Breast Edema After Breast-Conserving Surgery and Radiotherapy: Introduction of a Clinically Meaningful Classification and Evaluation of the Incidence After Normo- and Hypofractionated Treatments
Simple Summary
Abstract
1. Introduction
- -
- “Late effects on normal tissues, in subjective, objective, management and analytic categories “ (LENT-SOMA) differentiates between “asymptomatic—grade 1” and “symptomatic—grade 2,” with medical interventions being graded as “grade 3” and surgical interventions graded as “grade 4” [6]. However, this does not reflect clinical reality, as in everyday language use, “asymptomatic” would mean “the absence of any symptoms—grade 0.” Furthermore, “medical intervention” is not further specified, leading to significant uncertainties in applying this classification.
- -
- The second system is “CTC 2.0”, which provides general definitions [7]. It differentiates between “none/normal—grade 0,” “mild lymphedema—grade 1,” “moderate lymphedema requiring compression—grade 2,” “severe lymphedema limiting function—grade 3,” and “severe lymphedema limiting function with ulceration.”
2. Materials and Methods
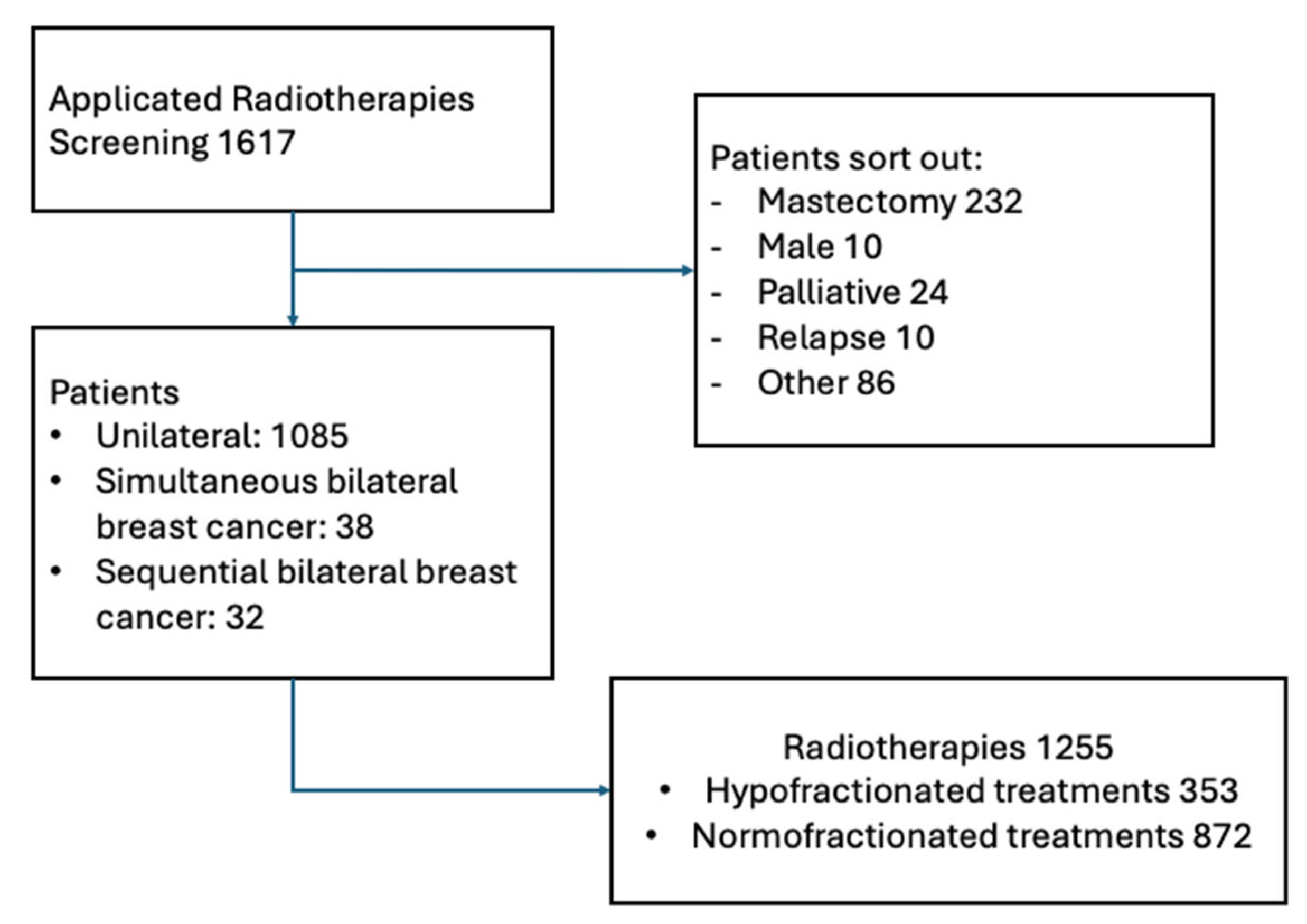
2.1. Data Collection
2.2. Westerstede (WST) Breast Edema Classification
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHT | Anti-hormonal therapy |
| BE | Breast edema |
| BMI | Body mass index |
| CTC | Common Toxicity Criteria |
| LENT-SOMA | Late effects on normal tissues, in subjective, objective, management and analytic categories |
| WST | Westerstede |
| CTX | Chemotherapy |
| RT | Radiotherapy |
| hfRT | Hypofractionated radiotherapy |
| nfRT | Normofractionated radiotherapy |
| ALND | Axillary lymph node dissection |
| SNLD | Sentinel node biopsy |
References
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.4, 2021, AWMF Registernummer: 032-045OL. Available online: http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ (accessed on 7 October 2024).
- Sjöström, M.; Fyles, A.; Liu, F.-F.; McCready, D.; Shi, W.; Rey-McIntyre, K.; Chang, S.L.; Feng, F.Y.; Speers, C.W.; Pierce, L.J.; et al. Development and Validation of a Genomic Profile for the Omission of Local Adjuvant Radiation in Breast Cancer. J. Clin. Oncol. 2023, 41, 1533–1540. [Google Scholar] [CrossRef]
- Boicean, A.; Boeras, I.; Birsan, S.; Ichim, C.; Todor, S.B.; Onisor, D.M.; Brusnic, O.; Bacila, C.; Dura, H.; Roman-Filip, C.; et al. In Pursuit of Novel Markers: Unraveling the Potential of miR-106, CEA and CA 19-9 in Gastric Adenocarcinoma Diagnosis and Staging. Int. J. Mol. Sci. 2024, 25, 7898. [Google Scholar] [CrossRef] [PubMed]
- Young-Afat, D.A.; Gregorowitsch, M.L.; van den Bongard, D.H.; Burgmans, I.; van der Pol, C.C.; Witkamp, A.J.; Bijlsma, R.M.; Koelemij, R.; Schoenmaeckers, E.J.; Jonasse, Y.; et al. Breast Edema Following Breast-Conserving Surgery and Radiotherapy: Patient-Reported Prevalence, Determinants, and Effect on Health-Related Quality of Life. JNCI Cancer Spectr. 2019, 3, pkz011. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, H.; Gebruers, N.; Beyers, T.; De Monie, A.C.; Tjalma, W. Breast edema in breast cancer patients following breast-conserving surgery and radiotherapy: A systematic review. Breast Cancer Res. Treat. 2014, 147, 463–471. [Google Scholar] [CrossRef]
- LENT SOMA scales for all anatomic sites. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1049–1091. [CrossRef]
- Trotti, A.; Byhardt, R.; Stetz, J.; Gwede, C.; Corn, B.; Fu, K.; Gunderson, L.; McCormick, B.; Morrisintegral, M.; Rich, T.; et al. Common toxicity criteria: Version 2.0. an improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 13–47. [Google Scholar] [CrossRef]
- Offersen, B.V.; Alsner, J.; Nielsen, H.M.; Jakobsen, E.H.; Nielsen, M.H.; Krause, M.; Stenbygaard, L.; Mjaaland, I.; Schreiber, A.; Kasti, U.-M.; et al. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients With Early Breast Cancer or Ductal Carcinoma In Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J. Clin. Oncol. 2020, 38, 3615–3625. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Coles, C.E.; Haviland, J.S.; Kirby, A.M.; Griffin, C.L.; Sydenham, M.A.; Titley, J.C.; Bhattacharya, I.; Brunt, A.M.; Chan, H.Y.C.; Donovan, E.M.; et al. Dose-escalated simultaneous integrated boost radiotherapy in early breast cancer (IMPORT HIGH): A multicentre, phase 3, non-inferiority, open-label, randomised controlled trial. Lancet 2023, 401, 2124–2137. [Google Scholar] [CrossRef]
- Whelan, T.J.; Pignol, J.P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; Cameron, D.A.; Dixon, J.M. Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. N. Engl. J. Med. 2023, 388, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Gruber, G. Escalation and De-Escalation of Adjuvant Radiotherapy in Early Breast Cancer: Strategies for Risk-Adapted Optimization. Cancers 2024, 16, 2946. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.E.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.S.; Brunt, A.M.; Ciurlionis, L.; Chan, C.; et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; De Santis, M.C.; Visani, L.; Scorsetti, M.; Fozza, A.; Meduri, B.; De Rose, F.; Bonzano, E.; Prisco, A.; Masiello, V.; et al. Single-modality endocrine therapy versus radiotherapy after breast-conserving surgery in women aged 70 years and older with luminal A-like early breast cancer (EUROPA): A preplanned interim analysis of a phase 3, non-inferiority, randomised trial. Lancet Oncol. 2025, 26, 37–50. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.; Barrett, J.M.; Barrett-Lee, P.J.; Bliss, J.M.; Brown, J.; Dewar, J.A.; Dobbs, H.J.; Haviland, J.S.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008, 9, 331–341. [Google Scholar] [CrossRef]
- Haviland, J.S.; Ashton, A.; Broad, B.; Gothard, L.; Owen, J.R.; Tait, D.; Sydenham, M.A.; Yarnold, J.R.; Bliss, J.M. Evaluation of a Method for Grading Late Photographic Change in Breast Appearance after Radiotherapy for Early Breast Cancer. Clin. Oncol. 2008, 20, 497–501. [Google Scholar] [CrossRef]
- LENT SOMA tables. Radiother. Oncol. 1995, 35, 17–60. [CrossRef]
- Fehlauer, F.; Tribius, S.; Höller, U.; Rades, D.; Kuhlmey, A.; Bajrovic, A.; Alberti, W. Long-term radiation sequelae after breast-conserving therapy in women with early-stage breast cancer: An observational study using the LENT-SOMA scoring system. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 651–658. [Google Scholar] [CrossRef]
- Hoeller, U.; Tribius, S.; Kuhlmey, A.; Grader, K.; Fehlauer, F.; Alberti, W. Increasing the rate of late toxicity by changing the score? A comparison of RTOG/EORTC and LENT/SOMA scores. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1013–1018. [Google Scholar] [CrossRef]
- Bogdan, R.-G.; Helgiu, A.; Cimpean, A.-M.; Ichim, C.; Todor, S.B.; Iliescu-Glaja, M.; Bodea, I.C.; Crainiceanu, Z.P. Assessing Fat Grafting in Breast Surgery: A Narrative Review of Evaluation Techniques. J. Clin. Med. 2024, 13, 7209. [Google Scholar] [CrossRef]
- Fogliata, A.; De Rose, F.; Stravato, A.; Reggiori, G.; Tomatis, S.; Scorsetti, M.; Cozzi, L. Evaluation of target dose inhomogeneity in breast cancer treatment due to tissue elemental differences. Radiat. Oncol. 2018, 13, 92. [Google Scholar] [CrossRef]
- Aluwini, S.; Pos, F.; Schimmel, E.; van Lin, E.; Krol, S.; van der Toorn, P.P.; de Jager, H.; Dirkx, M.; Alemayehu, W.G.; Heijmen, B.; et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015, 16, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Aluwini, S.; Pos, F.; Schimmel, E.; Krol, S.; van der Toorn, P.P.; de Jager, H.; Alemayehu, W.G.; Heemsbergen, W.; Heijmen, B.; Incrocci, L. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Bartels, S.A.L.; Donker, M.; Poncet, C.; Sauvé, N.; Straver, M.E.; van de Velde, C.J.H.; Mansel, R.E.; Blanken, C.; Orzalesi, L.; Klinkenbijl, J.H.G.; et al. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981–22023 AMAROS Trial. J. Clin. Oncol. 2023, 41, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Appelgren, M.; Sackey, H.; Wengström, Y.; Johansson, K.; Ahlgren, J.; Andersson, Y.; Bergkvist, L.; Frisell, J.; Lundstedt, D.; Rydén, L.; et al. Patient-reported outcomes one year after positive sentinel lymph node biopsy with or without axillary lymph node dissection in the randomized SENOMAC trial. Breast 2022, 63, 16–23. [Google Scholar] [CrossRef]
- Fansa, H.; Linder, S. The Local Rhombus-Shaped Flap—An Easy and Reliable Technique for Oncoplastic Breast Cancer Surgery and Defect Closure in Breast and Axilla. Cancers 2024, 16, 3101. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Gokavarapu, S.; Shen, Q.; Ji, T. Radiotherapy and Smoking History Are Significant Independent Predictors for Osteosynthesis-Associated Late Complications in Vascular Free Fibula Reconstruction of Mandible. J. Craniofac. Surg. 2017, 28, 1508–1513. [Google Scholar] [CrossRef]
- Campbell, A.R.; Didier, A.J.; Sheikh, T.M.; Ansari, S.; Watkins, D.E.; Fahoury, A.M.; Nandwani, S.V.; Rashid, M. The Effects of Radiotherapy on the Sequence and Eligibility of Breast Reconstruction: Current Evidence and Controversy. Cancers 2024, 16, 2939. [Google Scholar] [CrossRef]
- Garip, M.; Van Dessel, J.; Grosjean, L.; Politis, C.; Bila, M. The impact of smoking on surgical complications after head and neck reconstructive surgery with a free vascularised tissue flap: A systematic review and meta-analysis. Br. J. Oral. Maxillofac. Surg. 2021, 59, e79–e98. [Google Scholar] [CrossRef]
| Grade | 0 | I | II | III |
|---|---|---|---|---|
| CTC 2.0 | No symptoms | Symptomatic edema | Need for compression | Severe lymphedema limiting function with ulceration |
| WST | No therapy | Lymphatic drainage performed by the patient | Professional lymphatic drainage | Surgical intervention |
| Category | Total n = 1225 | nfRT n = 872 | hfRT n = 353 | Significant Difference? |
|---|---|---|---|---|
| Mean age | 60.4 | 58.0 | 66.4 | yes (p < 0.05) |
| <40 | 47 (3.8%) | 42 (4.8%) | 5 (1.4%) | yes (p < 0.05). |
| 40–49 | 171 (13.9%) | 153 (17.55%) | 18 (5.1%) | yes (p < 0.05). |
| 50–64 | 539 (44.0%) | 419 (48.0%) | 120 (34.0%) | yes (p < 0.05). |
| 65–74 | 310 (25.3%) | 199 (22.8%) | 111 (31.4%) | yes (p < 0.05). |
| >75 | 158 (12.9%) | 59 (6.7%) | 99 (28.0%) | yes (p < 0.05). |
| Mean BMI | 27.09 | 27.51 | 26.08 | yes (p< 0.05) * |
| BMI > 25 | 751 (61.3%) | 541 (62%) | 210 (59%) | no (p = 0.44) |
| Left/right | 640/585 | 462/410 | 178/175 | no (p = 0.45) |
| Risk factors | ||||
| Active smokers | 151 (12.3%) | 137 (15.7%) | 14 (4.0%) | yes (p < 0.05). |
| Former smokers | 97 (7.9%) | 60 (6.9%) | 37 (10.5%) | yes (p < 0.05). |
| Alcohol consumption | ||||
| 614 (50.1%) | 436 (50%) | 178 (50.42%) | no (p = 0.94) |
| 552 (45.0%) | 388 (44.5%) | 164 (46.5%) | no (p = 0.57) |
| 44 (3.6%) | 37 (4.2%) | 7 (2%) | yes (p < 0.05). |
| Hypertension | 693 (56.6%) | 532 (61.0%) | 161 (45.6%) | yes (p < 0.05). |
| Vascular diseases | 77 (6.29%) | 42 (4.82%) | 35 (9.9%) | yes (p < 0.05) |
| Non-insulin-dependent diabetes | 83 (6.8%) | 59 (6.8%) | 24 (6.8%) | no (p = 1) |
| Insulin-dependent diabetes | 21 (1.7%) | 16 (1.8%) | 5 (1.4%) | no (p = 0.80) |
| T-status | ||||
| pTis + Tmi/ypT0 + ypTis + ypTmi | 14 (1.1%)/90 (7.3%) | 12 (1.38%)/73 (8%) | 2 (0.5%)/17 (5%) | yes (p < 0.05). |
| pT1/ypT1 | 739 (60.3%)/61 (4.9%) | 484 (56%)/50 (6%) | 255 (72%)/11 (3%) | yes (p < 0.05). |
| pT2/ypT2 | 258 (21.0%)/24 (1.9%) | 199 (23%)/22 (3%) | 59 (17%)/2 (0.6%) | yes (p < 0.05). |
| pT3/ypT3 | 27 (2.2%)/4 (0.3%) | 22 (3%)/4 (0.5%) | 5 (1%)/0 | no (p = 0.15) |
| pT4/ypT4 | 7 (0.6%)/1 (0.08%) | 5 (0.6%)/1 (0.1%) | 2 (0.6%)/0 | no (p > 0.99) |
| N-Status | ||||
| pN0,pNmi/ypN0 | 832 (67.9%)/144 (11.7%) | 537 (62%)/116 (13%) | 295 (84%)/28 (8%) | yes (p < 0.05). |
| pN1/ypN1 | 176 (1.4%)/25 (2.0%) | 151 (17%)/23 (3%) | 25 (7%)/2 (0.6%) | yes (p < 0.05). |
| pN2/ypN2 | 24 (1.9%)/9 (0.7%) | 22 (3%)/9 (1%) | 2 (0.6%)/0 | yes (p < 0.05). |
| pN3/ypN3 | 13 (1%)/2 (0.2%) | 12 (1%)/2 (0.2%) | 1 (0.3%)/0 | yes (p < 0.05). |
| Primary CTX | 182 (14.9%) | 150 (17.2%) | 32 (9.0%) | yes (p < 0.05). |
| Adjuvant CTX | 316 (25.8%) | 269 (30.9%) | 47 (13.3%) | yes (p < 0.05). |
| AHT | 1037 (84.7%) | 728 (83.5%) | 309 (87.5%) | no (p = 0.09) |
| Sentinel lymphatic biopsy | 1061 (86.6%) | 722 (82.8%) | 339 (96.0%) | yes (p < 0.05). |
| Axillary dissection | 146 (11.9%) | 137 (15.7%) | 9 (2.6%) | yes (p < 0.05). |
| RT target volumes | ||||
| Breast only | 959 (78.3%) | 627 (71.9%) | 332 (94.0%) | yes (p < 0.05). |
| Lymphatics | 216 (17.6%) | 198 (22.7%) | 18 (5.1%) | yes (p < 0.05). |
| 105 (8.6%) | 100 (11.5%) | 5 (1.4%) | yes (p < 0.05). |
| 18 (1.5%) | 12 (1.4%) | 6 (1.7%) | no (p = 0.61) |
| 76 (6.2%) | 69 (7.9%) | 7 (2%) | yes (p < 0.05). |
| 17 (1.4%) | 17 (1.9%) | 0 (0.0%) | yes (p < 0.05). |
| Simultaneous boost (tumor bed) | 744 (60.73%) | 622 (71.3%) | 122 (34.6%) | yes (p < 0.05). |
| Sequential boost (tumor bed) | 102 (8.3%) | 97 (11.1%) | 5 (1.4%) | yes (p < 0.05). |
| All (n = 1225) | nfRT (n = 872) | hfRT (n = 353) | Significant Difference? | |
|---|---|---|---|---|
| WST classification | ||||
| All | 407 (33%) | 308 (35%) | 99 (28%) | yes (p < 0.05) |
| I° | 298 (24%) | 225 (26%) | 73 (21%) | yes (p < 0.05) |
| II° | 109 (9%) | 83 (9%) | 26 (7%) | no (p = 0.268) |
| CTC classification | ||||
| All | 213 (17%) | 162 (19%) | 51 (14%) | no (p = 0.07) |
| I° | 82 (7%) | 61 (7%) | 20 (6%) | no (p = 0.45) |
| II° | 131 (10%) | 101 (12%) | 31 (9%) | no (p = 0.19) |
| Clinical course of BE (according to WST) | 407 | 308 | 99 | |
| Complete remission | 281 (69%) | 218 (70%) | 63 (64%) | no (p = 0.21) |
| Persistent grade I on the last examination | 39 (9%) | 31 (10%) | 8 (8%) | no (p = 0.70) |
| Persistent grade II on the last examination | 23 (6%) | 17 (6%) | 6 (6%) | no (p = 0.80) |
| Unknown | 64 (16%) | 42 (14%) | 22 (22%) | Not applicable |
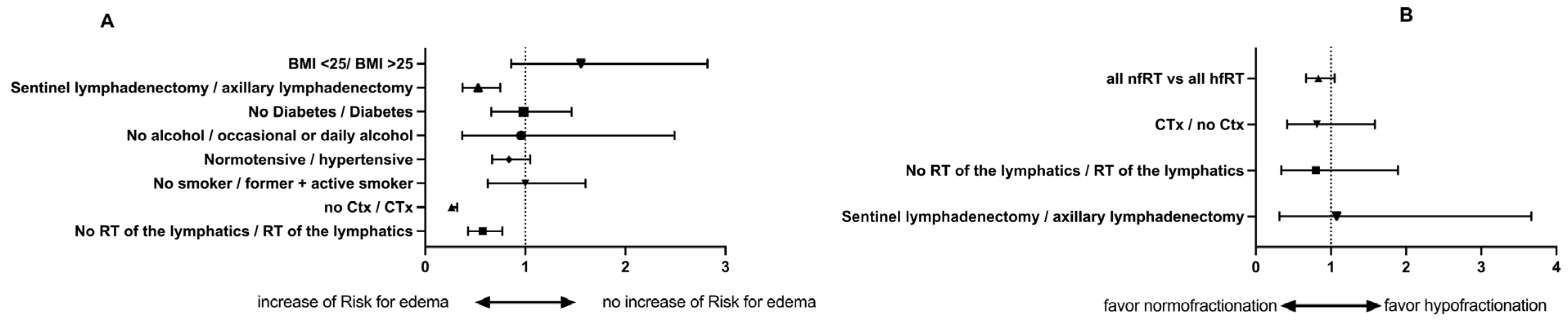
| All | Hazard | 95-% | Significant? | p Value |
|---|---|---|---|---|
| No RT of the lymphatics/RT of the lymphatics | 0.5744 | 0.4286–0.7698 | yes | 0.0002 |
| No hormonal therapy/hormonal therapy | 1.010 | 0.7402–1.378 | no | 0.9511 |
| No CTX/CTX | 0.263 | 0.263–0.3191 | yes | >0.0001 |
| Non-smoker/former + active smoker | 1 | 0.6245–1.602 | no | 0.9997 |
| Normotensive/hypertensive | 0.8374 | 0.6688–1.049 | no | 0.1219 |
| No alcohol/occasional or daily alcohol | 0.9594 | 0.3695–2.491 | no | 0.9322 |
| No Diabetes/diabetes | 0.9832 | 0.6596–1.463 | no | 0.9335 |
| Sentinel lymphadenectomy/axillary lymphadenectomy | 0.5288 | 0.3727–0.7505 | yes | 0.0004 |
| nfRT vs. hfRT | ||||
| No RT of the lymphatics/RT of the lymphatics | 0.8006 | 0.3386–1.893 | no | 0.6126 |
| No hormonal therapy/hormonal therapy | 1.166 | 0.8901–1.527 | no | 0.2648 |
| No CTX/CTX | 0.8138 | 0.4176–1.586 | no | 0.5448 |
| Non-smoker/former + active smoker | 1.892 | 0.4781–6.191 | no | 0.2919 |
| Normotensive/hypertensive | 1.327 | 0.0917–1.926 | no | 0.1374 |
| No alcohol/occasional or daily alcohol | 1.327 | 0.9137–1.926 | no | 0.1374 |
| No Diabetes/diabetes | 1.327 | 0.9137–2.195 | no | 0.1340 |
| Sentinel lymphadenectomy/axillary lymphadenectomy | 0.8535 | 0.3319–3.668 | no | 0.7224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyur, M.R.; Blach, R.M.; Christiansen, H.; Merten, R.; Becker, J.-N.; Knöchelmann, A.C.; Nitsche, M.; Hermann, R.M.; Sonnhoff, M.A. Breast Edema After Breast-Conserving Surgery and Radiotherapy: Introduction of a Clinically Meaningful Classification and Evaluation of the Incidence After Normo- and Hypofractionated Treatments. Cancers 2025, 17, 2368. https://doi.org/10.3390/cancers17142368
Oyur MR, Blach RM, Christiansen H, Merten R, Becker J-N, Knöchelmann AC, Nitsche M, Hermann RM, Sonnhoff MA. Breast Edema After Breast-Conserving Surgery and Radiotherapy: Introduction of a Clinically Meaningful Classification and Evaluation of the Incidence After Normo- and Hypofractionated Treatments. Cancers. 2025; 17(14):2368. https://doi.org/10.3390/cancers17142368
Chicago/Turabian StyleOyur, Melsa Rojin, Robert Maximilian Blach, Hans Christiansen, Roland Merten, Jan-Niklas Becker, Anne Caroline Knöchelmann, Mirko Nitsche, Robert Michael Hermann, and Mathias Alexander Sonnhoff. 2025. "Breast Edema After Breast-Conserving Surgery and Radiotherapy: Introduction of a Clinically Meaningful Classification and Evaluation of the Incidence After Normo- and Hypofractionated Treatments" Cancers 17, no. 14: 2368. https://doi.org/10.3390/cancers17142368
APA StyleOyur, M. R., Blach, R. M., Christiansen, H., Merten, R., Becker, J.-N., Knöchelmann, A. C., Nitsche, M., Hermann, R. M., & Sonnhoff, M. A. (2025). Breast Edema After Breast-Conserving Surgery and Radiotherapy: Introduction of a Clinically Meaningful Classification and Evaluation of the Incidence After Normo- and Hypofractionated Treatments. Cancers, 17(14), 2368. https://doi.org/10.3390/cancers17142368







