Transperineal Free-Hand Prostate Fusion Biopsy with AI-Driven Auto-Contouring: First Results of a Prospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI and Biopsy Technique
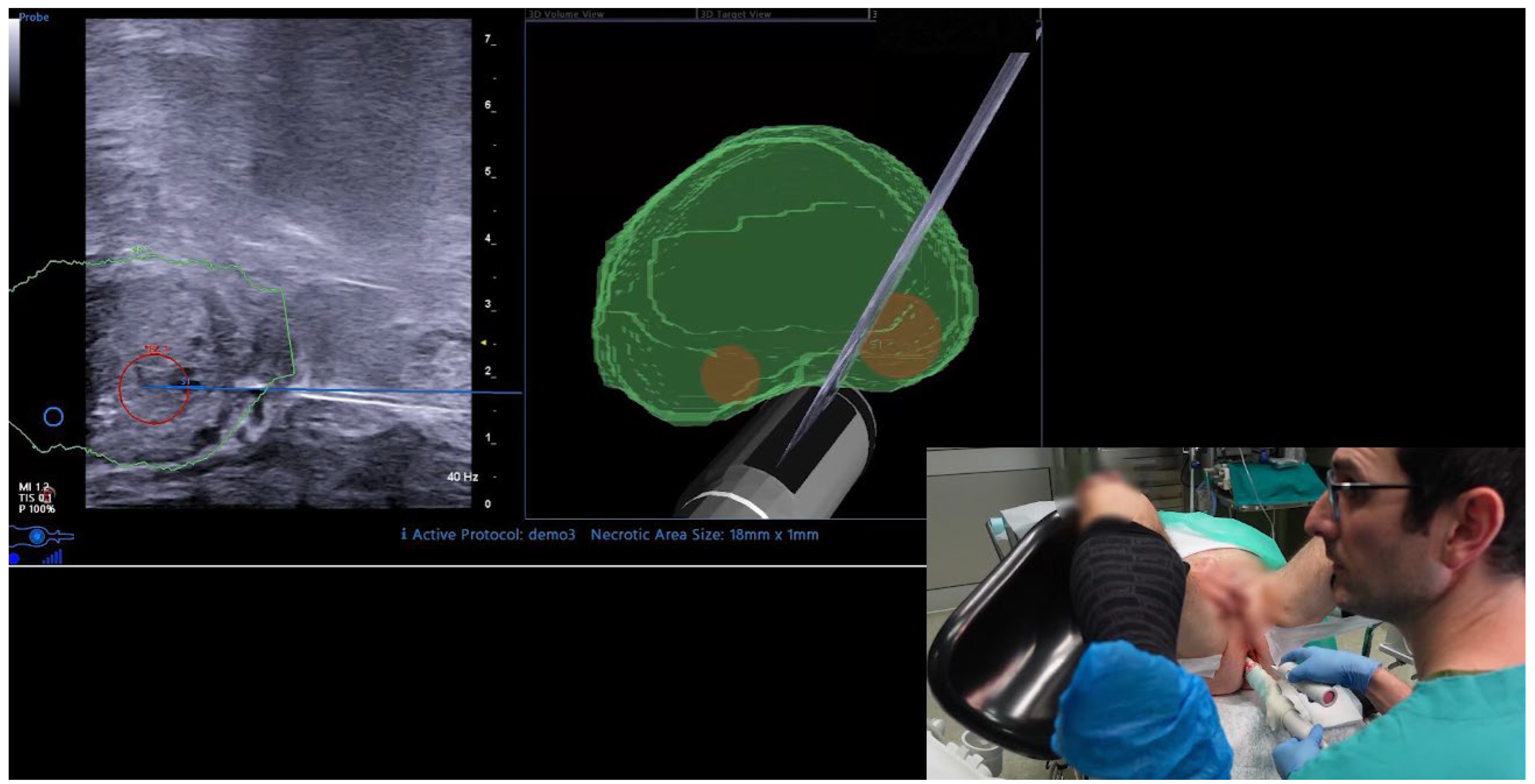
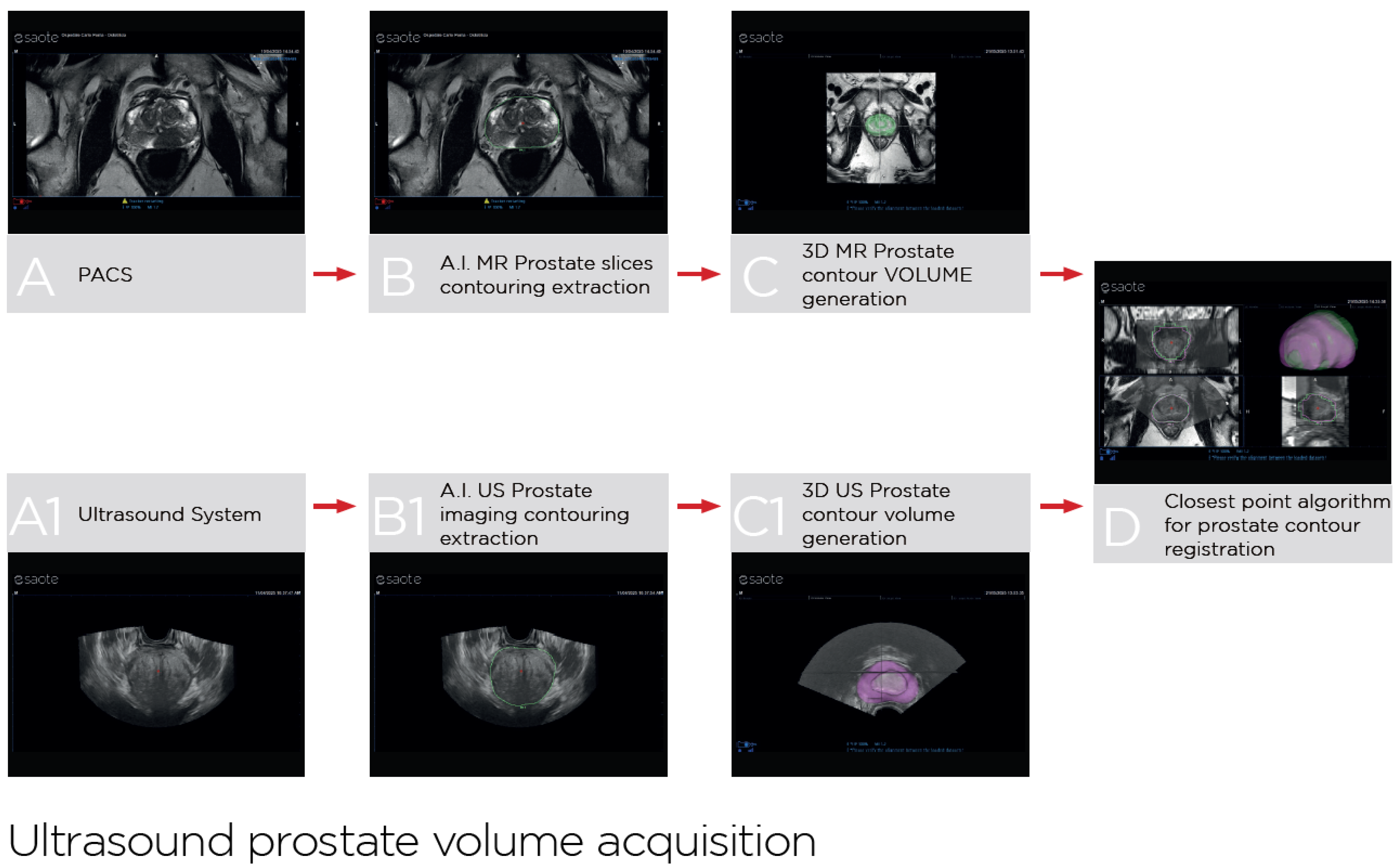
2.3. UroFusion Software
2.4. Endpoints and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornford, P.; Tilki, D.; van den Bergh, R.C.N.; Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Henry, A.M.; van Leenders, G.J.L.H.; Oldenburg, J.; et al. EAU Guidelines on Prostate Cancer; EAU Guidelines Office: Arnhem, The Netherlands, 2025; Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 15 July 2025).
- Tonttila, P.P.; Lantto, J.; Paakko, E.; Piippo, U.; Kauppila, S.; Lammentausta, E.; Ohtonen, P.; Vaarala, M.H. Prebiopsy Multiparametric Magnetic Resonance Imaging for Prostate Cancer Diagnosis in Biopsy-naive Men with Suspected Prostate Cancer Based on Elevated Prostate-specific Antigen Values: Results from a Randomized Prospective Blinded Controlled Trial. Eur. Urol. 2016, 69, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Izadpanahi, M.H.; Elahian, A.; Gholipour, F.; Khorrami, M.H.; Zargham, M.; Mohammadi Sichani, M.; Alizadeh, F.; Khorrami, F. Diagnostic yield of fusion magnetic resonance-guided prostate biopsy versus cognitive-guided biopsy in biopsy-naïve patients: A head-to-head randomized controlled trial. Prostate Cancer Prostatic Dis. 2021, 24, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Falagario, U.G.; Pellegrino, F.; Fanelli, A.; Guzzi, F.; Bartoletti, R.; Cash, H.; Pavlovich, C.; Emberton, M.; Carrieri, G.; Giannarini, G. Prostate cancer detection and complications of MRI-targeted prostate biopsy using cognitive registration, software-assisted image fusion or in-bore guidance: A systematic review and meta-analysis of comparative studies. Prostate Cancer Prostatic Dis. 2024, 28, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Liu, Y.; Ruan, M.; Wang, K.; Li, D.; Wu, J.; Qiu, J.; Wu, P.; Tian, P.; Yu, C.; et al. Comparison of MRI artificial intelligence-guided cognitive fusion-targeted biopsy versus routine cognitive fusion-targeted prostate biopsy in prostate cancer diagnosis: A randomized controlled trial. BMC Med. 2024, 22, 530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bao, J.; Qiao, X.; Jin, P.; Ji, Y.; Li, Z.; Zhang, J.; Su, Y.; Ji, L.; Shen, J.; et al. Predicting clinically significant prostate cancer with a deep learning approach: A multicentre retrospective study. Eur. J. Nucl. Med. Mol. Imaging. 2023, 50, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Marquis, A.; Tappero, S.; D’Agate, D.; Oderda, M.; Calleris, G.; Falcone, M.; Faletti, R.; Molinaro, L.; Zitella, A.; et al. Transperineal Free-hand mpMRI Fusion-targeted Biopsies Under Local Anesthesia: Technique and Feasibility From a Single-center Prospective Study. Urology 2020, 140, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wysock, J.S.; Rosenkrantz, A.B.; Huang, W.C.; Stifelman, M.D.; Lepor, H.; Deng, F.M.; Melamed, J.; Taneja, S.S. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: The PROFUS trial. Eur. Urol. 2014, 66, 343. [Google Scholar] [CrossRef] [PubMed]
- Oderda, M.; Faletti, R.; Battisti, G.; Dalmasso, E.; Falcone, M.; Marra, G.; Palazzetti, A.; Zitella, A.; Bergamasco, L.; Gandini, G.; et al. Prostate Cancer Detection Rate with Koelis Fusion Biopsies versus Cognitive Biopsies: A Comparative Study. Urol. Int. 2016, 97, 230. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Calleris, G.; Marquis, A.; Oderda, M.; Zhuang, J.; Guo, H.; Gontero, P. Transperineal freehand multiparametric MRI fusion targeted biopsies under local anaesthesia for prostate cancer diagnosis: A multicentre prospective study of 1014 cases. BJU Int. 2021, 128, 524. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xle, Y.; Zheng, X.; Liu, B.; Chen, H.; Li, J.; Ma, X.; Xiang, J.; Weng, G.; Zhu, W.; et al. A prospective multi-center randomized comparative trial evaluating outcomes of transrectal ultrasound (TRUS)-guided 12-core systematic biopsy, mpMRI-targeted 12-core biopsy, and artificial intelligence ultrasound of prostate (AIUSP) 6-core targeted biopsy for prostate cancer diagnosis. World J. Urol. 2023, 41, 653–662. [Google Scholar] [PubMed]
- Prata, F.; Anceschi, U.; Cordelli, E.; Faiella, E.; Civitella, A.; Tuzzolo, P.; Iannuzzi, A.; Ragusa, A.; Esperto, F.; Prata, S.M.; et al. Radiomic Machine-Learning Analysis of Multiparametric Magnetic Resonance Imaging in the Diagnosis of Clinically Significant Prostate Cancer: New Combination of Textural and Clinical Features. Curr. Oncol. 2023, 30, 2021–2031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santucci, D.; Ragone, R.; Vergantino, E.; Vaccarino, F.; Esperto, F.; Prata, F.; Scarpa, R.M.; Papalia, R.; Beomonte Zobel, B.; Grasso, F.R.; et al. Comparison between Three Radiomics Models and Clinical Nomograms for Prediction of Lymph Node Involvement in PCa Patients Combining Clinical and Radiomic Features. Cancers 2024, 16, 2731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marra, G.; Zhuang, J.; Marquis, A.; Zhao, X.; Calleris, G.; Kan, Y.; Oderda, M.; Huang, H.; Faletti, R.; Zhang, Q.; et al. Pain in Men Undergoing Transperineal Free-Hand Multiparametric Magnetic Resonance Imaging Fusion Targeted Biopsies under Local Anesthesia: Outcomes and Predictors from a Multicenter Study of 1,008 Patients. J. Urol. 2020, 204, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Oerther, B.; Nedelcu, A.; Engel, H.; Schmucker, C.; Schwarzer, G.; Brugger, T.; Schoots, I.G.; Eisenblaetter, M.; Sigle, A.; Gratzke, C.; et al. Update on PI-RADS Version 2.1 Diagnostic Performance Benchmarks for Prostate MRI: Systematic Review and Meta-Analysis. Radiology 2024, 312, e233337. [Google Scholar] [CrossRef] [PubMed]
- Oderda, M.; Marra, G.; Albisinni, S.; Altobelli, E.; Baco, E.; Beatrici, V.; Cantiani, A.; Carbone, A.; Ciccariello, M.; Descotes, J.L.; et al. Accuracy of elastic fusion biopsy in daily practice: Results of a multicenter study of 2115 patients. Int. J. Urol. 2018, 25, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Oderda, M.; Diamand, R.; Abou Zahr, R.; Anract, J.; Assenmacher, G.; Barry Delongchamps, N.; Bui, A.P.; Benamran, D.; Calleris, G.; Dariane, C.; et al. Transrectal versus transperineal prostate fusion biopsy: A pair-matched analysis to evaluate accuracy and complications. World J. Urol. 2024, 42, 535. [Google Scholar] [CrossRef] [PubMed]
- Oderda, M.; Dematteis, A.; Calleris, G.; Diamand, R.; Gatti, M.; Marra, G.; Adans-Dester, G.; Al Salhi, Y.; Pastore, A.; Faletti, R.; et al. MRI-Targeted Prostate Fusion Biopsy: What Are We Missing outside the Target? Implications for Treatment Planning. Curr. Oncol. 2024, 31, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
| Variable | n 148 |
|---|---|
| Age, years, median (IQR) | 69 (64–76) |
| PSA, ng/mL, median (IQR) | 6.5 (3.9–9.1) |
| PSA density, median (IQR) | 0.13 (0.06–0.18) |
| Positive familiar history, n (%) | 19 (13%) |
| Biopsy naïve, n (%) | 124 (84%) |
| Suspicious DRE, n (%) | 50 (34%) |
| Suspicious extracapsular extension at MRI, n (%) | 17 (11%) |
| Prostate volume at MRI, cc, median (IQR) | 47 (34–65) |
| Prostate volume at MRI auto-contouring from MRI, cc, median (IQR) | 46 (35–63) |
| Prostate volume at US auto-contouring, cc, median (IQR) | 44 (34–60) |
| Difference in volume estimation between US and MRI auto-contouring, cc, median (IQR) | −1 (−4–2) |
Number of targets, n (%)
| 118 (80%) 30 (20%) |
| Target diameter, mm, median (IQR) | 11 (8–15) |
PI-RADS score, n (%)
| 26 (18%) 85 (57%) 37 (25%) |
Target location, n (%)
| 43 (29%) 82 (55%) 30 (20%) 90 (61%) 18 (12%) 39 (26%) |
| Variable | n 148 |
|---|---|
| PROCEDURAL OUTCOMES | |
Fusion biopsy operator, n (%)
| 54 (36%) 94 (64%) |
| Time needed for fusion imaging, min, median (IQR) | 5 (3–6) |
| Time needed for biopsy, min, median (IQR) | 15 (12–17) |
| Total numbers of cores taken, median (IQR) | 15 (15–15) |
| Total number of positive cores, median (IQR) | 3 (1–6) |
| VAS score at the end of the biopsy, median (IQR) | 2 (1–3) |
| PATHOLOGIC OUTCOMES | |
| Cancer detection rate, n (%) | 95 (64%) |
ISUP grade, n (%)
| 8/95 (8%) 50/95 (53%) 25/95 (26%) 9/95 (10%) 3/95 (3%) |
| Cancer detection rate at targeted cores only, n (%) | 79 (53%) |
ISUP grade at targeted cores only, n (%)
| 6/79 (7%) 40/79 (51%) 25/79 (32%) 5/79 (6%) 3/79 (4%) |
Cancer detection rate according to PI-RADS score, n (%)
| 9/26 (35%) 55/85 (65%) 31/37 (84%) |
ISUP ≥ 2 cancer detection rate according to PI-RADS score, n (%)
| 6/26 (23%) 52/85 (61%) 29/37 (78%) |
Cancer detection rate according to surgeon’s experience, n (%)
| 33/54 (61%) 62/94 (66%) |
Outfield positive systematic cores, same lobe of the target, n (%)
| 66/95 (69%) 59/95 (62%) |
Outfield positive systematic cores in the contralateral lobe, n (%)
| 35/95 (37%) 31/95 (33%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oderda, M.; Calleris, G.; Dematteis, A.; Greco, A.; Marquis, A.; Marra, G.; Merani, U.; Sasia, A.; Venturi, A.; Zitella, A.; et al. Transperineal Free-Hand Prostate Fusion Biopsy with AI-Driven Auto-Contouring: First Results of a Prospective Study. Cancers 2025, 17, 2381. https://doi.org/10.3390/cancers17142381
Oderda M, Calleris G, Dematteis A, Greco A, Marquis A, Marra G, Merani U, Sasia A, Venturi A, Zitella A, et al. Transperineal Free-Hand Prostate Fusion Biopsy with AI-Driven Auto-Contouring: First Results of a Prospective Study. Cancers. 2025; 17(14):2381. https://doi.org/10.3390/cancers17142381
Chicago/Turabian StyleOderda, Marco, Giorgio Calleris, Alessandro Dematteis, Alessandro Greco, Alessandro Marquis, Giancarlo Marra, Umberto Merani, Alberto Sasia, Alessio Venturi, Andrea Zitella, and et al. 2025. "Transperineal Free-Hand Prostate Fusion Biopsy with AI-Driven Auto-Contouring: First Results of a Prospective Study" Cancers 17, no. 14: 2381. https://doi.org/10.3390/cancers17142381
APA StyleOderda, M., Calleris, G., Dematteis, A., Greco, A., Marquis, A., Marra, G., Merani, U., Sasia, A., Venturi, A., Zitella, A., & Gontero, P. (2025). Transperineal Free-Hand Prostate Fusion Biopsy with AI-Driven Auto-Contouring: First Results of a Prospective Study. Cancers, 17(14), 2381. https://doi.org/10.3390/cancers17142381







