1. Introduction
Lynch syndrome (LS) is an autosomal dominant disease first described by Lynch and collaborators in 1966 [
1]. LS is caused by germline pathogenetic variants in one of the four DNA mismatch repair (MMR) genes (
MLH1,
MSH2,
MSH6, and
PMS2) or genomic deletion at the 3′ end of the
EPCAM gene, which disrupts
MSH2 gene transcription [
1,
2]. LS presents with a highly heterogeneous phenotype, as shown by substantial differences in cancer penetrance (lifetime cancer risk) and expressivity (malignancies in specific organs) among individuals harboring genetic variants in a specific altered MMR gene [
3]. Patients with
MLH1 and
MSH2 germline pathogenic variants (PVs) or likely pathogenic variants (LPVs) have an increased risk of developing colorectal cancer (CRC) and endometrial cancer (EC) compared to patients with
MSH6 or
PMS2 germline PVs/LPVs [
3]. According to the most recent literature, PVs and LPVs involving the
PMS2 gene are detected in up to 15% of LS patients compared to 39% for
MLH1, 33% for
MSH2, and 19% for
MSH6. This lower incidence may be due to the lower cancer penetrance observed in individuals and families with
PMS2 PVs/LPVs [
4,
5].
Depending on the MMR germline variant, LS patients have an increased risk of other primary cancers, such as ovarian, small bowel, prostate, biliary tract, pancreatic, brain (glioblastoma), cutaneous, and urothelial (ureter, renal pelvis, and bladder) cancer [
6]. Patients with germline disease-causing variants affecting the
PMS2 gene have a lower risk of developing CRC and EC compared to patients with variants in other MMR genes or the
EPCAM gene [
7]. Based on current guidelines from the National Comprehensive Cancer Network (NCCN), the overall risk of developing LS-related cancers in individuals carrying a
PMS2 PV/LPV is similar to that of the general population (NCCN Clinical Practice Guidelines in Oncology for Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric. Version: 4.2024—2 April 2025. Available online at
https://www.nccn.org; accessed on 20 May 2025). Accordingly, the
PMS2 gene has been defined as a low-penetrance gene for cancers associated with LS. Moreover, the clinical phenotype associated with
PMS2 germline variants can vary among families and even among affected individuals of the same family [
8].
The gene encoding the mismatch repair endonuclease
PMS2 is located on chromosome 7 and spans approximately 38,000 base pairs, with a full-length protein of 862 amino acids translated from 15 exons [
5]. The PMS2 endonuclease is a post-replicative DNA MMR system component and heterodimerizes with MLH1 to form the MutLα complex [
9]. The DNA MMR system is essential for the maintenance of genomic integrity and improves replication fidelity by 100–1000-fold. MMR genes are responsible for correctly pairing nucleotide bases during DNA replication [
9].
Failure to resolve mismatch errors generates a high rate of microsatellite instability (MSI). Loss of MMR protein expression and/or the presence of MSI are considered hallmarks of LS-related tumors [
10]. However, these features can also be identified in sporadic tumors, in association with
MLH1 gene promoter hypermethylation and the presence of a somatic substitution at codon V600 of the
BRAF gene [
2,
10]. The presence of somatic MSI and loss of expression of an MMR protein is indicative of the involvement of an MMR gene germline pathogenic variant in patients with LS-related tumors. Thus, individuals with LS-related tumors showing defects in the MMR system are recommended to undergo germline genetic testing for MMR gene analysis. However, genetic testing in these individuals does not always identify germline PVs/LPVs in MMR genes since more than 20% of MMR germline variations are clinically classified as variants of uncertain significance (VUSs) [
11]. According to previous studies, the vast majority of germline variants identified in the
PMS2 MMR gene are classified as VUSs [
12,
13]. In fact, establishing the pathogenic potential of these variants in patients with a personal and/or family history of cancer remains challenging due to the variable clinical manifestations and lower penetrance associated with the
PMS2 gene. In this context, an integrated model combining tumor molecular profiling (e.g., immunohistochemistry for MMR proteins, MSI status), in silico protein variant predictions (e.g., protein structure modelling, evolutionary conservation), and the identification of the same rare variant in unrelated families may provide additional insight into the pathogenicity of VUSs involving the
PMS2 gene [
14]. Improving the interpretation of
PMS2 VUSs is crucial for tailoring surveillance and management strategies in individuals with a personal and/or family history of cancer, as well as for ensuring accurate genetic counselling in affected families.
In the present study, we describe two unrelated families of LS patients in which a novel missense variant (c.184G>A; p.Gly62Arg) was identified in the N-terminal region of PMS2. This region is approximately 364 amino acids long and contains an ATPase domain that can hydrolyze ATP and bind DNA [
15]. The C-terminus end of PMS2 contains an endonuclease domain that forms a heterodimer with MLH1, which is essential for the stability of PMS2 in vivo [
16]. Although structural data for the C-terminal domain are lacking, the endonuclease domain in this region is essential for MMR function [
5]. To evaluate the effect of this novel
PMS2 gene variant (c.184G>A; p.Gly62Arg), we used an integrated approach combining genetic and clinical findings of the affected families, the evaluation of the tumor phenotype, and bioinformatics analyses to predict its effect on protein structure and stability and assess its molecular impact on the clinical phenotype associated with LS.
2. Materials and Methods
2.1. Patient Recruitment
Genetic and molecular testing of blood and/or pathological tissue samples was conducted as part of our institute’s routine clinical diagnostic assessment. Before testing, written informed consent was obtained from all patients using a form approved by the competent ethics committee, in compliance with the Declaration of Helsinki and any other local ethical and legal requirements (protocol code N_170, approval date 31 October 2016).
2.2. Germline Genetic Analysis
Genomic DNA was extracted from peripheral blood using the MagCore® Genomic DNA Whole Blood Kit (Amerigo Scientific, New York, NY, USA) according to the manufacturer’s instructions. DNA samples (10 ng) from the index cases of Family 1 and Family 2 were subsequently processed to sequence and analyze the entire coding regions of 25 genes associated with hereditary cancer—including APC, ATM, BARD1, BMPR1A, BRIP1, CDH1, CDK4, CDKN2A, CHEK2, EPCAM, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PTEN, RAD50, RAD51C, RAD51D, SMAD4, STK11, and TP53—using a targeted next-generation sequencing (NGS) multigene panel (Ion AmpliSeq™ BRCA Reflex—Hereditary Cancer Research Panel) (Thermo Fisher Scientific, Waltham, MA, USA).
The sequencing library was generated using two premixed pools of primer pairs working with the Ion AmpliSeq Chef Solutions DL8 Kit (Thermo Fisher Scientific, Waltham, MA, USA) on an Ion Chef System (Thermo Fisher Scientific, Waltham, MA, USA).
Subsequently, the prepared libraries were sequenced on an Ion GeneStudio S5 Prime System (Thermo Fisher Scientific, Waltham, MA, USA) using the Ion 510™ & Ion 520™ & Ion 530™ Kit and the Ion 520 Chip Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Data analysis was performed using the Torrent Suite Software v.5.12.1 (Thermo Fisher Scientific, Waltham, MA, USA). Reads were aligned to the hg19 human reference genome. The mean average read depth and the percentage of reads that mapped to the region of interest (ROI) out of the total number of reads (reads on target) were calculated using the Coverage Analysis plugin (Torrent Suite v.5.12.1 software, Thermo Fisher Scientific, Waltham, MA, USA). For each sample, the percentage of the ROI with a minimum coverage of 20X was calculated using the amplicon coverage matrix file.
Gene-specific guidelines established by the Clinical Genome Consortium (ClinGen;
https://clinicalgenome.org/; accessed on 20 March 2025) were used to perform germline variant classification [
17]. The global population frequency of the identified genetic variants was retrieved from the gnomAD v4.1 dataset (
https://gnomad.broadinstitute.org/news/2024-04-gnomad-v4-1/; accessed on 24 June 2025) [
18]. Then, the genetic variants likely responsible for the clinical phenotype of the index cases were validated using Sanger sequencing. Specifically, to validate the presence of the identified
PMS2 variant, specific primers designed in genomic regions unique to the
PMS2 gene were used to perform a long-range PCR, as previously described [
19]. This method enables the preferential amplification of the
PMS2 gene and avoids interference from its homologous pseudogene [
19]. Successively, exon-specific amplification was performed using the primers and experimental conditions used by Vaughn et al. [
19]. Sanger sequencing was performed using the BigDye™ Terminator Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA), and each sample was analyzed with the SeqStudio™ Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. PREMM5 Risk Prediction
The PREdiction Model for gene Mutations (PREMM
5) is a clinical prediction algorithm that provides a comprehensive assessment of the risk of having LS. This model estimates the probability of the presence of a mutation in any of the five LS genes, supporting genetic testing in the case of a PREMM
5 score ≥ 2.5%. The PREMM
5 algorithm was used to quantify the overall LS risk (
https://premm.dfci.harvard.edu/; accessed on 24 June 2025) [
20].
2.4. Tumor Testing Screening
Genomic DNA was extracted from formalin-fixed paraffin-embedded (FFPE) CRC tissue sections from the index cases of Family 1 and Family 2 using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. MSI status was determined on FFPE tumor specimens using the Idylla MSI Test fully automated real-time PCR system (Biocartis, Mechel, Belgium) according to the manufacturer’s protocol and as previously described [
21]. Additionally, detection of the
BRAFV600E substitution on DNA extracted from FFPE CRC specimens was performed using the Idylla
BRAF Mutation Test (Biocartis, Mechel, Belgium) according to the manufacturer’s protocol and as previously described [
2]. Methylation analysis of the promoter region of the human
MLH1 gene was performed in two replicates for each DNA tumor sample by MS-MLPA using the ME011-Mismatch Repair Genes kit (MRC-Holland, Amsterdam, The Netherlands). Control leukocyte DNA specimens were included in this assay. MS-MLPA was performed according to the manufacturer’s instructions. PCR products were run on the SeqStudio™ Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). Data were analyzed using the Coffalyser.NET software v.250317.1029 (MRC Holland, Amsterdam, The Netherlands). The presence or absence of
MLH1 gene promoter methylation was determined as previously reported [
22]. For immunohistochemical analysis, an FFPE CRC tissue specimen of the index case of Family 1 was cut in sequential sections (4 µm thick). Immunohistochemical staining procedures were carried out on a BOND III automated immunostainer (Leica Biosystems, Deer Park, IL, USA), from deparaffinization to counterstaining with hematoxylin, using the Bond Polymer Refine Detection Kit (DS9800, Leica Biosystems, Deer Park, IL, USA). After deparaffinization, sections were rehydrated in dH2O and treated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. Then, they were incubated overnight with the primary antibody FLEX Monoclonal Mouse Anti-MLH1, Clone ES05 (GA079, Agilent Dako, Santa Clara, CA, USA), FLEX Monoclonal Mouse Anti-MSH2, Clone FE11 (GA085, Agilent Dako, Santa Clara, CA, USA), FLEX Monoclonal Rabbit Anti-MSH6, Clone EP49 (GA086, Agilent Dako, Santa Clara, CA, USA), and FLEX Monoclonal Rabbit Anti-PMS2, Clone EP51 (GA087, Agilent Dako, Santa Clara, CA, USA). Antigen retrieval was performed using the EnVition FLEX Target Solution, High pH (50×) (GV804, Agilent Dako, Santa Clara, CA, USA). Images were acquired using a Zeiss Axio Observer Z1 optical microscope (Carl Zeiss, Oberkochen, Germany). The expression pattern of the analyzed MMR proteins was considered normal when positive staining was detected in neoplastic cells. Conversely, the absence of nuclear staining, with positive internal control (normal mucosa or stromal cells, endothelial cells, lymphocytes), was considered defective for protein expression.
2.5. In Silico Analysis
The protein sequences of human PMS2 and homologous proteins from other species were aligned with the latest version of Align-GVGD (
http://agvgd.hci.utah.edu/, accessed on 20 April 2025 [
23]), a freely available, web-based program that combines the biophysical properties of amino acids with multiple protein sequence alignments to predict where missense mutations occur in selected genes, assessing their potential from highly deleterious to less deleterious to neutral. This alignment is based on two quantitative metrics for each missense variant: GV (Grantham Variation) and GD (Grantham Deviation). These scores are computed based on differences in amino acid composition, polarity, and molecular volume using the Grantham distance framework, with constants α = 1.833, β = 0.1018, and γ = 0.000399 and scaling factor ρ = 50.723, as described by Tavtigian et al. [
23].
The color coding of the alignment follows Taylor’s color code used by the Align-GVGD web-based program. In particular, Taylor’s classification groups amino acids based on their physicochemical properties, facilitating the interpretation of evolutionary conservation in multiple sequence alignments. This scheme is widely used in alignment visualization tools (e.g., Jalview, TexShade, CINEMA, and Align-GVGD) and enables the identification of conserved regions not only by identity but also by functional or structural similarity. In this system, the 20 standard amino acids are classified into 10 classes, primarily based on features such as size, charge, polarity, aromaticity, and hydrophobicity. Each Taylor’s class includes amino acids with similar characteristics indicated by the same color in the alignment [
24].
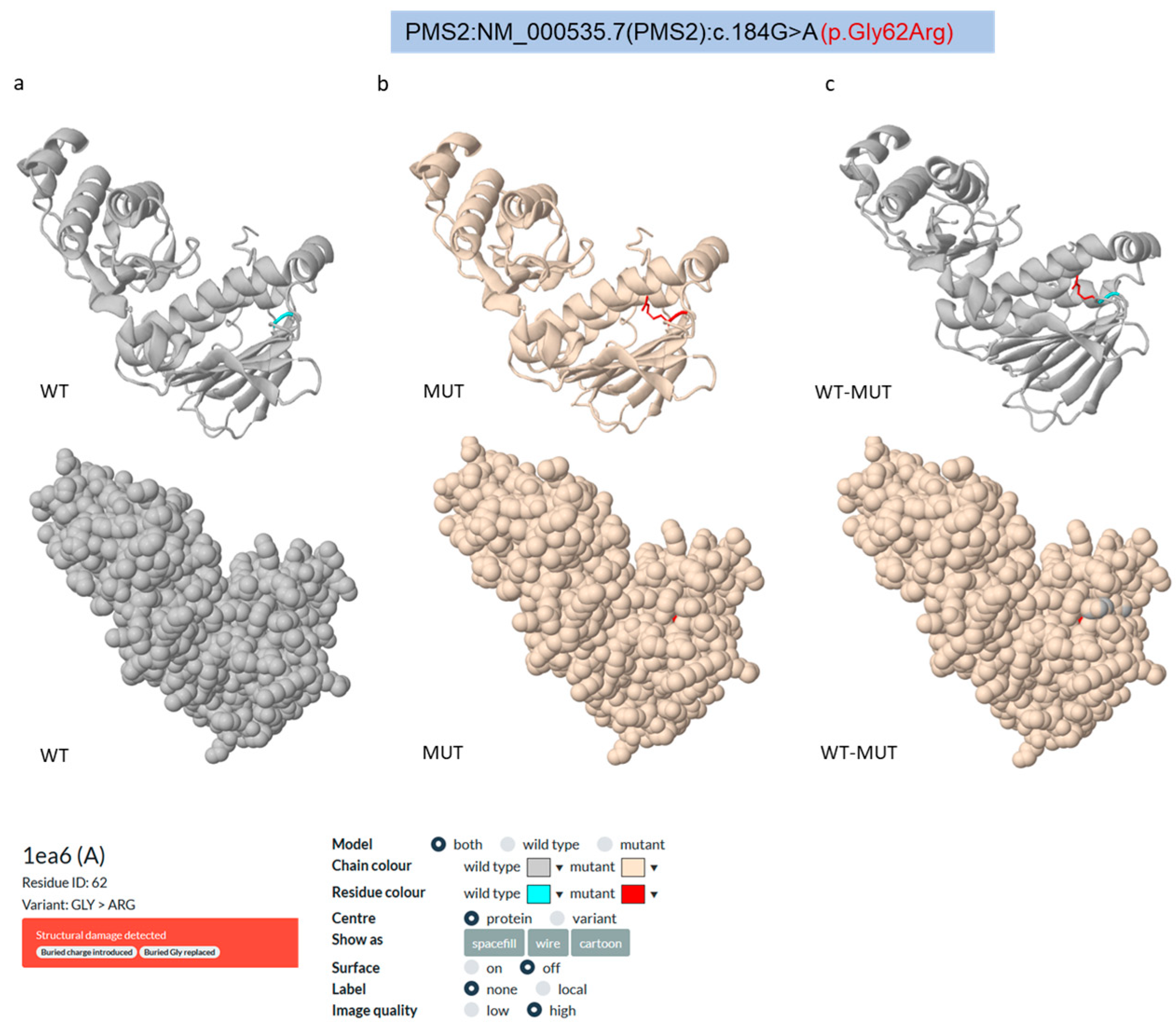
To investigate the structural and functional impact of the VUS (c.184G>A; p.Gly62Arg) identified in the
PMS2 gene, we assessed its effect on the molecular structure of the human PMS2 (hPMS2) protein (PDB entry: 1ea6, chain A; UniProt entry: P54278,
https://www.uniprot.org/uniprotkb/P54278/entry; accessed on 18 March 2025) through an in silico prediction meta-analysis using several computational tools, such as Missense 3D (
https://missense3d.bc.ic.ac.uk/; accessed on 20 March 2025 [
25]), Alpha Missense (
https://github.com/deepmind/alphamissense; accessed on 20 March 2025 [
26]), PremPS (
https://lilab.jysw.suda.edu.cn/research/PremPS/; accessed on 20 March 2025 [
27]), PolyPhen2 (
http://genetics.bwh.harvard.edu/pph2/; accessed on 20 March 2025 [
28]), PMut (
https://mmb.irbbarcelona.org/PMut/predictor/new/; accessed on 20 March 2025 [
29]), CUPSAT (
https://cupsat.brenda-enzymes.org/; accessed on 21 March 2025 [
30]), PROVEAN (
http://provean.jcvi.org/index.php; accessed on 21 March 2025 [
31]), mCSM (
https://biosig.lab.uq.edu.au/mcsm; accessed on 21 March 2025 [
32]), SDM (
https://compbio.medschl.cam.ac.uk/sdm2/; accessed on 21 March 2025 [
33]), DUET (
https://biosig.lab.uq.edu.au/duet/; accessed on 21 March 2025 [
34]), REVEL (
https://sites.google.com/site/revelgenomics/; accessed on 24 June 2025 [
35]), and MetaLR (
https://www.ensembl.org/info/genome/variation/prediction/protein_function.html#MetaLR; accessed on 24 June 2025 [
36]), which are based on different approaches and methodologies.
First, we used the Missense3D portal, a valuable server for predicting the structural consequences of missense variations on protein structure [
25]. The Missense3D tool predicts the structural impact of amino acid substitutions based on 16 critical features for protein structure, including the introduction of buried glycine or proline and charges, alterations in buried/exposed residues, introduction of buried hydrophilic residues, alteration of buried hydrogen bonds, switches in buried charges, replacement of cysteine with proline, disruption of a salt bridge, breakage of disulfide bonds, alterations in the secondary structure, and changes in cavity properties.
In 2023, Google DeepMind created AlphaMissense, an artificial intelligence (AI) model based on Alphafold v2.3.2. AlphaMissense predicts missense variant pathogenicity by combining structural context and amino acid evolutionary conservation. The algorithm assigns a score for pathogenicity prediction across various genetic and experimental criteria for each missense variant [
26].
PremPS (predicting the impact of missense mutations on protein stability) is an online computational method based on large-scale mutational scanning. It requires a few minutes to calculate the predicted stability score for a single amino acid variant per protein with ~300 residues [
27]. The PremPS approach consists of only ten evolutionary and structural variables, parameterized on a balanced dataset containing equal amounts of stabilizing and destabilizing variants [
27].
The PolyPhen2 server is an updated version of PolyPhen [
28]. The PolyPhen2 algorithm compares the sequence-based and structure-based predictive features of the wild-type (ancestral, normal) allele and the corresponding mutant (derived, disease-causing) allele, which together define an amino acid substitution [
28].
PMut is a tool that enables the quick identification of mutational hot regions. These regions can be found using genetically accessible mutations, large mutations, or alanine scanning. PMut has an 80% success rate in humans and can rapidly and reliably recognize the pathogenic nature of single-point amino acid changes [
29].
CUPSAT is an algorithm that predicts the structural impact of point mutations on protein stability. The prediction model evaluates the amino acid environment of the mutation site using torsion angle distribution and amino acid–atom potentials [
30].
PROVEAN v1.1.3 is a software that calculates sequence alignment scores, facilitating the development of precomputed predictions for 20 single amino acid changes and a single amino acid deletion at each amino acid position across all human and mouse protein sequences [
31,
37].
mCSM employs missense graph signatures that include atom–atom distance patterns to characterize the protein residue environment and develop prediction models. Each missense mutation is subsequently depicted as a signature vector, used to train and evaluate predictive machine learning algorithms for regression and classification tasks [
32]. SDM relies on statistical and potential energy (ΔΔG) variation to calculate a stability score using environment-specific amino acid substitution frequencies between homologous protein families [
33]. Finally, the DUET web server optimizes a predictor using support vector machines (SVMs) by combining the results of two complementary methodologies (mCSM and SDM) to provide a consensus prediction [
33]. In line with the “coincidence rule” criterion of the ClinGen Sequence Variant Interpretation Working Group (
https://clinicalgenome.org/working-groups/sequence-variant-interpretation; accessed on 24 June 2025) and recommended by the ACMG/AMP guidelines, rather than relying on individual outputs, we considered the variant as likely damaging or destabilizing only on the condition that the majority (≥70%) of the selected tools agreed on a predicted damaging effect. This consensus-based interpretation strategy improves overall prediction accuracy compared with each method alone and provides equal or superior performance with respect to similar methods [
38].
4. Discussion
The interpretation of a missense VUS in hereditary cancer-related genes, including those linked to LS, poses a significant diagnostic challenge in medical genetics [
40,
41]. Difficulties arise from limited clinical, molecular, and functional data related to the involvement of these variants in LS disease [
41]. As such, the identification of a germline VUS in patients with a personal and family history of cancer complicates risk assessment and clinical surveillance decisions [
42]. Based on current NCCN guidelines, patients with a germline VUS in genes associated with LS are not eligible for recommended clinical surveillance programs, which should only be based on patients’ family history (NCCN Clinical Practice Guidelines in Oncology for Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric. Version: 4.2024—2 April 2025. Available online at
https://www.nccn.org; accessed on 20 May 2025). In this study, we report the clinical and molecular characterization of a missense
PMS2 gene variant (NM_000535.7: c.184G>A; p.Gly62Arg) that was identified in two unrelated patients with a personal and family history of cancer. So far, several
PMS2 disease-causing variants deposited in the ClinVar database (
https://www.ncbi.nlm.nih.gov/clinvar?LinkName=gene_clinvar&from_uid=5395, accessed on 24 June 2025), including nonsense, frameshift, splicing, and missense variants, have been reported to be associated with LS clinical manifestation. However, the majority of these variants, most of which are missense variants, are classified as VUSs.
Several in silico tools have been developed to evaluate the impact of germline missense VUSs on protein structure and function [
42]. In this study, we performed an in silico prediction analysis of the structural and functional effects of the PMS2 Gly62Arg VUS using twelve tools based on different predictive algorithms, including structure-based (e.g., Missense3D, DUET, mCSM, SDM, PremPS, CUPSAT), sequence conservation- and function-based (e.g., PolyPhen2, PROVEAN, PMut), and machine learning ensemble approaches (e.g., AlphaMissense, Revel, and MetaLR). This strategy allowed us to evaluate various aspects of the impact of the variants (e.g., structural stability versus functional conservation), recognizing that no single tool is sufficient on its own [
43]. Interestingly, the PMS2 Gly62Arg variant was also evaluated using three of the in silico tools used to assess the PMS2 Gly62Arg variant, i.e., AlphaMissense, REVEL, and MetaLR are machine learning-based and yielded high scores indicative of potential pathogenicity (AlphaMissense = 0.9924; REVEL = 0.762; MetaLR = 0.8152), suggesting prediction consistency across different models. Of note, AlphaMissense, which incorporates protein structural data from AlphaFold2, has been shown to outperform other predictors, especially for rare variants in disease-associated genes [
26]. Accordingly, the integration of the structural context in the AlphaMissense prediction model provides added confidence in the results of our in silico analysis. Overall, these consensus in silico findings corroborate the potential pathogenic role of the PMS2 Gly62Arg variant, though further clinical and functional validation is required.
One important limitation of our multi-tool in silico analysis lies in the varying reliability of structure-based prediction tools, which may be affected by the quality, resolution, or availability of structural models. Several of the tools used in our analysis, such as Missense3D, mCSM, DUET, and SDM, rely on experimentally derived or computationally predicted protein structures (e.g., from PDB or AlphaFold) [
44]. For proteins with limited structural coverage, such as PMS2, predictions thus might be less accurate or unavailable for specific variants. Additionally, the heterogeneity of the algorithms—each evaluating different biophysical or evolutionary properties—may lead to discordant predictions.
For example, we are aware that tools, such as DUET and Missense3D, may occasionally yield conflicting results due to their differing underlying assumptions (e.g., atomic environment vs. statistical potential score). To mitigate the impact of individual tool biases, we employed a consensus-based approach, considering the identified variant as potentially pathogenic or destabilizing only on the condition that at least 70% of the tools agreed on a predicted damaging effect. While this approach is qualitative, it offers a practical and reproducible method for mitigating the impact of outliers and discrepancies between individual predictions. Moreover, we decided not to assign explicit weights to specific tools, as there is currently no universally accepted standard for weighting predictive tools, and their performance may vary depending on the protein family or mutational context. Anyway, we acknowledge that even consensus predictions remain probabilistic and should ideally be interpreted alongside experimental or clinical evidence, when available.
Recent studies have highlighted the value of combining in silico analysis with tumor immunohistochemistry (IHC) and molecular profiling to characterize genetic VUSs in MMR genes associated with LS [
45,
14]. Remarkably, a comprehensive systems biology study assessed 54 VUSs involving various MMR genes using multiple bioinformatics platforms. By integrating predictive scores with clinical and pathological features, the authors successfully reclassified a subset of variants as potentially pathogenic [
14]. The
PMS2 missense variant identified in the index cases of the present study (NM_000535.7: c.184G>A; p.Gly62Arg) has never been described in the literature in patients with a personal and/or family history of cancer. This variant has been reported in the ClinVar database in three different patients with hereditary cancer-predisposing syndromes and has been classified as a VUS. Both index cases harboring the PMS2 Gly62Arg missense variant developed late-onset CRC (66–79 years). Additionally, the index case of Family 1 developed an ampullary adenocarcinoma, and the index case of Family 2 developed a biliary tract cancer, which have recently been associated with the LS spectrum [
46,
47]. Additionally, two other LS-associated malignancies, i.e., prostate cancer and gynecological cancer (ovarian or endometrial), were diagnosed in these patients. Importantly, the families of both index cases include relatives with CRC , gastric cancer, leukemia, and lung cancer, suggesting an underlying hereditary cancer-predisposing syndrome. However, neither of the families involved in this study meets the diagnostic criteria established by the Amsterdam II or revised Bethesda guidelines. Specifically, the Amsterdam II criteria—which require at least three relatives with LS-associated cancers across two generations, with one diagnosed before age 50—were not met by either Family 1 or Family 2 [
48]. Moreover, various clinical criteria of the revised Bethesda guidelines were also not fulfilled, including (i) CRC diagnosed before the age of 50; (ii) CRC with MSI-H status in a family member under 60 years of age; and (iii) CRC diagnosed in an individual with two or more first- or second-degree relatives with LS-associated tumors, regardless of age [
49]. It is noteworthy that these criteria were primarily developed based on the clinical characteristics observed in families harboring
MLH1 and
MSH2 genetic variants and may not fully capture the cancer phenotypes associated with
PMS2 genetic variants.
Recently, the PREMM
5 statistical model has been applied in clinical practice to predict the likelihood of carrying a PV or LPV in DNA MMR genes [
20]. This model has shown good performance for
MLH1 and
MSH2 PV/LPV carriers, but only fair performance in discriminating
PMS2 PV/LPV carriers from non-carriers [
20]. The likelihood of having LS was identified as high (≥2.5%) in both Family 1 and Family 2. While neither family met the Amsterdam nor the revised Bethesda guidelines, the PREMM
5 model indicated that they both have an increased estimated probability of harboring an LS disease-causing variant, highlighting the utility of this prediction model to support genetic testing decisions.
To improve the identification of individuals with LS, a universal screening strategy, whereby all newly diagnosed CRC patients undergo either MSI testing or IHC assays for the loss of MMR proteins, has been analyzed [
50]. This approach has been demonstrated to achieve a sensitivity of 100% (95% CI, 99.3–100%) and a specificity of 93.0% (95% CI, 92.0–93.7%) for detecting individuals with LS [
50]. Our molecular analysis of microsatellite loci revealed that the tumors from both index cases exhibited a high level of microsatellite instability (MSI-high), consistent with an MMR-deficient phenotype. Additionally, IHC testing of CRC samples from the index case of Family 1 revealed a lack of PMS2 immunoreactivity, suggesting that the identified
PMS2 missense variant likely affects PMS2 protein expression. Together with the results from our in silico meta-analysis, these findings suggest that the Gly62Arg missense variant identified in this study negatively impacts PMS2 protein structure and stability, leading to reduced functional activity within the MMR system, which supports its potential clinical involvement in LS.
Taken together, these findings suggest that the Gly62Arg missense variant identified in this study negatively impacts PMS2 protein structure and stability, supporting its potential clinical involvement in LS.
Recent evidence highlights the importance of the ATPase domain at the N-terminal end of the PMS2 protein for its structural stability and biological function. In particular, a recent study investigated the effects of two
PMS2 VUSs (p.Gly207Glu and p.Leu42_Glu44del) and found that the p.Gly207Glu variant did not affect PMS2 endonuclease and ATPase activity in vitro. Conversely, the p.Leu42_Glu44del variant, which involves amino acids that are close to Gly62, caused a complete loss of both functions [
39]. Furthermore, the X-ray crystallography of the N-terminal domain of the PMS2 p.Leu42_Glu44del variant showed a disordered domain, leading to reduced protein expression levels [
39]. Despite substantial in silico and tumor characterization evidence, a limitation of our study is the lack of experimental in vitro validation of the functional role of the PMS2 Gly62Arg variant. In this light, additional studies are needed to better elucidate its impact on PMS2 stability. For instance, a knock-in cellular model could provide valuable data to better define the functional consequences of this variant in cell biology. Then, deep human proteomic profiling of this model, such as mass spectrometry-based analysis, could be used to further explore the effect of the Gly62Arg substitution on PMS2 post-translational modifications, thereby providing additional insight into how this variant may influence protein function and its potential role in LS pathogenesis. Another limitation of the present study is the small number of families used for the clinical and molecular characterization of the PMS2 Gly62Arg variant, which may reduce the generalizability of our findings. Further studies involving individuals carrying this variant would thus be helpful to support the validation of its potential clinical relevance in LS. Additionally, the integration of tumor genomic data evaluating PMS2 somatic alterations in affected families’ members carrying the germline PMS2 Gly62Arg variant may also contribute to establishing its pathogenic role in LS.
Based on our patients’ personal and family history, tumor pathology, and in silico protein structure analysis, the new PMS2 gene variant described in this paper is likely associated with hereditary LS. According to these results, in our view, individuals carrying the PMS2 Gly62Arg variant would benefit from personalized surveillance strategies based on current NCCN guidelines for LS patients carrying PV/LPVs in the PMS2 gene. These include a colonoscopy every 2 to 5 years starting at age 35 and a risk-reducing hysterectomy for EC prevention. Moreover, additional screening procedures should be tailored based on cancer occurrence in affected family members.