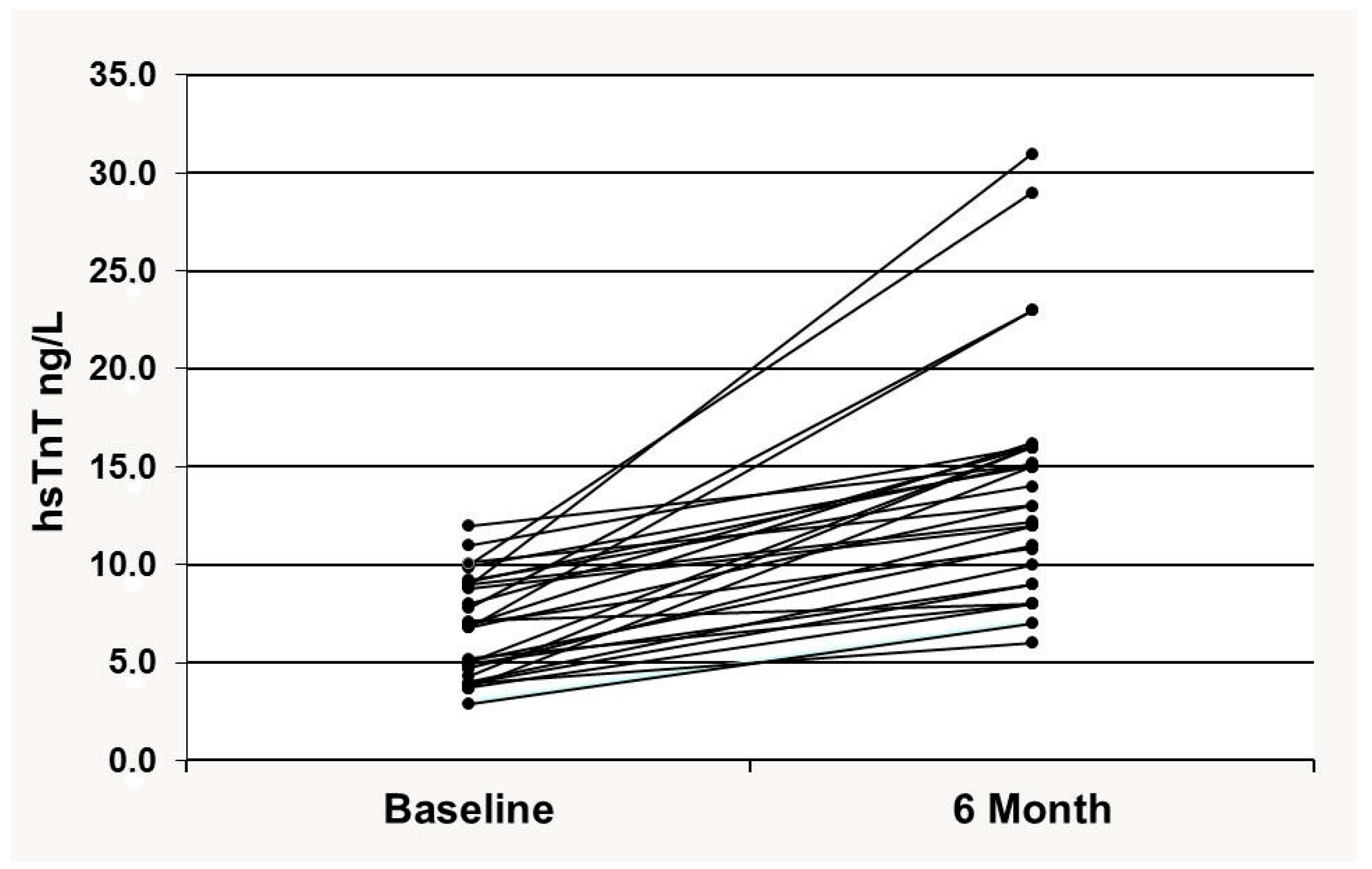
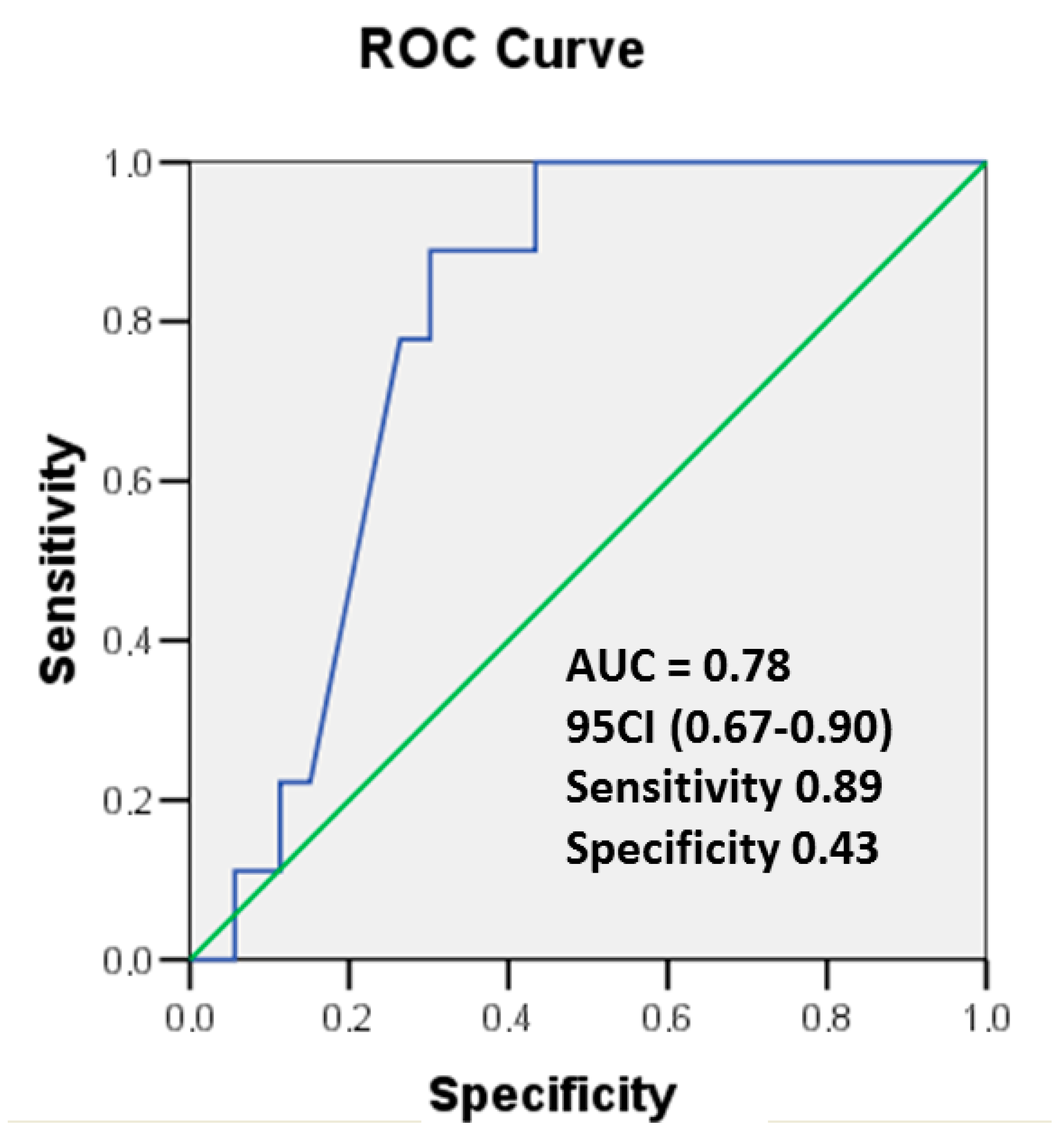
3.2. Cardiac hsTnT
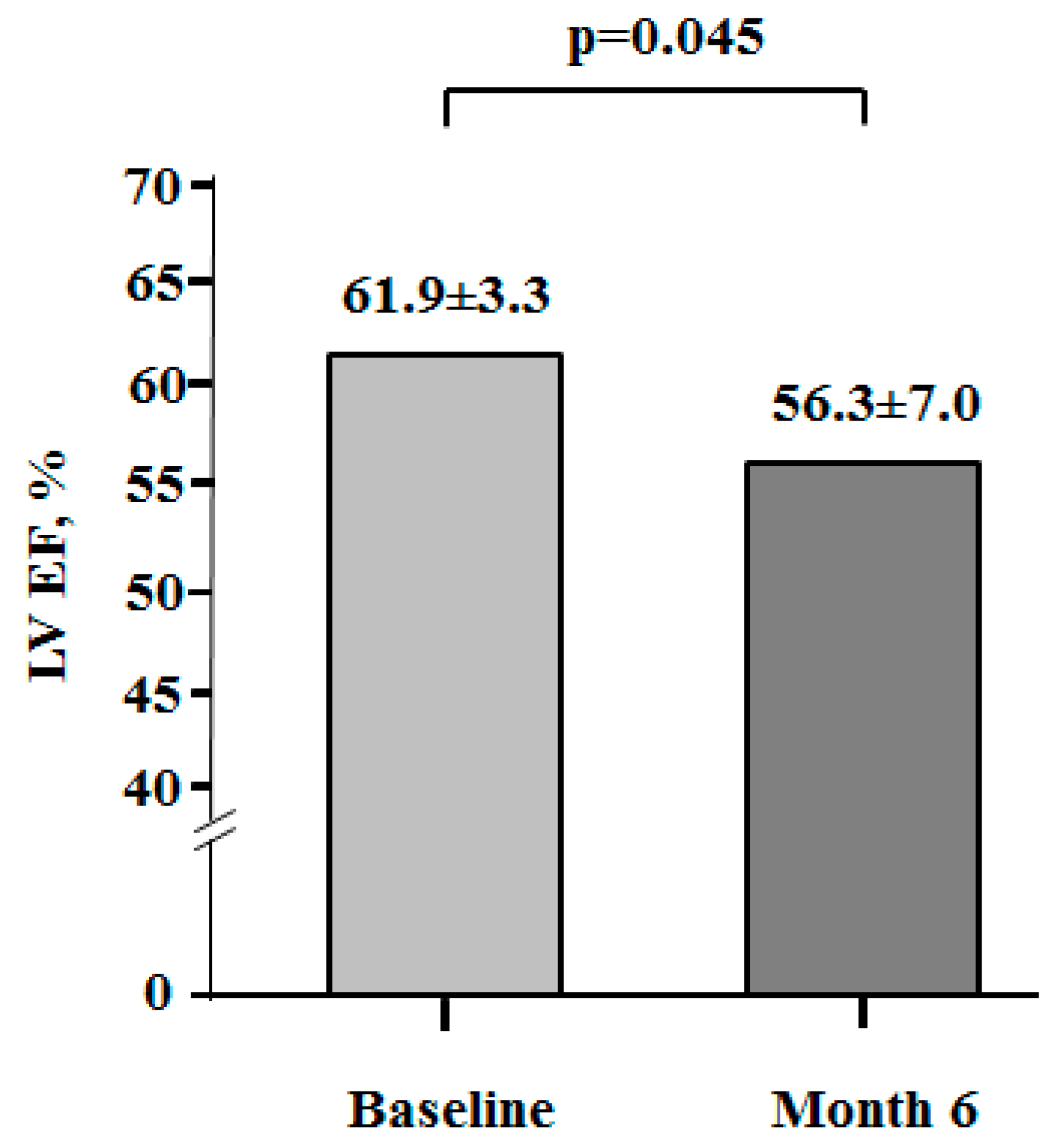
3.3. Myocardial Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | breast cancer |
| hsTnT | high-sensitive cardiac troponin T |
| ULN | upper limit of normal |
| CTRCD | cancer therapy-related cardiac disfunction |
| CV | cardiovascular |
| Echo | echocardiography |
| LV EF | left ventricle ejection fraction |
| BMI | body mass index |
| BP | blood pressure |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
References
- Cancer in Ukraine 2022–2023: Incidence, mortality, prevalence and other relevant statistics. In Bulletin of the National Cancer Registry of Ukraine; National Cancer Institute: Kyiv, Ukraine, 2024; Volume 25, 132p.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Han, S.A.; Sohn, I.S. Breast Cancer and Therapy-Related Cardiovascular Toxicity. J. Breast Cancer 2024, 27, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, U.; Di Sarro, E.; Tricarico, L.; Di Lisi, D.; Armentaro, G.; Miceli, S.; Fioretti, F.; Deidda, M.; Correale, M.; Novo, G.; et al. Cardiovascular Biomarkers in Cardio-Oncology: Antineoplastic Drug Cardiotoxicity and Beyond. Biomolecules 2024, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Trans. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.W.; Kristin Newby, L. High-sensitivity troponin assays: Evidence, indications, and reasonable use. J. Am. Heart Assoc. 2014, 3, e000403. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Q.; Tan, Y.; Qiao, J.; Liu, Q.; Yang, B.; Yang, S.; Cui, L. Early detection of anthracycline-induced cardiotoxicity. Clin. Chim. Acta. 2025, 565, 120000. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [PubMed]
- Lancellotti, P.; Cosyns, B. The EACVI Echo Handbook, online ed.; ESC Publications: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Saenger, A.K.; Beyrau, R.; Braun, S.; Cooray, R.; Dolci, A.; Freidank, H.; Giannitsis, E.; Gustafson, S.; Handy, B.; Katus, H.; et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin. Chim. Acta. 2011, 412, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wei, S.; Zhang, B.; Li, W. Molecular Mechanisms of Cardiomyocyte Death in Drug-Induced Cardiotoxicity. Front. Cell Dev. Biol. 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Cipolla, C.M.; Dent, S.; Minotti, G.; Cardinale, D.M. Anthracycline Cardiotoxicity in Adult Cancer Patients: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ. 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Giannoni, A.; Prontera, C.; Giovannini, S. High-sensitivity troponin: A new tool for pathophysiological investigation and clinical practice. Adv. Clin. Chem. 2009, 49, 1–30. [Google Scholar] [PubMed]
- Tan, S.; Kader, Z.; Day, D.; Chen, D.; Nicholls, S.J.; Ramkumar, S. Cardiotoxicity in Oncology Guidelines: Discrepancies Do Matter. Heart Lung Circ. 2024, 33, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Barac, A.; Brazile, T.L. Did You Check Diastolic Function During Cancer Treatment?: It’s Not Just Systole’s Show. JACC Cardiovasc. Imaging 2025, 18, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Finke, D.; Romann, S.W.; Heckmann, M.B.; Hund, H.; Bougatf, N.; Kantharajah, A.; Katus, H.A.; Müller, O.J.; Frey, N.; Giannitsis, E.; et al. High-sensitivity cardiac troponin T determines all-cause mortality in cancer patients: A single-centre cohort study. ESC Heart Fail. 2021, 8, 3709–3719. [Google Scholar] [CrossRef] [PubMed]
- Demissei, B.G.; Hubbard, R.A.; Zhang, L.; Smith, A.M.; Sheline, K.; McDonald, C.; Narayan, V.; Domchek, S.M.; DeMichele, A.; Shah, P.; et al. Changes in Cardiovascular Biomarkers With Breast Cancer Therapy and Associations with Cardiac Dysfunction. J. Am. Heart Assoc. 2020, 9, e014708. [Google Scholar] [CrossRef] [PubMed]
- Skyttä, T.; Tuohinen, S.; Boman, E.; Virtanen, V.; Raatikainen, P.; Kellokumpu-Lehtinen, P.L. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat. Oncol. 2015, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Mincu, R.I.; Mahabadi, A.A.; Settelmeier, S.; Al-Rashid, F.; Rassaf, T.; Totzeck, M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: A meta-analysis. Eur. J. Heart Fail. 2020, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; D’Agostino, R., Jr.; Crotts, T.; Craver, K.; Hackney, M.H.; Jordan, J.H.; Ky, B.; Wagner, L.I.; Herrington, D.M.; Yeboah, J.; et al. Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid. 2022, 1, EVIDoa2200097. [Google Scholar] [CrossRef] [PubMed]

| Variable | Value (n = 60) |
|---|---|
| History and Demography | |
| Sex (female), n (%) | 60 (100) |
| Age, years | 48.6 ± 1.3 |
| Patients of age > 65 years, n (%) | 5 (7) |
| Smoker, n (%) | 2 (3) |
| BMI, kg/m2 | 27.8 ± 0.8 |
| Obesity, n (%) | 12 (16) |
| Dyslipidemia, n (%) | 21 (27) |
| Hemodynamic Measurements | |
| Systolic BP, mm Hg | 128.2 ± 3.8 |
| Diastolic BP, mm Hg | 82.9 ± 1.8 |
| Heart rate, b.p.m. | 84.1 ± 2.5 |
| LV EF, % | 61.6 ± 0.5 |
| Comorbidities | |
| Coronary Artery Disease, n (%) | 3 (4) |
| Diabetes Mellitus, n (%) | 2 (3) |
| Hypertension, n (%) | 23 (30) |
| Biomarkers | |
| hsTnT level in plasma, ng/L | 5.5 ± 1.4 |
| Variable | Value (n = 60) |
|---|---|
| Cancer Stage | |
| I | 2 (3) |
| II | 18 (30) |
| III | 35 (59) |
| IV | 5 (8) |
| Cancer Treatment | |
| Anthracyclines, n (%) | 60 (79) |
| Cumulative antracycline dose, (mg/m2) | 258.6 ± 13.2 |
| Trastuzumab, n (%) | 30 (39) |
| Radiation therapy, n (%) | 25 (32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhukhov, S.; Dovganych, N. The Prognostic Value of High-Sensitive Troponin T Rise Within the Upper Reference Limit in Breast Cancer: A Prospective Pilot Study. Cancers 2025, 17, 2412. https://doi.org/10.3390/cancers17142412
Kozhukhov S, Dovganych N. The Prognostic Value of High-Sensitive Troponin T Rise Within the Upper Reference Limit in Breast Cancer: A Prospective Pilot Study. Cancers. 2025; 17(14):2412. https://doi.org/10.3390/cancers17142412
Chicago/Turabian StyleKozhukhov, Sergey, and Nataliia Dovganych. 2025. "The Prognostic Value of High-Sensitive Troponin T Rise Within the Upper Reference Limit in Breast Cancer: A Prospective Pilot Study" Cancers 17, no. 14: 2412. https://doi.org/10.3390/cancers17142412
APA StyleKozhukhov, S., & Dovganych, N. (2025). The Prognostic Value of High-Sensitive Troponin T Rise Within the Upper Reference Limit in Breast Cancer: A Prospective Pilot Study. Cancers, 17(14), 2412. https://doi.org/10.3390/cancers17142412








