Toxicity Profiles of Antibody–Drug Conjugates: Synthesis and Graphical Insights to Optimize Patient-Centered Treatment Strategies for HER2-Negative Metastatic Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Trastuzumab-Deruxtecan (T-DXd)
3.1. Management of ILD
3.2. Prophylaxis of AE for T-DXd
4. Sacituzumab Govitecan (SG)
4.1. Prophylaxis of AE for SG
4.2. Management of AE
4.3. Graphical Representations Propositions to Guide Therapeutic Decision-Making
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Female Breast Cancer Subtypes—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 29 June 2025).
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Lipatov, O.; Im, S.-A.; Gonçalves, A.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; Ohtani, S.; et al. LBA21—KEYNOTE-119: Phase III Study of Pembrolizumab (Pembro) versus Single-Agent Chemotherapy (Chemo) for Metastatic Triple Negative Breast Cancer (mTNBC). Ann. Oncol. 2019, 30, v859–v860. [Google Scholar] [CrossRef]
- Géraud, A.; Gougis, P.; de Nonneville, A.; Beaufils, M.; Bertucci, F.; Billon, E.; Brisou, G.; Gravis, G.; Greillier, L.; Guerin, M.; et al. Pharmacology and Pharmacokinetics of Antibody-Drug Conjugates, Where Do We Stand? Cancer Treat. Rev. 2025, 135, 102922. [Google Scholar] [CrossRef]
- De Nonneville, A.; Goncalves, A.; Mamessier, E.; Bertucci, F. Sacituzumab Govitecan in Triple-Negative Breast Cancer. Ann. Transl. Med. 2022, 10, 647. [Google Scholar] [CrossRef]
- Elias, D.J.; Hirschowitz, L.; Kline, L.E.; Kroener, J.F.; Dillman, R.O.; Walker, L.E.; Robb, J.A.; Timms, R.M. Phase I Clinical Comparative Study of Monoclonal Antibody KS1/4 and KS1/4-Methotrexate Immunconjugate in Patients with Non-Small Cell Lung Carcinoma. Cancer Res. 1990, 50, 4154–4159. [Google Scholar]
- Saleh, M.N.; Sugarman, S.; Murray, J.; Ostroff, J.B.; Healey, D.; Jones, D.; Daniel, C.R.; LeBherz, D.; Brewer, H.; Onetto, N.; et al. Phase I Trial of the Anti-Lewis Y Drug Immunoconjugate BR96-Doxorubicin in Patients with Lewis Y-Expressing Epithelial Tumors. J. Clin. Oncol. 2000, 18, 2282–2292. [Google Scholar] [CrossRef]
- Amiri-Kordestani, L.; Blumenthal, G.M.; Xu, Q.C.; Zhang, L.; Tang, S.W.; Ha, L.; Weinberg, W.C.; Chi, B.; Candau-Chacon, R.; Hughes, P.; et al. FDA Approval: Ado-Trastuzumab Emtansine for the Treatment of Patients with HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2014, 20, 4436–4441. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- André, F.; Hee Park, Y.; Kim, S.-B.; Takano, T.; Im, S.-A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab Deruxtecan versus Treatment of Physician’s Choice in Patients with HER2-Positive Metastatic Breast Cancer (DESTINY-Breast02): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hu, X.; Dent, R.; Yonemori, K.; Barrios, C.H.; O’Shaughnessy, J.A.; Wildiers, H.; Pierga, J.-Y.; Zhang, Q.; Saura, C.; et al. Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. N. Engl. J. Med. 2024, 391, 2110–2122. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Overall Survival with Sacituzumab Govitecan in Hormone Receptor-Positive and Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer (TROPiCS-02): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Collineau, B.; Gonçalves, A.; Bertucci, F.; de Nonneville, A. Treatment-related adverse events associated with antibody drug conjugate in breast cancer. Bull. Cancer 2024, 111, 765–781. [Google Scholar] [CrossRef]
- Barry, M.J.; Edgman-Levitan, S. Shared Decision Making—The Pinnacle of Patient-Centered Care. N. Engl. J. Med. 2012, 366, 780–781. [Google Scholar] [CrossRef]
- Shay, L.A.; Lafata, J.E. Where Is the Evidence? A Systematic Review of Shared Decision Making and Patient Outcomes. Med. Decis. Mak. 2015, 35, 114–131. [Google Scholar] [CrossRef]
- Bomhof-Roordink, H.; Gärtner, F.R.; Stiggelbout, A.M.; Pieterse, A.H. Key Components of Shared Decision Making Models: A Systematic Review. BMJ Open 2019, 9, e031763. [Google Scholar] [CrossRef] [PubMed]
- Coombs, L.A.; Crowder, V.; Black, M.; Tan, K.; Ray, E.M.; Ferrari, R.M.; Kent, E.E.; Reuland, D.S.; Bryant, A.L. Co-Creating and Refining a Values Assessment Tool (VAsT) for Women With Metastatic Breast Cancer. Psychooncology 2025, 34, e70173. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Elwyn, G.; Percac-Lima, S.; Grande, S.; Durand, M.-A. Assessing the Acceptability and Feasibility of Encounter Decision Aids for Early Stage Breast Cancer Targeted at Underserved Patients. BMC Med. Inf. Decis. Mak. 2016, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Sargent, D.J.; Köhne, C.H.; Sanoff, H.K.; Bot, B.M.; Seymour, M.T.; de Gramont, A.; Porschen, R.; Saltz, L.B.; Rougier, P.; Tournigand, C.; et al. Pooled Safety and Efficacy Analysis Examining the Effect of Performance Status on Outcomes in Nine First-Line Treatment Trials Using Individual Data From Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 1948–1955. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Corti, C.; Giugliano, F.; Nicolò, E.; Ascione, L.; Curigliano, G. Antibody-Drug Conjugates for the Treatment of Breast Cancer. Cancers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Chen, N.; Michaels, E.; Howard, F.; Nanda, R. The Evolving Therapeutic Landscape of Antibody–Drug Conjugates in Breast Cancer. Expert. Rev. Anticancer. Ther. 2022, 22, 1325–1331. [Google Scholar] [CrossRef]
- Jerusalem, G.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Modi, S.; Andre, F.; Krop, I.E.; Gonzàlez Farré, X.; You, B.; Saura, C.; et al. Trastuzumab Deruxtecan in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A DESTINY-Breast01 Subgroup Analysis. Cancer Discov. 2022, 12, 2754–2762. [Google Scholar] [CrossRef]
- Tarantino, P.; Modi, S.; Tolaney, S.M.; Cortés, J.; Hamilton, E.P.; Kim, S.-B.; Toi, M.; Andrè, F.; Curigliano, G. Interstitial Lung Disease Induced by Anti-ERBB2 Antibody-Drug Conjugates: A Review. JAMA Oncol. 2021, 7, 1873–1881. [Google Scholar] [CrossRef]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, Pharmacokinetics, and Antitumour Activity of Trastuzumab Deruxtecan (DS-8201), a HER2-Targeting Antibody-Drug Conjugate, in Patients with Advanced Breast and Gastric or Gastro-Oesophageal Tumours: A Phase 1 Dose-Escalation Study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef]
- Rugo, H.S.; Crossno, C.L.; Gesthalter, Y.B.; Kelley, K.; Moore, H.N.; Rimawi, M.F.; Westbrook, K.E.; Buys, S.S. Real-World Perspectives and Practices for Pneumonitis/Interstitial Lung Disease Associated With Trastuzumab Deruxtecan Use in Human Epidermal Growth Factor Receptor 2–Expressing Metastatic Breast Cancer. JCO Oncol. Pr. 2023, 19, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bianchini, G.; Cortes, J.; Henning, J.-W.; Untch, M. Optimizing Treatment Management of Trastuzumab Deruxtecan in Clinical Practice of Breast Cancer. ESMO Open 2022, 7, 100553. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Tolaney, S.M. Clinical Development of New Antibody-Drug Conjugates in Breast Cancer: To Infinity and Beyond. BioDrugs 2021, 35, 159–174. [Google Scholar] [CrossRef]
- Spring, L.M.; Nakajima, E.; Hutchinson, J.; Viscosi, E.; Blouin, G.; Weekes, C.; Rugo, H.; Moy, B.; Bardia, A. Sacituzumab Govitecan for Metastatic Triple-Negative Breast Cancer: Clinical Overview and Management of Potential Toxicities. Oncologist 2021, 26, 827–834. [Google Scholar] [CrossRef]
- Poumeaud, F.; Morisseau, M.; Cabel, L.; Gonçalves, A.; Rivier, C.; Trédan, O.; Volant, E.; Frenel, J.-S.; Ladoire, S.; Jacot, W.; et al. Efficacy of Administration Sequence: Sacituzumab Govitecan and Trastuzumab Deruxtecan in HER2-Low Metastatic Breast Cancer. Br. J. Cancer 2024, 131, 702–708. [Google Scholar] [CrossRef]
- Guidi, L.; Boldrini, L.; Trapani, D.; Curigliano, G. Antibody-Drug Conjugates in Metastatic Breast Cancer: Sequencing, Combinations and Resistances. Curr. Opin. Oncol. 2024, 36, 487. [Google Scholar] [CrossRef]
- Bertucci, F.; de Nonneville, A.; Finetti, P.; Cohendet, A.; Guille, A.; Mamessier, E. The Co-Expression of Antigen Targets as a Rationale for ADC Combination in Urothelial Cancer. Ann. Oncol. 2024, 35, 477–478. [Google Scholar] [CrossRef]
- Bardia, A.; Jhaveri, K.; Kalinsky, K.; Pernas, S.; Tsurutani, J.; Xu, B.; Hamilton, E.; Im, S.-A.; Nowecki, Z.; Sohn, J.; et al. TROPION-Breast01: Datopotamab Deruxtecan vs Chemotherapy in Pre-Treated Inoperable or Metastatic HR+/HER2– Breast Cancer. Future Oncol. 2024, 20, 423–436. [Google Scholar] [CrossRef]
- Spira, A.; Lisberg, A.; Sands, J.; Greenberg, J.; Phillips, P.; Guevara, F.; Tajima, N.; Kawasaki, Y.; Gu, J.; Kobayashi, F.; et al. OA03.03 Datopotamab Deruxtecan (Dato-DXd; DS-1062), a TROP2 ADC, in Patients With Advanced NSCLC: Updated Results of TROPION-PanTumor01 Phase 1 Study. J. Thorac. Oncol. 2021, 16, S106–S107. [Google Scholar] [CrossRef]
- Krop, I.E.; Masuda, N.; Mukohara, T.; Takahashi, S.; Nakayama, T.; Inoue, K.; Iwata, H.; Yamamoto, Y.; Alvarez, R.H.; Toyama, T.; et al. Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3–Directed Antibody-Drug Conjugate, in Patients With Previously Treated Human Epidermal Growth Factor Receptor 3–Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial. JCO 2023, 41, 5550–5560. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, B.; Pierotti, L.; Lacroix-Triki, M.; Vicier, C.; Frenel, J.-S.; D’Hondt, V.; Dalenc, F.; Bachelot, T.; Ducoulombier, A.; Benderra, M.-A.; et al. 340O Efficacy, Safety and Biomarker Analysis of ICARUS-BREAST01: A Phase II Study of Patritumab Deruxtecan (HER3-DXd) in Patients (Pts) with HR+/HER2- Advanced Breast Cancer (ABC). Ann. Oncol. 2024, 35, S357. [Google Scholar] [CrossRef]
- Hahlweg, P.; Lindig, A.; Frerichs, W.; Zill, J.; Hanken, H.; Müller, V.; Peters, M.-C.; Scholl, I. Major Influencing Factors on Routine Implementation of Shared Decision-Making in Cancer Care: Qualitative Process Evaluation of a Stepped-Wedge Cluster Randomized Trial. BMC Health Serv. Res. 2023, 23, 840. [Google Scholar] [CrossRef] [PubMed]
- French National Authority for Health (Haute Autorité de Santé). Developing a Shared Decision-Making Aid: Methodological Guide. Saint-Denis La Plaine: HAS. 2018. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2018-03/elaborer_une_aide_a_la_prise_de_decision_partagee_mel.pdf (accessed on 8 July 2025).
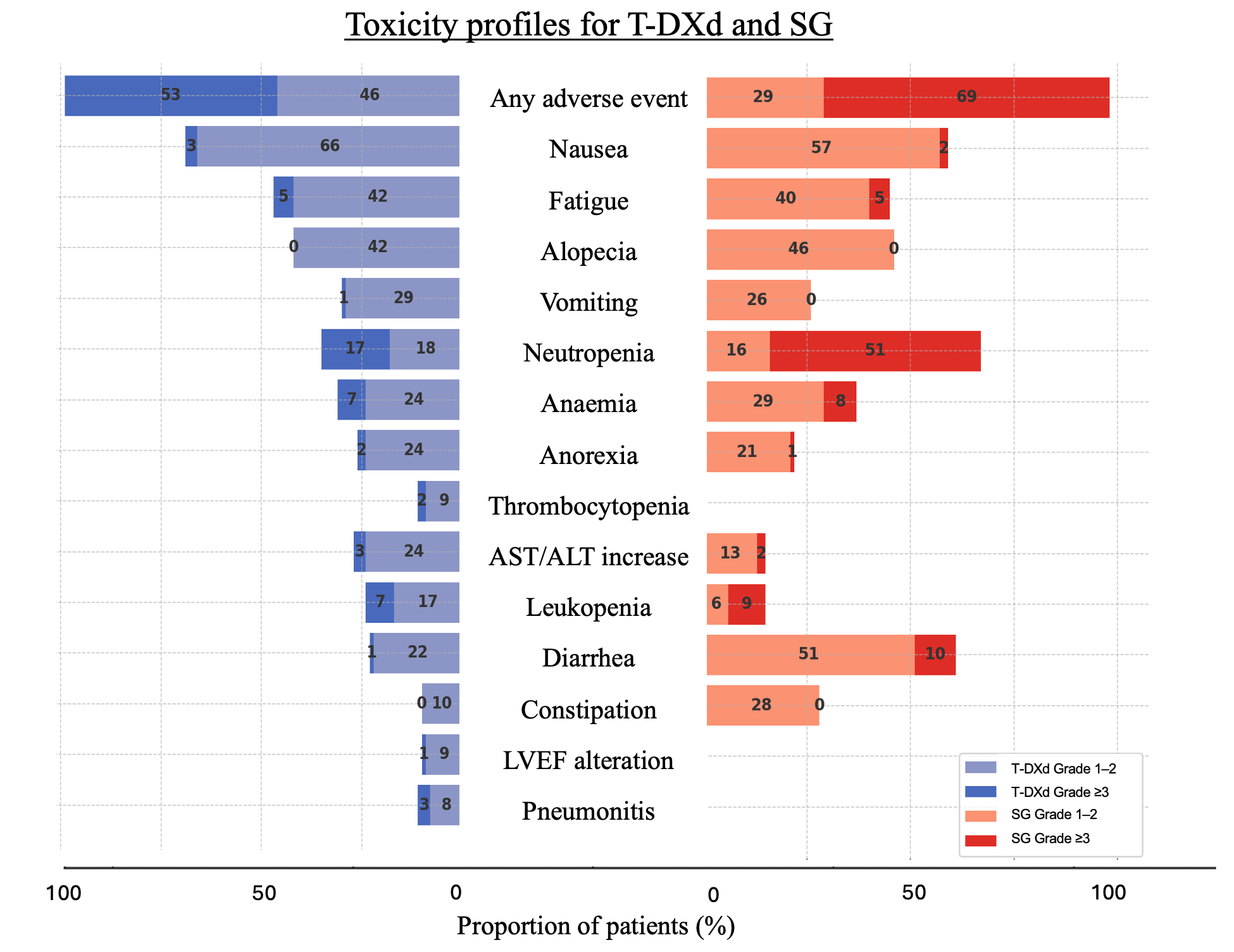
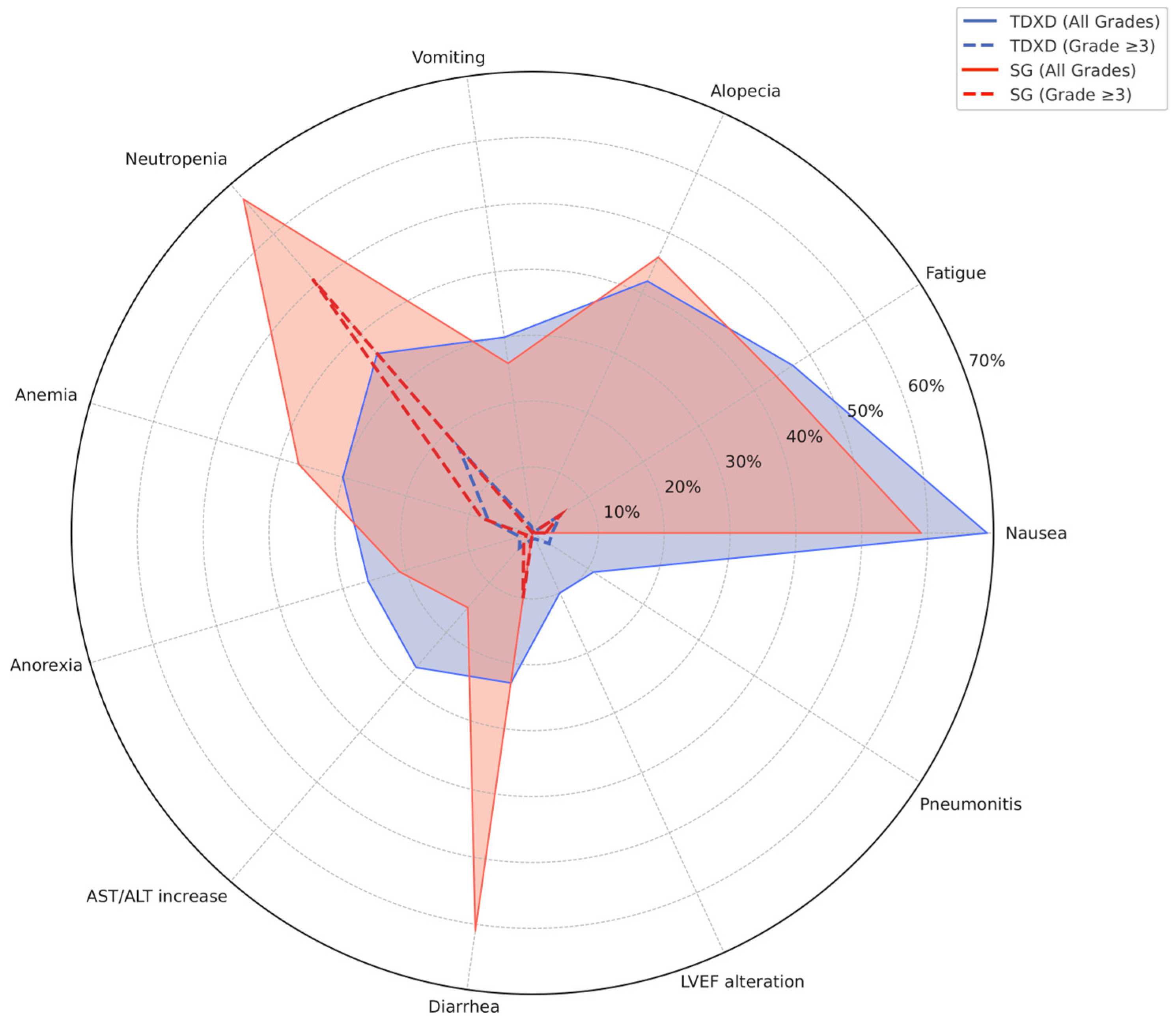
| All Grade (%) | Grade ≥ 3 (%) | |||||
|---|---|---|---|---|---|---|
| wMean | DB04 | DB06 | wMean | DB04 | DB06 | |
| Any adverse event | 99.1 | 99.5 | 98.8 | 52.7 | 52.6 | 52.8 |
| Nausea | 69.2 | 73 | 65.9 | 3.0 | 4.6 | 1.6 |
| Fatigue | 47.2 | 47.7 | 46.8 | 5.5 | 7.5 | 3.7 |
| Alopecia | 41.9 | 37.7 | 45.4 | 0 | 0 | 0 |
| Neutropenia | 35.6 | 33.2 | 37.6 | 17.5 | 13.7 | 20.7 |
| Anemia | 30.5 | 33.2 | 28.1 | 6.9 | 8.1 | 5.8 |
| Vomiting | 30.3 | 34 | 27.2 | 1.4 | 1.3 | 1.4 |
| AST/ALT increase | 26.7 | 23.5 | 29.5 | 2.7 | 3.2 | 2.3 |
| Anorexia | 25.9 | 28.6 | 23.5 | 1.9 | 2.4 | 1.4 |
| Thrombocytopenia | 23.7 | 23.7 | NA | 5.1 | 5.1 | NA |
| Leukopenia | 23.3 | 23.2 | 23.3 | 6.7 | 6.5 | 6.9 |
| Diarrhea | 23.1 | 22.4 | 23.7 | 1.5 | 1.1 | 1.8 |
| Constipation | 21.3 | 21.3 | NA | 0.0 | 0 | NA |
| Pneumonitis | 10.7 | 10 | 11.3 | 2.6 | 4.1 | 1.4 |
| LVEF alteration | 9.9 | 11.9 | 8.1 | 1.1 | 1.5 | 0.7 |
| All Grade (%) | Grade ≥ 3 (%) | |||||
|---|---|---|---|---|---|---|
| wMean | ASCENT | TROPICS-02 | wMean | ASCENT | TROPIC-02 | |
| Neutropenia | 67.1 | 64 | 70 | 51.3 | 52 | 51 |
| Diarrhea | 60.8 | 65 | 57 | 10.3 | 11 | 9 |
| Nausea | 58.7 | 62 | 55 | 2.1 | 3 | 1 |
| Alopecia | 46.4 | 47 | 46 | 0 | 0 | 0 |
| Fatigue | 44.5 | 52 | 38 | 4.9 | 4 | 6 |
| Anemia | 36.7 | 40 | 34 | 7.8 | 9 | 6 |
| Constipation | 27.8 | 37 | 19 | 0.2 | 0 | 0 |
| Vomiting | 26.0 | 33 | 19 | 1.0 | 3 | 0 |
| Anorexia | 21.5 | 28 | 16 | 1.0 | 2 | 0 |
| Asthenia | 17.7 | 16 | 20 | 1.7 | 2 | 2 |
| Abdominal pain | 16.9 | 21 | 13 | 1.7 | 3 | 1 |
| Leukopenia | 15.2 | 17 | 14 | 9.3 | 10 | 9 |
| Lymphopenia | 12.0 | NA | 12 | 4.0 | NA | 4 |
| Neuropathy | 9.0 | NA | 9 | 1.0 | NA | 1 |
| Febrile neutropenia | 5.5 | 6 | 5 | 5.5 | 6 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collineau, B.; Gonçalves, A.; Domon, M.; Bruyat, D.; Bertucci, F.; de Nonneville, A. Toxicity Profiles of Antibody–Drug Conjugates: Synthesis and Graphical Insights to Optimize Patient-Centered Treatment Strategies for HER2-Negative Metastatic Breast Cancer. Cancers 2025, 17, 2307. https://doi.org/10.3390/cancers17142307
Collineau B, Gonçalves A, Domon M, Bruyat D, Bertucci F, de Nonneville A. Toxicity Profiles of Antibody–Drug Conjugates: Synthesis and Graphical Insights to Optimize Patient-Centered Treatment Strategies for HER2-Negative Metastatic Breast Cancer. Cancers. 2025; 17(14):2307. https://doi.org/10.3390/cancers17142307
Chicago/Turabian StyleCollineau, Bérénice, Anthony Gonçalves, Marie Domon, Damien Bruyat, François Bertucci, and Alexandre de Nonneville. 2025. "Toxicity Profiles of Antibody–Drug Conjugates: Synthesis and Graphical Insights to Optimize Patient-Centered Treatment Strategies for HER2-Negative Metastatic Breast Cancer" Cancers 17, no. 14: 2307. https://doi.org/10.3390/cancers17142307
APA StyleCollineau, B., Gonçalves, A., Domon, M., Bruyat, D., Bertucci, F., & de Nonneville, A. (2025). Toxicity Profiles of Antibody–Drug Conjugates: Synthesis and Graphical Insights to Optimize Patient-Centered Treatment Strategies for HER2-Negative Metastatic Breast Cancer. Cancers, 17(14), 2307. https://doi.org/10.3390/cancers17142307







