Somatostatin Receptor Expression of Gastroenteropancreatic Neuroendocrine Tumors: A Comprehensive Analysis in the Era of Somatostatin Receptor PET Imaging
Simple Summary
Abstract
1. Introduction
2. Methods
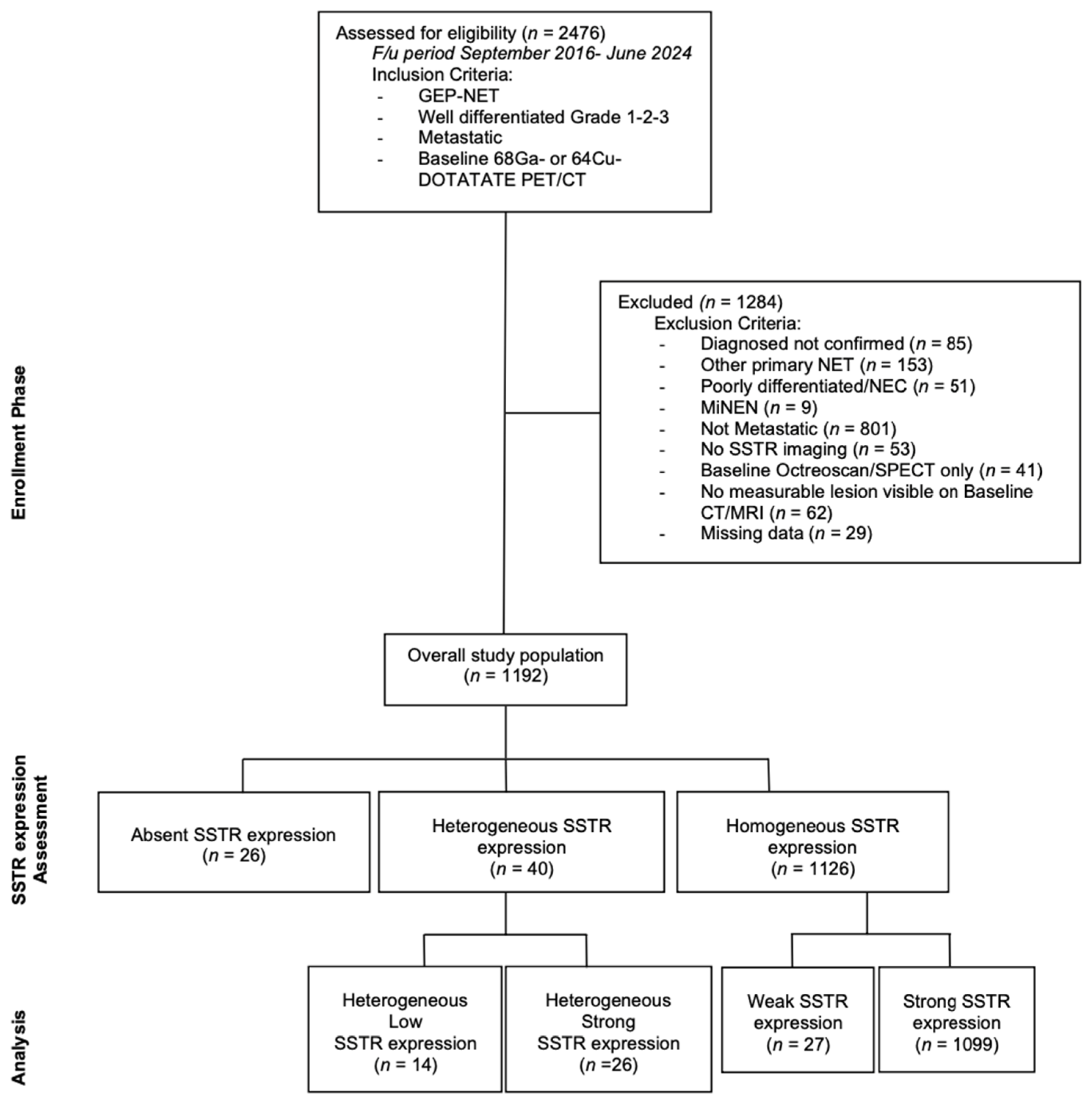
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cakir, M.; Dworakowska, D.; Grossman, A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: Part 1—Molecular pathways. J. Cell Mol. Med. 2010, 14, 2570–2584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zamora, V.; Cabanne, A.; Salanova, R.; Bestani, C.; Domenichini, E.; Marmissolle, F.; Giacomi, N.; O’Connor, J.; Méndez, G.; Roca, E.; et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig. Liver Dis. 2010, 42, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R.; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763, Erratum in Lancet Oncol. 2022, 23, e59. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Naqvi, S.; Cohn, A.L.; Delpassand, E.S.; Wagner, V.J.; Torgue, J.; Woloski, R.; Manuel, A.; Maluccio, M.A. Safety, tolerability and efficacy of 212Pb-DOTAMTATE as a targeted alpha therapy for subjects with unresectable or metastatic somatostatin receptor-expressing gastroenteropancreatic neuroendocrine tumors (SSTR+ GEP-NETs): A phase 2 study. J. Clin. Oncol. 2024, 42, 4020. [Google Scholar] [CrossRef]
- Morris, M.; Ulaner, G.A.; Halperin, D.M.; Strosberg, J.; Mehr, S.H.; Li, D.; Soares, H.P.; Anthony, L.B.; Kotiah, S.D.; Jarcene, H.; et al. ACTION-1 phase Ib/3 trial of RYZ101 in somatostatin receptor subtype 2–expressing (SSTR2+) gastroenteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177Lu somatostatin analogue (SSA) therapy: Initial safety analysis. J. Clin. Oncol. 2023, 41, 4132. [Google Scholar] [CrossRef]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimura, N.; Pilichowska, M.; Date, F.; Kimura, I.; Schindler, M. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine tumors. Clin. Cancer Res. 1999, 5, 3483–3487. [Google Scholar] [PubMed]
- Volante, M.; Brizzi, M.P.; Faggiano, A.; La Rosa, S.; Rapa, I.; Ferrero, A.; Mansueto, G.; Righi, L.; Garancini, S.; Capella, C.; et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod. Pathol. 2007, 20, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Gonzalez, R.S.; Zhao, Z.; Koyama, T.; Cornish, T.C.; Hande, K.R.; Walker, R.; Sandler, M.; Berlin, J.; Liu, E.H. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am. J. Clin. Pathol. 2015, 143, 398–404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grillo, F.; Valle, L.; Ferone, D.; Albertelli, M.; Brisigotti, M.P.; Cittadini, G.; Vanoli, A.; Fiocca, R.; Mastracci, L. KI-67 heterogeneity in well differentiated gastro-entero-pancreatic neuroendocrine tumors: When is biopsy reliable for grade assessment? Endocrine 2017, 57, 494–502, Erratum in Endocrine 2017, 57, 503. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, L.H.; Klimstra, D.S. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: Implications for prognostic stratification. Am. J. Surg. Pathol. 2011, 35, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cao, F.; Zhao, X.; Xie, Q.; Lu, M.; Li, J.; Yang, Z.; Sun, Y. Correlation and Comparison of Somatostatin Receptor Type 2 Immunohistochemical Scoring Systems with 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography Imaging in Gastroenteropancreatic Neuroendocrine Neoplasms. Neuroendocrinology 2022, 112, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Laffi, A.; Spada, F.; Bagnardi, V.; Frassoni, S.; Pisa, E.; Rubino, M.; Barberis, M.; Fazio, N. Gastroenteropancreatic grade 3 neuroendocrine tumors: A single entity or a heterogeneous group? A retrospective analysis. J. Endocrinol. Investig. 2022, 45, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, K.; Venkataraman, H.; Hughes, S.; Shah, H.; Vickrage, S.; Smith, S.; Humpries, S.; Elshafie, M.; Taniere, P.; Diaz-Cano, S.; et al. Well-differentiated gastroenteropancreatic G3 NET: Findings from a large single centre cohort. Sci. Rep. 2021, 11, 17947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Toubah, T.; Montilla-Soler, J.; El-Haddad, G.; Haider, M.; Strosberg, J. Somatostatin Receptor Expression in Lung Neuroendocrine Tumors: An Analysis of DOTATATE PET Scans. J. Nucl. Med. 2023, 64, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Has Simsek, D.; Kuyumcu, S.; Turkmen, C.; Sanlı, Y.; Aykan, F.; Unal, S.; Adalet, I. Can complementary 68Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? J. Nucl. Med. 2014, 55, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Naswa, N.; Sharma, P.; Gupta, S.K.; Karunanithi, S.; Reddy, R.M.; Patnecha, M.; Lata, S.; Kumar, R.; Malhotra, A.; Bal, C. Dual tracer functional imaging of gastroenteropancreatic neuroendocrine tumors using 68Ga-DOTA-NOC PET-CT and 18F-FDG PET-CT: Competitive or complimentary? Clin. Nucl. Med. 2014, 39, e27–e34. [Google Scholar] [CrossRef] [PubMed]
- Laffi, A.; Colandrea, M.; Buonsanti, G.; Frassoni, S.; Bagnardi, V.; Spada, F.; Pisa, E.; Barberis, M.; Rubino, M.; Grana, C.M.; et al. A Retrospective Analysis of the Correlation between Functional Imaging and Clinical Outcomes in Grade 3 Neuroendocrine Tumors (NETs G3). Diagnostics 2021, 11, 2401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Li, L.; Wang, H.; Huang, H.X.; Cao, D.; Ke, N.W.; Su, M.G.; Tian, R. Heterogeneous Uptake of 68 Ga-DOTATATE and 18 F-FDG in Initial Diagnosed Neuroendocrine Tumors Patients: Which Patients Are Suitable for Dual-Tracer PET Imaging? Clin. Nucl. Med. 2024, 49, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, D.L.; Hayes, A.R.; Karfis, I.; Conner, A.; Furtado O’Mahony, L.; Mileva, M.; Bernard, E.; Roach, P.; Marin, G.; Pavlakis, N.; et al. Dual [68Ga]DOTATATE and [18F]FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasms: A multicentre validation of the NETPET score. Br. J. Cancer 2023, 128, 549–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, D.L.; Hayes, A.R.; Karfis, I.; Conner, A.; Furtado O’Mahony, L.; Mileva, M.; Bernard, E.; Roach, P.; Marin, G.; Pavlakis, N.; et al. [18F]FDG PET/CT-Avid Discordant Volume as a Biomarker in Patients with Gastroenteropancreatic Neuroendocrine Neoplasms: A Multicenter Study. J. Nucl. Med. 2024, 65, 185–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, D.L.; Ulaner, G.A.; Pattison, D.; Wyld, D.; Ladwa, R.; Kirchner, J.; Li, B.T.; Lai, W.V.; Pavlakis, N.; Roach, P.J.; et al. Dual PET Imaging in Bronchial Neuroendocrine Neoplasms: The NETPET Score as a Prognostic Biomarker. J. Nucl. Med. 2021, 62, 1278–1284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, T.; Brixi-Benmansour, H.; Lombard-Bohas, C.; Cadiot, G. New treatment strategies in advanced neuroendocrine tumours. Dig. Liver Dis. 2012, 44, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Liccardi, A.; Minotta, R.; Cannavale, G.; Benevento, E.; Colao, A. Therapeutic strategies for patients with neuroendocrine neoplasms: Current perspectives. Expert Rev. Endocrinol. Metab. 2022, 17, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Citterio, D.; Pusceddu, S.; Facciorusso, A.; Coppa, J.; Milione, M.; Buzzoni, R.; Bongini, M.; deBraud, F.; Mazzaferro, V. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur. J. Surg. Oncol. 2017, 43, 380–387. [Google Scholar] [CrossRef] [PubMed]
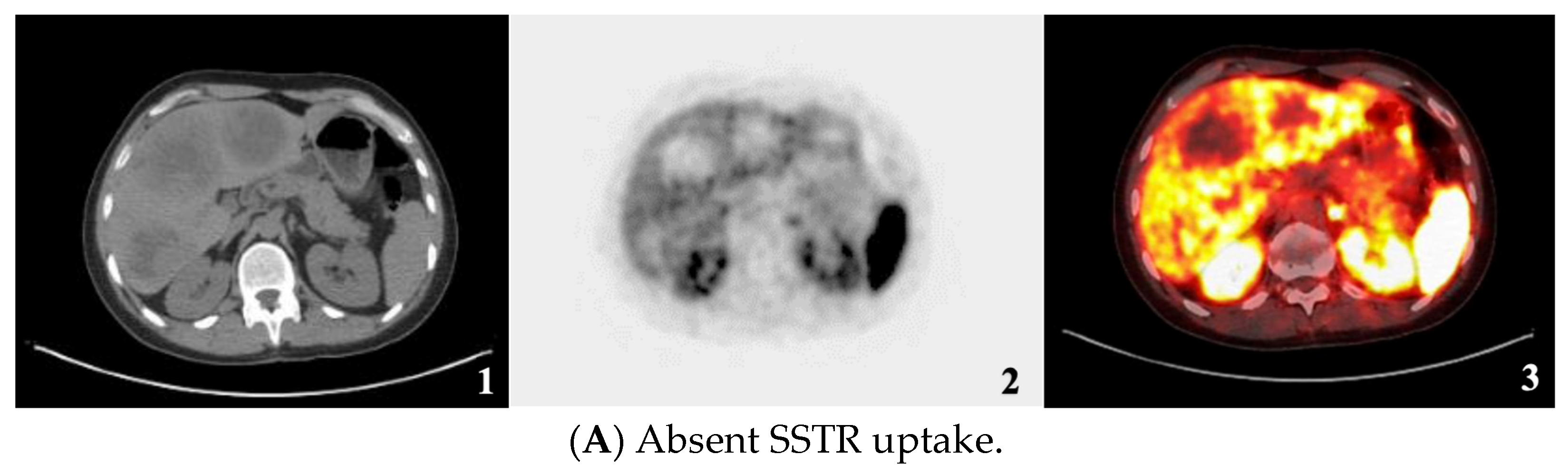
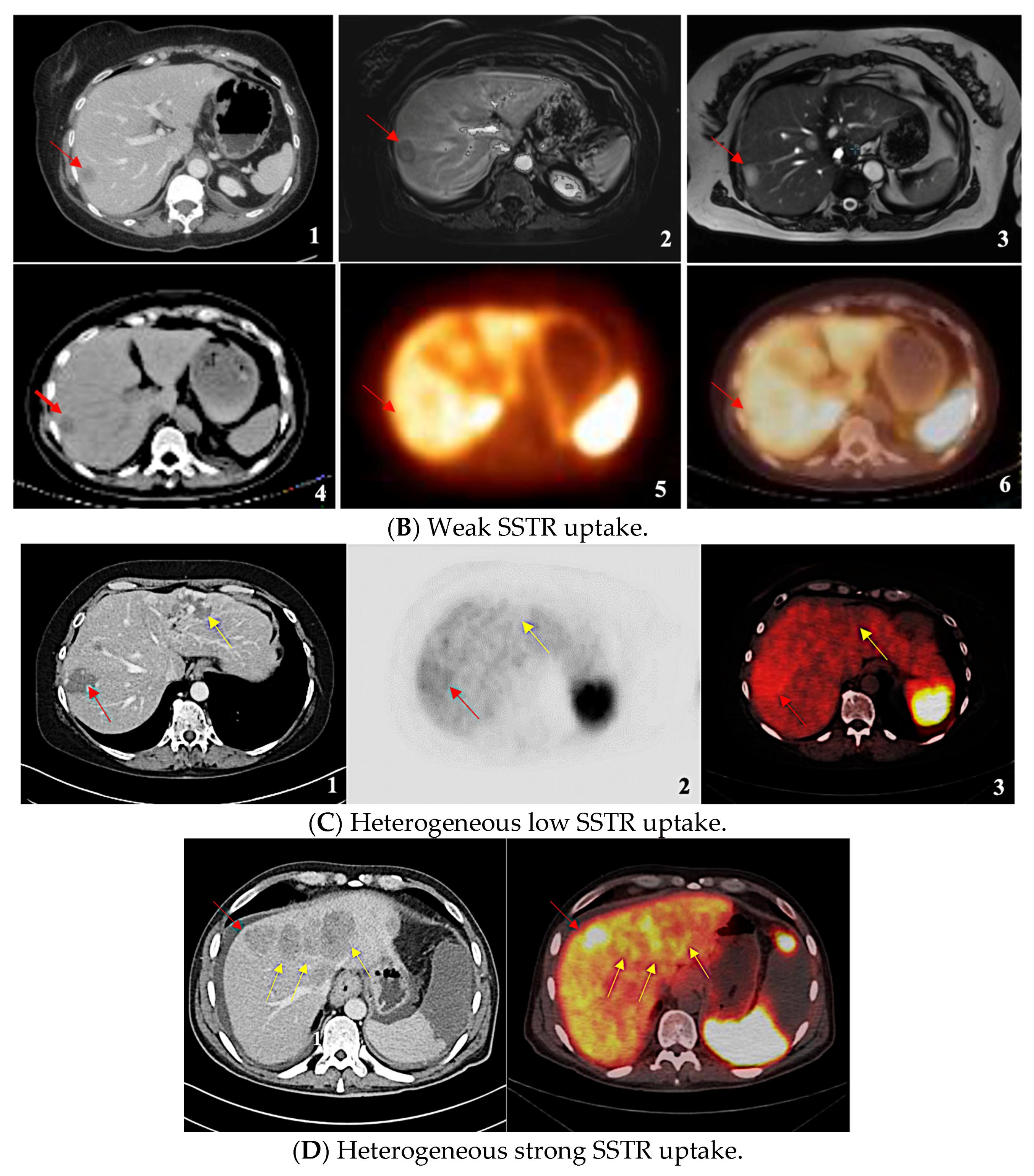
| SSTR Expression Assessment per Study | Krenning Score | Definition |
|---|---|---|
| Negative/Absent | 0 | No uptake |
| Homogeneous Weak | 1 | Much lower than liver |
| 2 | Slightly less than or equal to liver | |
| Homogeneous Strong | 3 | Greater than liver |
| 4 | Greater than spleen | |
| Heterogeneous Low | - | Mixture of absent and less than or equal to liver uptake lesions |
| Heterogeneous Strong | - | Mixture of strongly avid lesions combined with the absence or near absence of SSTR expression in at least one measurable tumor (primary or metastasis). |
| Parameter | N (=1192) | % |
|---|---|---|
| Sex | ||
| Female | 537 | 45.10% |
| Male | 655 | 54.90% |
| Grade | ||
| 1 | 389 | 32.60% |
| 2 | 613 | 51.40% |
| 3 | 95 | 8% |
| Unknown | 95 | 8% |
| Ki-67 | ||
| ≤2% | 389 | 32.60% |
| 3–20% | 581 | 48.70% |
| >20% | 95 | 8% |
| Unknown | 127 | 10.70% |
| Primary | ||
| Small Bowel | 784 | 65.80% |
| Pancreas | 302 | 25.30% |
| Gastric | 20 | 1.70% |
| Biliary-Tract | 8 | 0.70% |
| Appendix | 3 | 0.20% |
| Colorectal | 24 | 2% |
| Unknown | 51 | 4.30% |
| Hormone syndrome | ||
| Yes | 415 | 34.80% |
| No | 777 | 65.20% |
| SSTR Expression Assessment per Study | Total N = 1192 (100%) | [68Ga]-DOTATATE PET/CT N (%) | [64Cu]DOTATATE PET/CT N (%) |
|---|---|---|---|
| Negative/Absent | 26 (2.2%) | 20 (1.7%) | 6 (0.5%) |
| Homogeneous Weak | 27 (2.3%) | 26 (2.2%) | 1 (0.1%) |
| Homogeneous Strong | 1099 (92.2%) | 896 (75.2%) | 203 (17.0%) |
| Heterogeneous Low | 14 (1.2%) | 12 (1.0%) | 2 (0.2%) |
| Heterogeneous Strong | 26(2.2%) | 20 1.7(%) | 6 (0.5%) |
| SSTR Expression | G1 | G2 | G3 | N/A |
|---|---|---|---|---|
| Absent | 3 (0.8%) | 15 (2.4%) | 7 (7.4%) | 1 (1.1%) |
| Uniformly present low expression (<liver) | 12 (3.1%) | 13 (2.1%) | 2 (2.1%) | - |
| Uniformly present high expression (>liver) | 370 (95.1%) | 566 (92.3%) | 70 (73.7%) | 93 (97.9%) |
| Heterogeneous low expression (mixture of low and absent expression) | - | 4 (0.7%) | 10 (10.5%) | - |
| Heterogeneous strong expression (mixture of high and absent expression) | 4 (1.0%) | 15 (2.4%) | 6 (6.3%) | 1 (1.1%) |
| Total (100%) | 389 | 613 | 95 | 95 |
| SSTR Expression | SI-NET | Pan-NET | Other |
|---|---|---|---|
| Absent | 5 (0.6%) | 14 (4.6%) | 7 (6.6%) |
| Uniformly present low expression (<liver) | 17 (2.2%) | 7 (2.3%) | 3 (2.8%) |
| Uniformly present high expression (>liver) | 748 (95.4%) | 259 (85.8%) | 92 (86.8%) |
| Heterogeneous low expression (mixture of low and absent expression) | 2 (0.3%) | 8 (2.6%) | 4 (3.8%) |
| Heterogeneous strong expression (mixture of high and absent expression) | 12 (1.5%) | 14 (4.6%) | - |
| Total (100%) | 784 | 302 | 106 |
| SSTR Expression Assessment per Study | Krenning Score | Definition | Mean SUVmax |
|---|---|---|---|
| Negative/Absent | 0 | No uptake | - |
| Homogeneous Weak | 1 | Much lower than liver | 9 |
| 2 | Slightly less than or equal to liver | ||
| Homogeneous Strong | 3 | Greater than liver | 31.2 |
| 4 | Greater than spleen | ||
| Heterogeneous Low | - | Mixture of absent and less than or equal to liver uptake lesions | 9.5 |
| Heterogeneous Strong | - | Mixture of strongly avid lesions combined with the absence or near absence of SSTR expression in at least one measurable tumor (primary or metastasis). | 42.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maratta, M.G.; Al-Toubah, T.; Montilla-Soler, J.; Pelle, E.; Haider, M.; El-Haddad, G.; Strosberg, J. Somatostatin Receptor Expression of Gastroenteropancreatic Neuroendocrine Tumors: A Comprehensive Analysis in the Era of Somatostatin Receptor PET Imaging. Cancers 2025, 17, 1937. https://doi.org/10.3390/cancers17121937
Maratta MG, Al-Toubah T, Montilla-Soler J, Pelle E, Haider M, El-Haddad G, Strosberg J. Somatostatin Receptor Expression of Gastroenteropancreatic Neuroendocrine Tumors: A Comprehensive Analysis in the Era of Somatostatin Receptor PET Imaging. Cancers. 2025; 17(12):1937. https://doi.org/10.3390/cancers17121937
Chicago/Turabian StyleMaratta, Maria Grazia, Taymeyah Al-Toubah, Jaime Montilla-Soler, Eleonora Pelle, Mintallah Haider, Ghassan El-Haddad, and Jonathan Strosberg. 2025. "Somatostatin Receptor Expression of Gastroenteropancreatic Neuroendocrine Tumors: A Comprehensive Analysis in the Era of Somatostatin Receptor PET Imaging" Cancers 17, no. 12: 1937. https://doi.org/10.3390/cancers17121937
APA StyleMaratta, M. G., Al-Toubah, T., Montilla-Soler, J., Pelle, E., Haider, M., El-Haddad, G., & Strosberg, J. (2025). Somatostatin Receptor Expression of Gastroenteropancreatic Neuroendocrine Tumors: A Comprehensive Analysis in the Era of Somatostatin Receptor PET Imaging. Cancers, 17(12), 1937. https://doi.org/10.3390/cancers17121937








