Serum Visfatin/eNAMPT as a Biomarker in Pancreatic and Small Intestine Neuroendocrine Tumors: A Cross-Sectional Study and Future Perspectives
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Laboratory Analysis
2.3. Statistical Calculations
3. Results
3.1. Baseline Characteristics
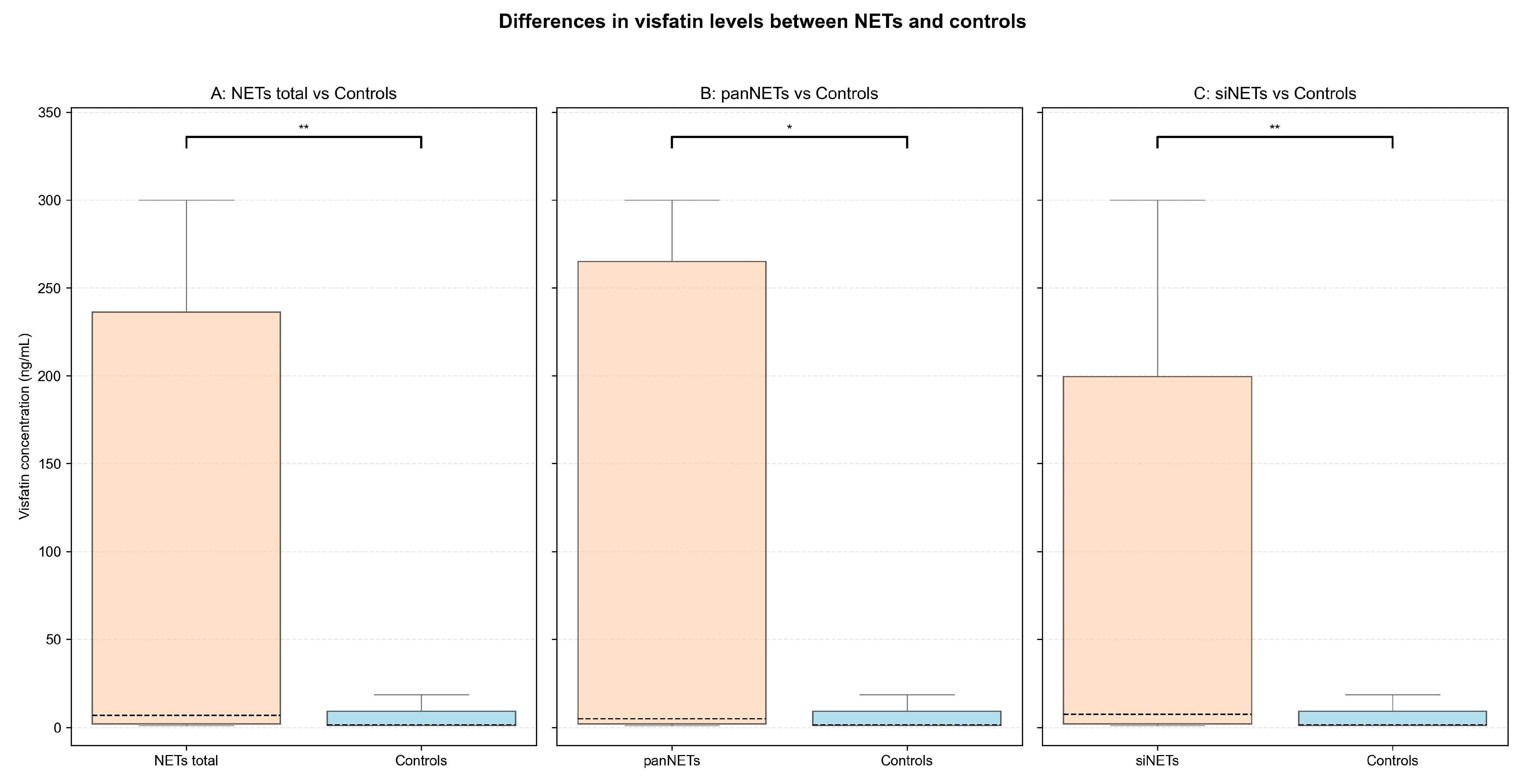
3.2. Serum Visfatin Concentrations in NETs vs. Controls
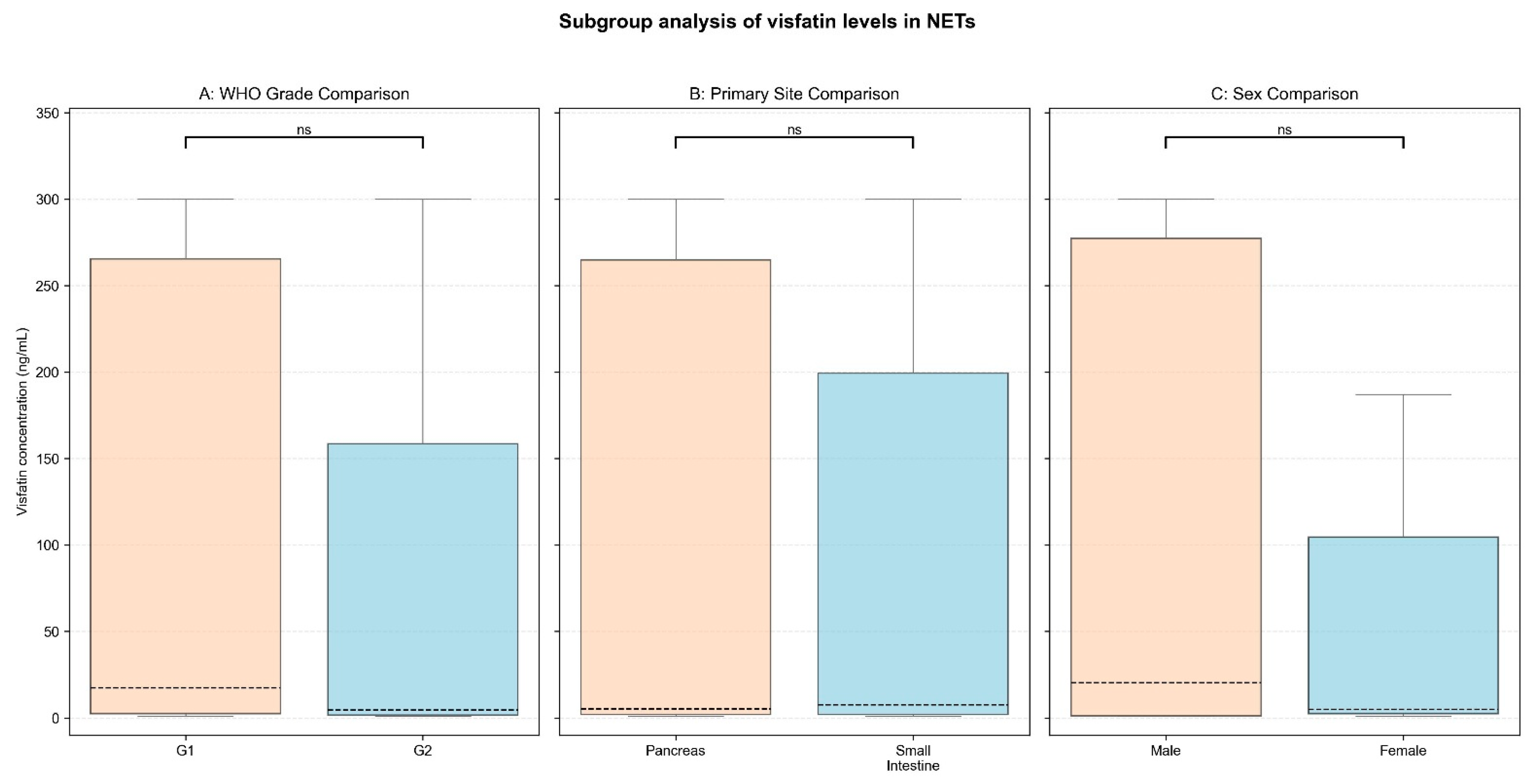
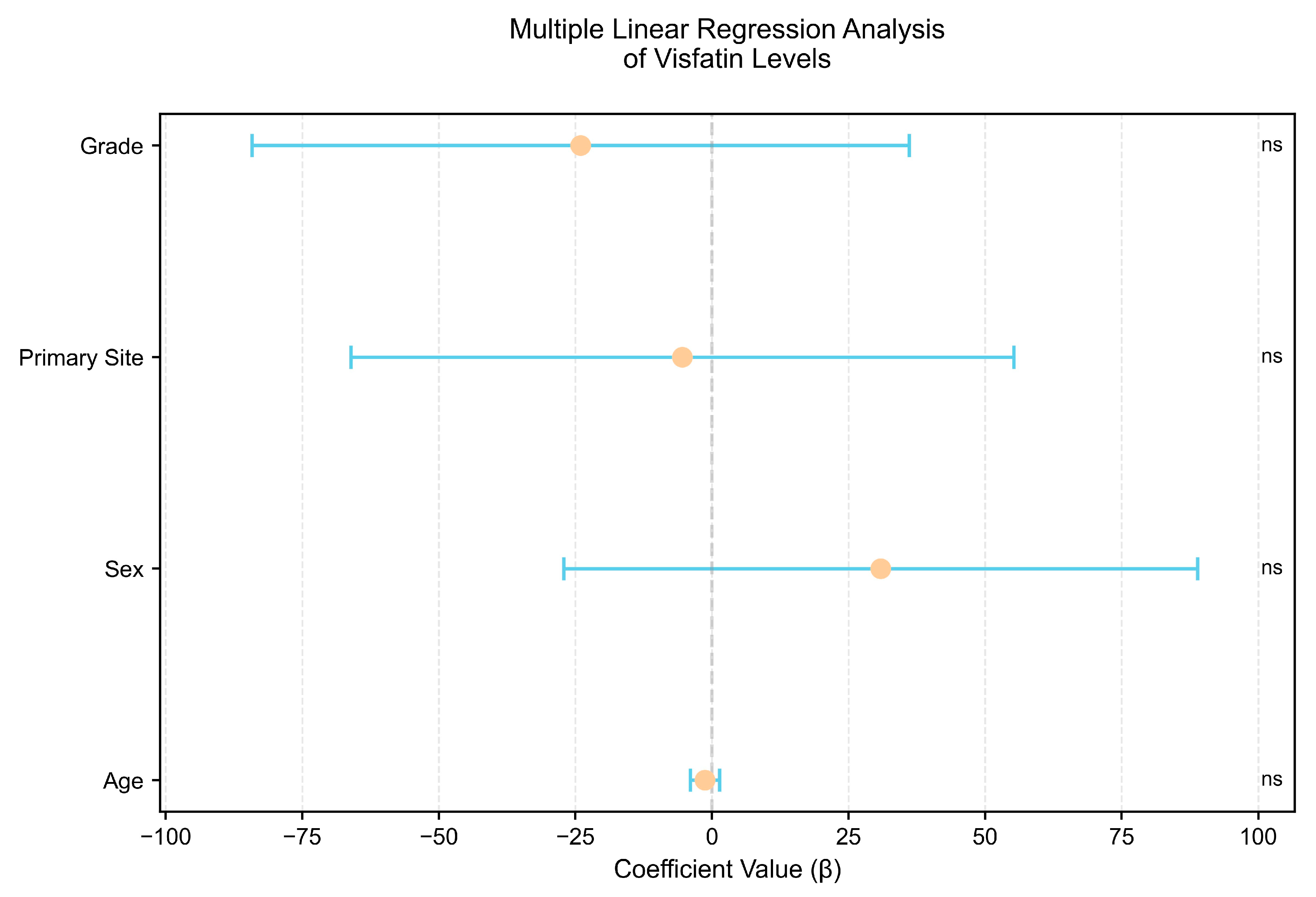
3.3. Subgroup Comparison and Correlation Analysis
3.4. Serum Visfatin’s Diagnostic Performance
4. Discussion
4.1. Overview of Visfatin and Its Role in Tumorigenesis
4.2. Diagnostic Utility of Serum Visfatin in NETs
4.3. Association of Visfatin with Tumor and Patient Characteristics
4.4. Visfatin as a Therapeutic Target—NAMPT Inhibitors
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NENs | Neuroendocrine neoplasms |
| NETs | Neuroendocrine tumors |
| NECs | Neuroendocrine carcinomas |
| WHO | World Health Organization |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| PBEF1 | Pre-B-cell colony-enhancing factor 1 |
| NAD+ | Nicotinamide adenine dinucleotide |
| eNAMPT | Extracellular NAMPT |
| iNAMPT | Intracellular NAMPT |
| siNETs | Small intestinal NETs |
| panNETs | Pancreatic NETs |
| TSH | Thyroid-stimulating hormone |
| NAMPT-i | NAMPT inhibitors |
| SCLC | Small cell lung cancer |
References
- Hofland, J.; Kaltsas, G.; De Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Komarnicki, P.; Musiałkiewicz, J.; Stańska, A.; Maciejewski, A.; Gut, P.; Mastorakos, G.; Ruchała, M. Circulating Neuroendocrine Tumor Biomarkers: Past, Present and Future. J. Clin. Med. 2022, 11, 5542. [Google Scholar] [CrossRef] [PubMed]
- Woliński, K.; Komarnicki, P.; Maciejewski, A.; Musiałkiewicz, J.; Gut, P.; Ruchała, M. Prevalence of Second Primary Malignancies in Patients With Well-Differentiated Neuroendocrine Tumors. Endocr. Pract. 2025, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- De Herder, W.W.; Rehfeld, J.F.; Kidd, M.; Modlin, I.M. A Short History of Neuroendocrine Tumours and Their Peptide Hormones. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 3–17. [Google Scholar] [CrossRef]
- Oberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.; Strosberg, J.; et al. Consensus on Biomarkers for Neuroendocrine Tumour Disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef] [PubMed]
- Komarnicki, P.; Gut, P.; Cieślewicz, M.; Musiałkiewicz, J.; Maciejewski, A.; Czupińska, M.; Mastorakos, G.; Ruchała, M. Serum β-HCG as a Biomarker in Pancreatic Neuroendocrine Tumors: Rethinking Single-Analyte Approach. Cancers. 2024, 16, 2060. [Google Scholar] [CrossRef]
- Semerena, E.; Nencioni, A.; Masternak, K. Extracellular Nicotinamide Phosphoribosyltransferase: Role in Disease Pathophysiology and as a Biomarker. Front. Immunol. 2023, 14, 1268756. [Google Scholar] [CrossRef]
- Navas, L.E.; Carnero, A. NAD+ Metabolism, Stemness, the Immune Response, and Cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef]
- Grolla, A.A.; Torretta, S.; Gnemmi, I.; Amoruso, A.; Orsomando, G.; Gatti, M.; Caldarelli, A.; Lim, D.; Penengo, L.; Brunelleschi, S.; et al. Nicotinamide Phosphoribosyltransferase (NAMPT/PBEF/Visfatin) Is a Tumoural Cytokine Released from Melanoma. Pigment Cell Melanoma Res. 2015, 28, 718–729. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Ziółkowska, P.; Derwich, A.; Gut, P.; Czarnywojtek, A.; Kloska, M.; Ruchała, M. Is ENAMPT/Visfatin a Potential Serum Marker of Papillary Thyroid Cancer? Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221090005. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mianabadi, F.; Mehrad-Majd, H. Circulating Visfatin Levels and Cancers Risk: A Systematic Review and Meta-Analysis. J. Cell. Physiol. 2019, 234, 5011–5022. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chang, D.M.; Lin, K.C.; Shin, S.J.; Lee, Y.J. Visfatin in Overweight/Obesity, Type 2 Diabetes Mellitus, Insulin Resistance, Metabolic Syndrome and Cardiovascular Diseases: A Meta-Analysis and Systemic Review. Diabetes. Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef]
- Yu, P.L.; Wang, C.; Li, W.; Zhang, F.X. Visfatin Level and The Risk of Hypertension and Cerebrovascular Accident: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2019, 51, 220–229. [Google Scholar] [CrossRef]
- Guiu, B.; Petit, J.M.; Bonnetain, F.; Ladoire, S.; Guiu, S.; Cercueil, J.-P.; Krause, D.; Hillon, P.; Borg, C.; Chauffert, B.; et al. Visceral Fat Area Is an Independent Predictive Biomarker of Outcome after First-Line Bevacizumab-Based Treatment in Metastatic Colorectal Cancer. Gut 2010, 59, 341–347. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Wang, L.; Qiu, S.; Yao, Y.; Yan, C.; Xiong, X.; Chen, X.; Ji, Q.; Cao, J.; et al. NAD+ Supplement Potentiates Tumor-Killing Function by Rescuing Defective TUB-Mediated NAMPT Transcription in Tumor-Infiltrated T Cells. Cell Rep. 2021, 36, 109516. [Google Scholar] [CrossRef] [PubMed]
- Miethe, C.; Torres, L.; Beristain, J.; Zamora, M.; Price, R.S. The Role of Visfatin and Resistin in an in Vitro Model of Obesity-Induced Invasive Liver Cancer. Can. J. Physiol. Pharmacol. 2021, 99, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, A.; Ntanasis-Stathopoulos, I.; Terpos, E. Obesity and Multiple Myeloma: Emerging Mechanisms and Perspectives. Semin. Cancer Biol. 2023, 92, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; Tenti, S.; Bedogni, G.; Fioravanti, A. Circulating Mir-140 and Leptin Improve the Accuracy of the Differential Diagnosis between Psoriatic Arthritis and Rheumatoid Arthritis: A Case-Control Study. Transl. Res. 2022, 239, 18–34. [Google Scholar] [CrossRef]
- Mohammed Ali, D.M.; Al-Fadhel, S.Z.; Al-Ghuraibawi, N.H.A.; Al-Hakeim, H.K. Serum Chemerin and Visfatin Levels and Their Ratio as Possible Diagnostic Parameters of Rheumatoid Arthritis. Reumatologia 2020, 58, 67–75. [Google Scholar] [CrossRef]
- Askari, A.; Arasteh, P.; Homayounfar, R.; Naghizadeh, M.M.; Ehrampoush, E.; Mousavi, S.M.; Alipoor, R. The Role of Adipose Tissue Secretion in the Creation and Pain Level in Osteoarthritis. Endocr. Regul. 2020, 54, 6–13. [Google Scholar] [CrossRef]
- Fioravanti, A.; Cheleschi, S.; De Palma, A.; Addimanda, O.; Mancarella, L.; Pignotti, E.; Pulsatelli, L.; Galeazzi, M.; Meliconi, R. Can Adipokines Serum Levels Be Used as Biomarkers of Hand Osteoarthritis? Biomarkers 2018, 23, 265–270. [Google Scholar] [CrossRef]
- Colombo, G.; Caviglia, G.P.; Ravera, A.; Tribocco, E.; Frara, S.; Rosso, C.; Travelli, C.; Genazzani, A.A.; Ribaldone, D.G. NAMPT and NAPRT Serum Levels Predict Response to Anti-TNF Therapy in Inflammatory Bowel Disease. Front. Med. 2023, 10, 1116862. [Google Scholar] [CrossRef]
- Neubauer, K.; Bednarz-Misa, I.; Walecka-Zacharska, E.; Wierzbicki, J.; Agrawal, A.; Gamian, A.; Krzystek-Korpacka, M. Oversecretion and Overexpression of Nicotinamide Phosphoribosyltransferase/Pre-B Colony-Enhancing Factor/Visfatin in Inflammatory Bowel Disease Reflects the Disease Activity, Severity of Inflammatory Response and Hypoxia. Int. J. Mol. Sci. 2019, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Bime, C.; Casanova, N.G.; Camp, S.M.; Oita, R.C.; Ndukum, J.; Hernon, V.R.; Oh, D.K.; Li, Y.; Greer, P.J.; Whitcomb, D.C.; et al. Circulating ENAMPT as a Biomarker in the Critically Ill: Acute Pancreatitis, Sepsis, Trauma, and Acute Respiratory Distress Syndrome. BMC Anesthesiol. 2022, 22, 182. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, C.Y.; Chen, Y.H.; Chiu, W.C.; Wang, Y.Y.; Hsu, C.; Hu, S.C.S.; Su, Y.H.; Yuan, S.S.F. Essential Role of Visfatin in Lipopolysaccharide and Colon Ascendens Stent Peritonitis-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 1678. [Google Scholar] [CrossRef]
- Mir, M.M.; Mir, R.; Alghamdi, M.A.A.; Wani, J.I.; Sabah, Z.U.; Jeelani, M.; Marakala, V.; Sohail, S.K.; O’haj, M.; Alharthi, M.H.; et al. Differential Association of Selected Adipocytokines, Adiponectin, Leptin, Resistin, Visfatin and Chemerin, with the Pathogenesis and Progression of Type 2 Diabetes Mellitus (T2DM) in the Asir Region of Saudi Arabia: A Case Control Study. J. Pers. Med. 2022, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, T.M.; El-Gharbawy, N.M.; Werida, R.H. Circulating IRAPe, Irisin, and IL-34 in Relation to Insulin Resistance in Patients With Type 2 Diabetes. Clin. Ther. 2021, 43, e230–e240. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, M.; Nourbakhsh, M.; Gholinejad, Z.; Razzaghy-Azar, M. Visfatin in Obese Children and Adolescents and Its Association with Insulin Resistance and Metabolic Syndrome. Scand. J. Clin. Lab. Investig. 2015, 75, 183–188. [Google Scholar] [CrossRef]
- Chen, C.C.; Li, T.C.; Li, C.I.; Liu, C.S.; Lin, W.Y.; Wu, M.T.; Lai, M.M.; Lin, C.C. The Relationship between Visfatin Levels and Anthropometric and Metabolic Parameters: Association with Cholesterol Levels in Women. Metabolism 2007, 56, 1216–1220. [Google Scholar] [CrossRef]
- Yin, C.; Hu, W.; Wang, M.; Xiao, Y. The Role of the Adipocytokines Vaspin and Visfatin in Vascular Endothelial Function and Insulin Resistance in Obese Children. BMC Endocr. Disord. 2019, 19, 127. [Google Scholar] [CrossRef]
- Zhong, M.; Tan, H.W.; Gong, H.P.; Wang, S.F.; Zhang, Y.; Zhang, W. Increased Serum Visfatin in Patients with Metabolic Syndrome and Carotid Atherosclerosis. Clin. Endocrinol. 2008, 69, 878–884. [Google Scholar] [CrossRef]
- Gunes, F.; Akbal, E.; Cakir, E.; Akyurek, O.; Altunbas, M.; Ozbek, M. Visfatin May Be a Novel Marker for Identifying Stages of Essential Hypertension in Advanced Age Patients. Intern. Med. 2012, 51, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Liakos, C.I.; Sanidas, E.A.; Perrea, D.N.; Grassos, C.A.; Chantziara, V.; Viniou, N.A.; Barbetseas, J.D.; Papadopoulos, D.P. Apelin and Visfatin Plasma Levels in Healthy Individuals With High Normal Blood Pressure. Am. J. Hypertens. 2016, 29, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.J.; Xuan, H.F.; Lu, M.; Chen, X.Z.; Dong, W.F.; Yan, X.F.; Si, Y.; Gao, G.L.; Hu, D.X.; Miao, J.Q. Admission Plasma Visfatin Level Strongly Correlates with Hematoma Growth and Early Neurologic Deterioration in Patients with Acute Spontaneous Basal Ganglia Hemorrhage. Clin. Chim. Acta 2013, 425, 85–89. [Google Scholar] [CrossRef]
- Huang, Q.; Dai, W.M.; Jie, Y.Q.; Yu, G.F.; Fan, X.F.; Wu, A. High Concentrations of Visfatin in the Peripheral Blood of Patients with Acute Basal Ganglia Hemorrhage Are Associated with Poor Outcome. Peptides 2013, 39, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Hou, Y.; Ruan, S.P.; Wang, J.; Hu, X.M. Plasma Visfatin, a Possible Prognostic Marker in Aneurysmal Subarachnoid Hemorrhage. Peptides 2013, 50, 8–12. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, R.; Wang, H.; Tao, Q.; Lin, X.; Ge, S.; Zhai, Z. Nicotinamide Phosphate Transferase (NAMPT) Increases in Plasma in Patients with Acute Coronary Syndromes, and Promotes Macrophages to M2 Polarization. Int. Heart J. 2018, 59, 1116–1122. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Xu, X.; Wan, R.H.; Xia, S.; Lu, J.; Huang, Q. Association between Serum Visfatin Levels and Atherosclerotic Plaque in Patients with Type 2 Diabetes. Diabetol. Metab. Syndr. 2019, 11, 60. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Sailer, N.; Moumtzouoglou, A.; Kapelouzou, A.; Gerasimidis, T.; Kostakis, A.; Liapis, C.D. Adipokines: A Novel Link between Adiposity and Carotid Plaque Vulnerability. Eur. J. Clin. Investig. 2012, 42, 1278–1286. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Waligórska-Stachura, J.; Andrusiewicz, M.; Biczysko, M.; Sowiński, J.; Skrobisz, J.; Ruchała, M. Nicotinamide Phosphorybosiltransferase Overexpression in Thyroid Malignancies and Its Correlation with Tumor Stage and with Survivin/Survivin DEx3 Expression. Tumor Biol. 2015, 36, 7859–7863. [Google Scholar] [CrossRef]
- Szymanska, K.; Rytelewska, E.; Zaobidna, E.; Kiezun, M.; Gudelska, M.; Kopij, G.; Dobrzyn, K.; Mlyczynska, E.; Kurowska, P.; Kaminska, B.; et al. The Effect of Visfatin on the Functioning of the Porcine Pituitary Gland: An In Vitro Study. Cells 2023, 12, 2835. [Google Scholar] [CrossRef]
- Szymanska, K.; Zaobidna, E.; Rytelewska, E.; Mlyczynska, E.; Kurowska, P.; Dobrzyn, K.; Kiezun, M.; Kaminska, B.; Smolinska, N.; Rak, A.; et al. Visfatin in the Porcine Pituitary Gland: Expression and Regulation of Secretion during the Oestrous Cycle and Early Pregnancy. Sci. Rep. 2023, 13, 18253. [Google Scholar] [CrossRef]
- Marini, D.; Cappai, M.G.; Palmioli, E.; Battacone, G.; Maranesi, M.; Dobrzyń, K.; Mercati, F.; Dall’Aglio, C. Morphological Digital Assessment and Transcripts of Gastric and Duodenal Visfatin in Growing Piglets Fed with Increasing Amounts of Polyphenols from Olive Mill Waste Extract. Ann. Anat. Anat. Anzeiger 2025, 258, 152369. [Google Scholar] [CrossRef] [PubMed]
- Mpilla, G.; Aboukameel, A.; Muqbil, I.; Kim, S.; Beydoun, R.; Philip, P.A.; Mohammad, R.M.; Kamgar, M.; Shidham, V.; Senapedis, W.; et al. PAK4-NAMPT Dual Inhibition as a Novel Strategy for Therapy Resistant Pancreatic Neuroendocrine Tumors. Cancers 2019, 11, 1902. [Google Scholar] [CrossRef]
- Olesen, U.H.; Hastrup, N.; Sehested, M. Expression Patterns of Nicotinamide Phosphoribosyltransferase and Nicotinic Acid Phosphoribosyltransferase in Human Malignant Lymphomas. APMIS 2011, 119, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, S.; Sun, C.; Li, J.; Gao, W.; Yu, L. Clinical Significance of Serum Adiponectin and Visfatin Levels in Endometrial Cancer. Int. J. Gynaecol. Obstet. 2019, 145, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhu, Y.; Wang, Y.; Teng, F.; Zhang, H.; Liu, G.; Ma, X.; Sun, D.; Rohan, T.; Xue, F. Visfatin, a Potential Biomarker and Prognostic Factor for Endometrial Cancer. Gynecol. Oncol. 2013, 129, 505–512. [Google Scholar] [CrossRef]
- Dalamaga, M.; Archondakis, S.; Sotiropoulos, G.; Karmaniolas, K.; Pelekanos, N.; Papadavid, E.; Lekka, A. Could Serum Visfatin Be a Potential Biomarker for Postmenopausal Breast Cancer? Maturitas 2012, 71, 301–308. [Google Scholar] [CrossRef]
- Koike Folgueira, M.A.A.; Carraro, D.M.; Brentani, H.; Da Costa Patrão, D.F.; Mantovani Barbosa, E.; Mourão Netto, M.; Fígaro Caldeira, J.R.; Hirata Katayama, M.L.; Soares, F.A.; Tosello Oliveira, C.; et al. Gene Expression Profile Associated with Response to Doxorubicin-Based Therapy in Breast Cancer. Clin. Cancer Res. 2005, 11, 7434–7443. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, E.O.; Jeong, B.R.; Min, Y.J.; Park, J.W.; Kim, E.S.; Namgoong, I.S.; Kim, Y.I.; Lee, B.J. Visfatin Stimulates Proliferation of MCF-7 Human Breast Cancer Cells. Mol. Cells 2010, 30, 341–345. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, B.; Zhang, P.; Zhang, Z.; Chen, P.; Pu, Y.; Song, Y.; Zhang, L. Prognostic Value of Serum Nicotinamide Phosphoribosyltransferase in Patients with Bladder Cancer. Croat. Med. J. 2014, 55, 507–513. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, U.M.; Saber, M.M.; Abdellateif, M.S.; Nassar, H.R.; Namour, A.E.; Ismail, Y.M.; Zekri, A.R.N. The Possible Role of Adipokines in HCV Associated Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.S.; Dashti, H.; Akbarzadeh, S.; Assadi, M.; Aminian, A.; Keramati, M.R.; Nabipour, I. Circulating Levels of Novel Adipocytokines in Patients with Colorectal Cancer. Cytokine 2013, 62, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kosova, F.; Coskun, T.; Kaya, Y.; Kara, E.; Ari, Z. Adipocytokine Levels of Colon Cancer Patients before and after Treatment. Bratisl. Lek. Listy 2013, 114, 394–397. [Google Scholar] [CrossRef]
- Hufton, S.E.; Moerkerk, P.T.; Brandwijk, R.; De Bruïne, A.P.; Arends, J.W.; Hoogenboom, H.R. A Profile of Differentially Expressed Genes in Primary Colorectal Cancer Using Suppression Subtractive Hybridization. FEBS Lett. 1999, 463, 77–82. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Moerkerk, P.T.M.; Gerbers, A.J.; De Brune, A.P.; Arends, J.W.; Hoogenboom, H.R.; Hufton, S.E. Target Validation for Genomics Using Peptide-Specific Phage Antibodies: A Study of Five Gene Products Overexpressed in Colorectal Cancer. Int. J. Cancer 2002, 101, 118–127. [Google Scholar] [CrossRef]
- Wang, B.; Hasan, M.K.; Alvarado, E.; Yuan, H.; Wu, H.; Chen, W.Y. NAMPT Overexpression in Prostate Cancer and Its Contribution to Tumor Cell Survival and Stress Response. Oncogene 2011, 30, 907–921. [Google Scholar] [CrossRef]
- Patel, S.T.; Mistry, T.; Brown, J.E.P.; Digby, J.E.; Adya, R.; Desai, K.M.; Randeva, H.S. A Novel Role for the Adipokine Visfatin/Pre-B Cell Colony-Enhancing Factor 1 in Prostate Carcinogenesis. Peptides 2010, 31, 51–57. [Google Scholar] [CrossRef]
- Bi, T.Q.; Che, X.M.; Liao, X.H.; Zhang, D.J.; Long, H.L.; Li, H.J.; Zhao, W. Overexpression of Nampt in Gastric Cancer and Chemopotentiating Effects of the Nampt Inhibitor FK866 in Combination with Fluorouracil. Oncol. Rep. 2011, 26, 1251–1257. [Google Scholar] [CrossRef]
- Long, H.L.; Che, X.M.; Bi, T.Q.; Li, H.J.; Liu, J.S.; Li, D.W. The Expression of Nicotinamide Phosphoribosyl Transferase and Vascular Endothelial Growth Factor-A in Gastric Carcinoma and Their Clinical Significance. Zhonghua Wai Ke Za Zhi 2012, 50, 839–842. [Google Scholar]
- Wang, Y.Y.; Hung, A.C.; Lo, S.; Yuan, S.S.F. Adipocytokines Visfatin and Resistin in Breast Cancer: Clinical Relevance, Biological Mechanisms, and Therapeutic Potential. Cancer Lett. 2021, 498, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Sompolska-Rzechuła, A.; Machaliński, B.; Menkiszak, J. Circulating Serum Level of Visfatin in Patients with Endometrial Cancer. Biomed Res. Int. 2018, 2018, 8576179. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.F.; Chen, Y.L.; Lee, C.H.; Chou, F.H.; Wu, L.C.; Jong, S.B.; Tsai, E.M. Decreased Plasma Visfatin Concentrations in Women with Gestational Diabetes Mellitus. J. Soc. Gynecol. Investig. 2006, 13, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Derdemezis, C.S.; Kiortsis, D.N.; Tselepis, A.D.; Elisaf, M.S. Increased Plasma Levels of Visfatin/Pre-B Cell Colony-Enhancing Factor in Obese and Overweight Patients with Metabolic Syndrome. J. Endocrinol. Investig. 2007, 30, 323–326. [Google Scholar] [CrossRef]
- Nomura, M.; Ohuchi, M.; Sakamoto, Y.; Kudo, K.; Yaku, K.; Soga, T.; Sugiura, Y.; Morita, M.; Hayashi, K.; Miyahara, S.; et al. Niacin Restriction with NAMPT-Inhibition Is Synthetic Lethal to Neuroendocrine Carcinoma. Nat. Commun. 2023, 14, 8095. [Google Scholar] [CrossRef]
- Audrito, V.; Moiso, E.; Ugolini, F.; Messana, V.G.; Brandimarte, L.; Manfredonia, I.; Bianchi, S.; De Logu, F.; Nassini, R.; Szumera-Ciećkiewicz, A.; et al. Tumors Carrying BRAF-Mutations over-Express NAMPT That Is Genetically Amplified and Possesses Oncogenic Properties. J. Transl. Med. 2022, 20, 118. [Google Scholar] [CrossRef]
- Safari, M.; Scotto, L.; Litman, T.; Petrukhin, L.A.; Zhu, H.; Shen, M.; Robey, R.W.; Hall, M.D.; Fojo, T.; Bates, S.E. Novel Therapeutic Strategies Exploiting the Unique Properties of Neuroendocrine Neoplasms. Cancers 2023, 15, 4960. [Google Scholar] [CrossRef]
- Winter, J.; Kunze, R.; Veit, N.; Kuerpig, S.; Meisenheimer, M.; Kraus, D.; Glassmann, A.; Probstmeier, R. Targeting of Glucose Transport and the NAD Pathway in Neuroendocrine Tumor (NET) Cells Reveals New Treatment Options. Cancers 2023, 15, 1415. [Google Scholar] [CrossRef]
- Mpilla, G.B.; Uddin, M.H.; Al-Hallak, M.N.; Aboukameel, A.; Li, Y.; Kim, S.H.; Beydoun, R.; Dyson, G.; Baloglu, E.; Senapedis, W.T.; et al. PAK4-NAMPT Dual Inhibition Sensitizes Pancreatic Neuroendocrine Tumors to Everolimus. Mol. Cancer Ther. 2021, 20, 1836–1845. [Google Scholar] [CrossRef]
- Elf, A.K.; Bernhardt, P.; Hofving, T.; Arvidsson, Y.; Forssell-Aronsson, E.; Wängberg, B.; Nilsson, O.; Johanson, V. NAMPT Inhibitor GMX1778 Enhances the Efficacy of 177Lu-DOTATATE Treatment of Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 288–292. [Google Scholar] [CrossRef]
- Wen, F.; Gui, G.; Wang, X.; Ye, L.; Qin, A.; Zhou, C.; Zha, X. Drug Discovery Targeting Nicotinamide Phosphoribosyltransferase (NAMPT): Updated Progress and Perspectives. Bioorg. Med. Chem. 2024, 99, 117595. [Google Scholar] [CrossRef] [PubMed]
- Dayton, T.L.; Alcala, N.; Moonen, L.; den Hartigh, L.; Geurts, V.; Mangiante, L.; Lap, L.; Dost, A.F.M.; Beumer, J.; Levy, S.; et al. Druggable Growth Dependencies and Tumor Evolution Analysis in Patient-Derived Organoids of Neuroendocrine Neoplasms from Multiple Body Sites. Cancer Cell 2023, 41, 2083–2099. [Google Scholar] [CrossRef] [PubMed]
- Komarnicki, P.; Gut, P.; Musiałkiewicz, J.; Czupińska, M.; Mastorakos, G.; Ruchala, M. Increased Serum Visfatin in Neuroendocrine Tumors Shows Promise as a Diagnostic Biomarker: A Single-Center, Cross-Sectional Study. Endocr. Abstr. 2025, 110, P465. [Google Scholar]

| Variable | NETs Patients | ||||||
|---|---|---|---|---|---|---|---|
| Total NETs (n = 77) | Pancreatic NETs (n = 33) | Small Intestinal NETs (n = 44) | Controls (n = 29) | ||||
| Demographics | |||||||
| Age, years | 71.0 [63.0–77.0] * | 70.0 [59.0–78.0] * | 71.0 [65.8–76.0] * | 56.0 [43.0–64.0] * | |||
| Sex | |||||||
| Male | 39 (50.7%) | 17 (51.5%) | 22 (50%) | 4 (13.8%) | |||
| Female | 38 (49.3%) | 16 (48.5%) | 22 (50%) | 25 (86.2%) | |||
| Clinical Characteristics | |||||||
| NET primary site | |||||||
| Pancreas | 33 (42.9%) | ||||||
| Small Intestine | 44 (57.1%) | ||||||
| Functioning NETs | |||||||
| Total | 26 (33.8%) | 5 (15.2%) | 21 (47.7%) | ||||
| Carcinoid Syndrome | 21 (27.3%) | - | 21 (47.7%) | ||||
| Insulinoma | 4 (5.2%) | 4 (12.1%) | - | ||||
| Glucagonoma | 1 (0.1%) | 1 (0.3%) | - | ||||
| WHO Grade | |||||||
| G1 | 35 (45.5%) | 10 (30.3%) | 25 (56.8%) | ||||
| G2 | 42 (54.5%) | 23 (69.7%) | 19 (43.2%) | ||||
| Subgroup | Median [IQR] (ng/mL) | Mann–Whitney U Statistic | Effect Size (r) | p-Value | |
|---|---|---|---|---|---|
| NETs | Controls | ||||
| Total NETs | 6.94 [2.11–236.17] | 1.59 [1.10–9.24] | 1518.5 | 0.36 | 0.004 |
| panNETs | 4.98 [2.13–264.96] | 642.0 | 0.34 | 0.019 | |
| siNETs | 7.46 [2.01–199.44] | 876.5 | 0.37 | 0.007 | |
| Variable | Subgroup | Median [IQR] (ng/mL) | Mann–Whitney U Statistic | Effect Size (r) | p-Value |
|---|---|---|---|---|---|
| Primary Site | panNETs | 4.98 [2.13–264.96] | 732.5 | 0.01 | 0.95 |
| siNETs | 7.46 [2.01–199.44] | ||||
| WHO Grade | G1 | 17.23 [2.44–265.55] | 834.0 | 0.11 | 0.31 |
| G2 | 4.45 [1.62–158.45] | ||||
| Sex (NETs) | Male | 20.27 [1.23–277.34] | 755.5 | 0.02 | 0.89 |
| Female | 4.84 [2.42–104.56] | ||||
| Kruskal–Wallis H statistic | Degrees of freedom | ||||
| Primary Site * WHO Grade | 4.9124 | 3 | 0.18 | ||
| R Spearman | |||||
| Age (NETs) | −0.1731 | 0.13 | |||
| Variable | Regression Coefficient (5–95% CI) | Standard Error | p-Value |
|---|---|---|---|
| Age | −1.28 (−3.96–1.41) | 1.35 | 0.35 |
| Sex (Male) | 30.88 (−27.14–88.90) | 29.11 | 0.29 |
| Primary Site (siNET) | −5.41 (−66.10–55.28) | 30.44 | 0.86 |
| Grade (G2) | −24.04 (−84.18–36.10) | 30.17 | 0.43 |
| R-squared | Adjusted R-squared | ||
| Model Fit | 0.036 | −0.018 | |
| Conditions with Elevated Serum Visfatin | Study | |
|---|---|---|
| Inflammatory diseases | Rheumatoid arthritis | Cheleschi [18] Ali [19] |
| Osteoarthritis | Askari [20] Fioravanti [21] | |
| Inflammatory bowel disease | Colombo [22] Neubauer [23] | |
| Lung injury | Bime [24] Lee [25] | |
| Metabolic disorders | Type 2 diabetes | Mir [26] Mostafa [27] |
| Insulin resistance | Nourbakhsh [28] Chen [29] | |
| Obesity | Yin [30] Nourbakhsh [28] | |
| Metabolic syndrome | Zhong [31] | |
| Cardiovascular diseases | Hypertension | Gunes [32] Liakos [33] |
| Cerebrovascular accidents | Gu [34] Huang [35] Wang [36] | |
| Acute coronary syndrome | Zhang [37] | |
| Atherosclerosis | Zheng [38] Kadoglou [39] | |
| Study | Malignancy | Assessment | Alteration |
|---|---|---|---|
| Olesen [45] | Hematopoietic malignancies | Tumor | ↑ |
| Wang [46] | Endometrial cancer | Circulating | ↑ |
| Tian [47] | Endometrial cancer | Tumor | ↑ |
| Dalamaga [48] | Breast cancer | Circulating | ↑ |
| Folgueira [49] Kim [50] | Breast cancer | Tumor | ↑ |
| Zhang [51] | Bladder cancer | Circulating | ↑ |
| Sawicka-Gutaj [10] | Thyroid cancer | Circulating | ↔ |
| Sawicka- Gutaj [40] | Thyroid cancer | Tumor | ↑ |
| El-Daly [52] | Hepatocellular carcinoma | Circulating | ↑ |
| Fazeli [53] Kosova [54] | Colorectal cancer | Circulating | ↑/↔ |
| Hufton [55] Beijnum [56] | Colorectal cancer | Tumor | ↑ |
| Wang [57] Patel [58] | Prostate cancer | Tumor | ↑ |
| Bi [59] Long [60] | Gastric cancer | Tumor | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarnicki, P.; Maciejewski, A.; Musiałkiewicz, J.; Czupińska, M.; Mastorakos, G.; Ruchała, M.; Gut, P. Serum Visfatin/eNAMPT as a Biomarker in Pancreatic and Small Intestine Neuroendocrine Tumors: A Cross-Sectional Study and Future Perspectives. Cancers 2025, 17, 2343. https://doi.org/10.3390/cancers17142343
Komarnicki P, Maciejewski A, Musiałkiewicz J, Czupińska M, Mastorakos G, Ruchała M, Gut P. Serum Visfatin/eNAMPT as a Biomarker in Pancreatic and Small Intestine Neuroendocrine Tumors: A Cross-Sectional Study and Future Perspectives. Cancers. 2025; 17(14):2343. https://doi.org/10.3390/cancers17142343
Chicago/Turabian StyleKomarnicki, Paweł, Adam Maciejewski, Jan Musiałkiewicz, Michalina Czupińska, George Mastorakos, Marek Ruchała, and Paweł Gut. 2025. "Serum Visfatin/eNAMPT as a Biomarker in Pancreatic and Small Intestine Neuroendocrine Tumors: A Cross-Sectional Study and Future Perspectives" Cancers 17, no. 14: 2343. https://doi.org/10.3390/cancers17142343
APA StyleKomarnicki, P., Maciejewski, A., Musiałkiewicz, J., Czupińska, M., Mastorakos, G., Ruchała, M., & Gut, P. (2025). Serum Visfatin/eNAMPT as a Biomarker in Pancreatic and Small Intestine Neuroendocrine Tumors: A Cross-Sectional Study and Future Perspectives. Cancers, 17(14), 2343. https://doi.org/10.3390/cancers17142343







