What Cachexia-Related Outcomes Are Measured in Lung Cancer Chemotherapy Clinical Trials?
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Trial Eligibility
2.2. Data Extraction, Items and Synthesis
2.3. Statistical Analysis
3. Results
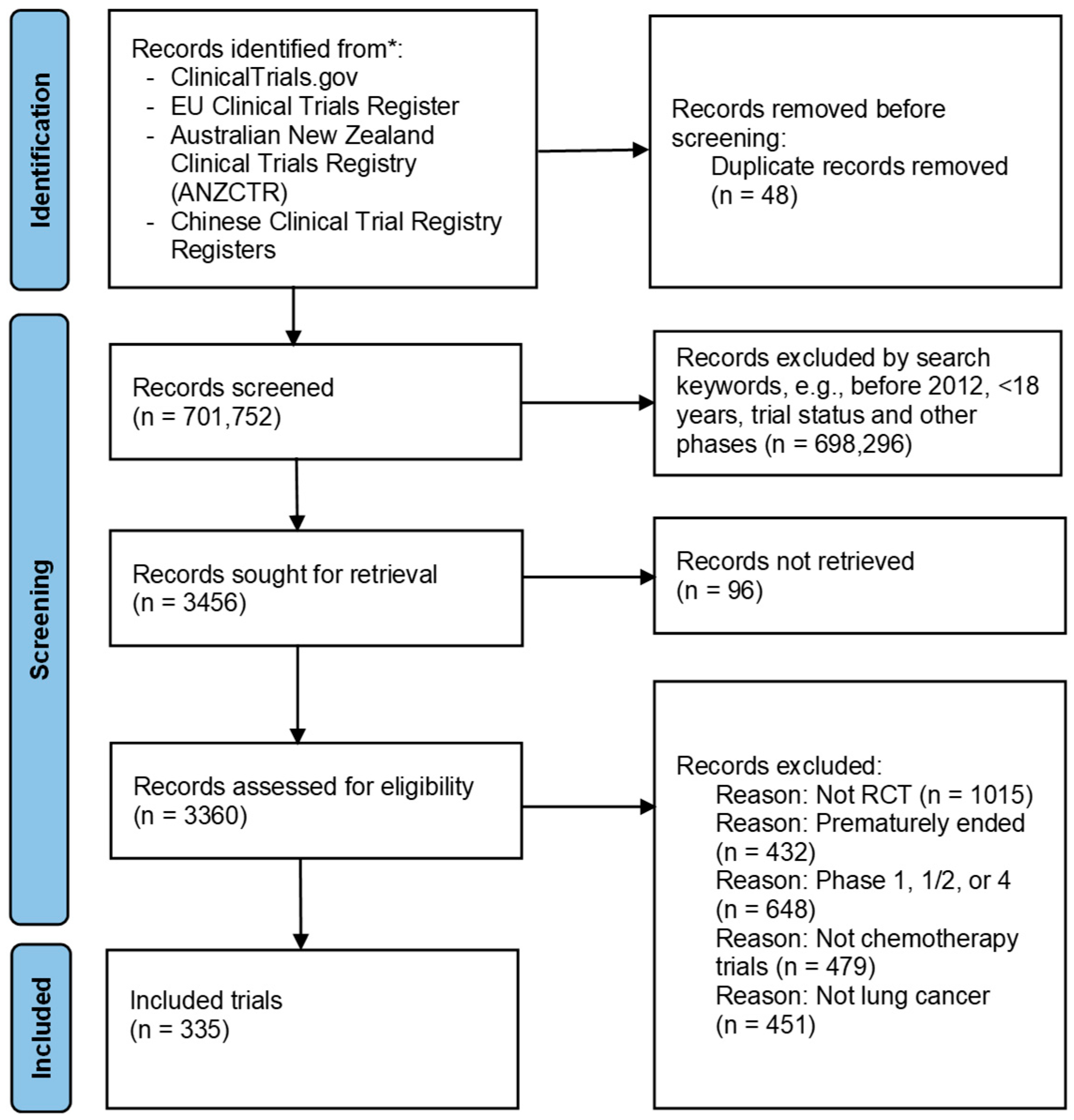
3.1. Search and Screening Process of the Registered Trials
3.2. Trial Characteristics
3.3. Trial Outcomes (Measures and Symptoms Reported)
3.3.1. Associations Between Trial Outcomes and Trial Characteristics
Trial Outcomes Across Lung Cancer Type
Trial Outcomes Across Trial Location
Trial Outcomes Across Lead Investigator
3.3.2. Changes in Outcomes Reported in Trials by Trial Commencement Year
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CXI | Cachexia index |
| ECOG | Eastern Cooperative Oncology Group |
| EORTC QLQ-C30 | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire |
| FAACT ACS | Functional Assessment of Anorexia/Cachexia Therapy Anorexia Cachexia Subscale |
| NCI CTCAE | National Cancer Institute’s Common Terminology Criteria for Adverse Events |
| NSCLC | Non-small-cell lung cancer |
| PROs | Patient-reported outcomes |
| RCTs | Randomised controlled trials |
| SCLC | Small-cell lung cancer |
References
- World Health Organization. Lung Cancer. 26 June 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 6 June 2024).
- Kimura, M.; Naito, T.; Kenmotsu, H.; Taira, T.; Wakuda, K.; Oyakawa, T.; Hisamatsu, Y.; Tokito, T.; Imai, H.; Akamatsu, H. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support. Care Cancer 2015, 23, 1699–1708. [Google Scholar] [CrossRef]
- Morley, J.E.; Thomas, D.R.; Wilson, M.-M.G. Cachexia: Pathophysiology and clinical relevance1, 2. Am. J. Clin. Nutr. 2006, 83, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, Z.; Jiao, R.; Zhang, C.; Yu, Q.; Han, S.; Duan, Z. Updates on the pathogenesis of advanced lung cancer-induced cachexia. Thorac. Cancer 2019, 10, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Agar, M.; Razmovski-Naumovski, V. Cachexia, Anorexia, and Feeding Difficulties in Palliative Care Patients. In Textbook of Palliative Care; Springer: Cham, Switzerland, 2025; pp. 287–307. [Google Scholar]
- Shiono, M.; Huang, K.; Downey, R.J.; Consul, N.; Villanueva, N.; Beck, K.; Fenn, K.; Dietz, D.; Yamaguchi, T.; Kato, S. An analysis of the relationship between metastases and cachexia in lung cancer patients. Cancer Med. 2016, 5, 2641–2648. [Google Scholar] [CrossRef]
- LeBlanc, T.W.; Nipp, R.D.; Rushing, C.N.; Samsa, G.P.; Locke, S.C.; Kamal, A.H.; Cella, D.F.; Abernethy, A.P. Correlation between the international consensus definition of the Cancer Anorexia-Cachexia Syndrome (CACS) and patient-centered outcomes in advanced non-small cell lung cancer. J. Pain Symptom Manag. 2015, 49, 680–689. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Willmann, J.; Olaechea, S.; Gilmore, B.W.; Dee, E.C.; Rao, M.; Gannavarapu, B.S.; Venkateswaran, S.; Alvarez, C.M.; Ahn, C. Prevalence and development of cachexia before and after diagnosis of non–small cell lung cancer. Am. J. Clin. Oncol. 2025. [Google Scholar] [CrossRef]
- Morita-Tanaka, S.; Yamada, T.; Takayama, K. The landscape of cancer cachexia in advanced non-small cell lung cancer: A narrative review. Transl. Lung Cancer Res. 2023, 12, 168–180. [Google Scholar] [CrossRef]
- Coletti, D. Chemotherapy-induced muscle wasting: An update. Eur. J. Transl. Myol. 2018, 28, 7587. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: A narrative review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Wong, M.L.; Paul, S.M.; Cooper, B.A.; Dunn, L.B.; Hammer, M.J.; Conley, Y.P.; Wright, F.; Levine, J.D.; Walter, L.C.; Cartwright, F. Predictors of the multidimensional symptom experience of lung cancer patients receiving chemotherapy. Support. Care Cancer 2017, 25, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.; Eisen, T.; Smith, I.; O’Brien, M. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef]
- Go, S.-I.; Park, M.J.; Lee, G.-W. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer 2021, 21, 563. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Okayama, T.; Aoyama, T.; Ohashi, T.; Masuda, Y.; Kimura, M.; Shiozaki, H.; Murakami, H.; Kenmotsu, H.; Taira, T. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non–small-cell lung cancer. BMC Cancer 2017, 17, 571. [Google Scholar] [CrossRef] [PubMed]
- Aktas, A.; Couto, M.M.; Walsh, D. We report performance status in oncology-but not nutritional status? Support. Care Cancer 2020, 28, 5605–5607. [Google Scholar] [CrossRef]
- Valaire, R.; Garden, F.; Razmovski-Naumovski, V. Are measures and related symptoms of cachexia recorded as outcomes in gastrointestinal cancer chemotherapy clinical trials? J. Cachexia Sarcopenia Muscle 2024, 15, 1146–1156. [Google Scholar] [CrossRef]
- Den Kamp, C.O.; Langen, R.; Minnaard, R.; Kelders, M.; Snepvangers, F.; Hesselink, M.; Dingemans, A.; Schols, A. Pre-cachexia in patients with stages I–III non-small cell lung cancer: Systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer 2012, 76, 112–117. [Google Scholar] [CrossRef]
- Del Ferraro, C.; Grant, M.; Koczywas, M.; Dorr-Uyemura, L.A. Management of anorexia-cachexia in late stage lung cancer patients. J. Hosp. Palliat. Nurs. JHPN Off. J. Hosp. Palliat. Nurses Assoc. 2012, 14, 397–402. [Google Scholar] [CrossRef]
- Royle, K.-L.; Meads, D.; Visser-Rogers, J.K.; White, I.R.; Cairns, D.A. How is overall survival assessed in randomised clinical trials in cancer and are subsequent treatment lines considered? A systematic review. Trials 2023, 24, 708. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. US National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 28 June 2025).
- Australia New Zealand Clinical Trials Registry. Available online: https://anzctr.org.au/Default.aspx (accessed on 28 June 2025).
- EU Clinical Trials Registry. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search (accessed on 28 June 2025).
- Wu Taixiang, L.Y. Chinese Clinical Trial Registry. 2005. Available online: https://www.chictr.org.cn/indexEN.html (accessed on 28 June 2025).
- Naito, T. Evaluation of the true endpoint of clinical trials for cancer cachexia. Asia-Pac. J. Oncol. Nurs. 2019, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Campelj, D.G.; Goodman, C.A.; Rybalka, E. Chemotherapy-induced myopathy: The dark side of the cachexia sphere. Cancers 2021, 13, 3615. [Google Scholar] [CrossRef]
- Oswalt, C.; Liu, Y.; Pang, H.; Le-Rademacher, J.; Wang, X.; Crawford, J. Associations between body mass index, weight loss and overall survival in patients with advanced lung cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.; Lopez, C.; Wolfe, E.; Foster, N.R.; Mandrekar, S.J.; Wang, X.; Kumar, R.; Adjei, A.; Jatoi, A. Weight loss over time and survival: A landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J. Cachexia Sarcopenia Muscle 2020, 11, 1501–1508. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 25 August 2024).
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cehreli, R.; Yavuzsen, T.; Ates, H.; Akman, T.; Ellidokuz, H.; Oztop, I. Can inflammatory and nutritional serum markers predict chemotherapy outcomes and survival in advanced stage nonsmall cell lung cancer patients? BioMed Res. Int. 2019, 2019, 1648072. [Google Scholar] [CrossRef]
- Liu, C.-A.; Liu, T.; Li, H.-C.; Song, M.-M.; Ge, Y.-Z.; Ruan, G.-T.; Deng, L.; Zhang, Q.; Xie, H.-L.; Lin, S.-Q. Nutrition impact symptoms: Noteworthy prognostic indicators for lung cancer. Clin. Nutr. 2023, 42, 550–558. [Google Scholar] [CrossRef]
- Lin, T.; Yang, J.; Hong, X.; Yang, Z.; Ge, T.; Wang, M. Nutritional status in patients with advanced lung cancer undergoing chemotherapy: A prospective observational study. Nutr. Cancer 2020, 72, 1225–1230. [Google Scholar] [CrossRef]
- Doshita, K.; Naito, T.; Matsuda, S.; Morita, M.; Sekikawa, M.; Miura, K.; Kodama, H.; Yabe, M.; Morikawa, N.; Iida, Y. Exploring the relationship between anorexia and therapeutic efficacy in advanced lung cancer treatment: A retrospective study. Thorac. Cancer 2024, 15, 1831–1841. [Google Scholar] [CrossRef]
- Carnio, S.; Di Stefano, R.F.; Novello, S. Fatigue in lung cancer patients: Symptom burden and management of challenges. Lung Cancer: Targets Ther. 2016, 7, 73–82. [Google Scholar] [CrossRef]
- de Jong, C.; Chargi, N.; Herder, G.J.; van Haarlem, S.W.; van der Meer, F.; van Lindert, A.S.; Ten Heuvel, A.; Brouwer, J.; de Jong, P.A.; Devriese, L.A. The association between skeletal muscle measures and chemotherapy-induced toxicity in non-small cell lung cancer patients. J. Cachexia Sarcopenia Muscle 2022, 13, 1554–1564. [Google Scholar] [CrossRef]
- Kinsey, E.; Ajazi, E.; Wang, X.; Johnston, M.A.M.; Crawford, J. Predictors of physical and functional loss in advanced-stage lung cancer patients receiving platinum chemotherapy. J. Thorac. Oncol. 2018, 13, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.E.; Mazzola, E.; Xiong, N.; Hong, F.; Lobach, D.F.; Braun, I.M.; Halpenny, B.; Rabin, M.S.; Johns, E.; Finn, K. Clinical decision support for symptom management in lung cancer patients: A group RCT. J. Pain Symptom Manag. 2022, 63, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Anggondowati, T.; Deviany, P.; Ryan, J.; Fetrick, A.; Bagenda, D.; Copur, M.; Tolentino, A.; Vaziri, I.; McKean, H. Patient preferences of chemotherapy treatment options and tolerance of chemotherapy side effects in advanced stage lung cancer. BMC Cancer 2019, 19, 835. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, T.W.; Nickolich, M.; Rushing, C.N.; Samsa, G.P.; Locke, S.C.; Abernethy, A.P. What bothers lung cancer patients the most? A prospective, longitudinal electronic patient-reported outcomes study in advanced non-small cell lung cancer. Support. Care Cancer 2015, 23, 3455–3463. [Google Scholar] [CrossRef]
- Tsao, T.C.; Chen, C.-H.; Chang, J.W.; Lee, C.-H. Weekly short infusion of taxotere at a 4 week cycle in Chinese patients with advanced NSCLC who have failed or relapsed after the frontline platinum-based non-taxane chemotherapy—A phase II trial. Jpn. J. Clin. Oncol. 2006, 36, 80–84. [Google Scholar] [CrossRef]
- Konwinska, M.D.; Mehr, K.; Owecka, M.; Kulczyk, T. Oral health status in patients undergoing chemotherapy for lung cancer. Open J. Dent. Oral Med. 2014, 2, 17–21. [Google Scholar] [CrossRef]
- Hopkinson, J.B. The psychosocial components of multimodal interventions offered to people with cancer cachexia: A scoping review. Asia-Pac. J. Oncol. Nurs. 2021, 8, 450–461. [Google Scholar] [CrossRef]
- Maddocks, M.; Hopkinson, J.; Conibear, J.; Reeves, A.; Shaw, C.; Fearon, K.C. Practical multimodal care for cancer cachexia. Curr. Opin. Support. Palliat. Care 2016, 10, 298–305. [Google Scholar] [CrossRef]
- Garcia, J.M.; Dunne, R.F.; Santiago, K.; Martin, L.; Birnbaum, M.J.; Crawford, J.; Hendifar, A.E.; Kochanczyk, M.; Moravek, C.; Piccinin, D.; et al. Addressing unmet needs for people with cancer cachexia: Recommendations from a multistakeholder workshop. J. Cachexia Sarcopenia Muscle 2022, 13, 1418–1425. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Mendoza, T.R.; Wang, X.S.; Woodruff, J.F.; Palos, G.R.; Richman, S.P.; Nazario, A.; Lynch, G.R.; Liao, K.-P.; Mobley, G.M. Levels of symptom burden during chemotherapy for advanced lung cancer: Differences between public hospitals and a tertiary cancer center. J. Clin. Oncol. 2011, 29, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer progress and priorities: Lung cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Ravina, A.; Provencio, M.; de Juan, V.C.; Carcereny, E.; Moran, T.; Rodriguez-Abreu, D.; Lopez-Castro, R.; Albite, E.C.; Guirado, M.; González, L.G. Lung cancer symptoms at diagnosis: Results of a nationwide registry study. ESMO Open 2020, 5, e001021. [Google Scholar] [CrossRef] [PubMed]
- Akin, S.; Can, G.; Aydiner, A.; Ozdilli, K.; Durna, Z. Quality of life, symptom experience and distress of lung cancer patients undergoing chemotherapy. Eur. J. Oncol. Nurs. 2010, 14, 400–409. [Google Scholar] [CrossRef]
- European Commission-European Cancer Information System. 2022 New Cancer Cases and Cancer Deaths on the Rise in the EU. 2022. Available online: https://ecis.jrc.ec.europa.eu/sites/default/files/2024-01/jrc_CancerEstimates2022_factsheet.pdf (accessed on 28 June 2025).
- Huang, J.; Ngai, C.H.; Deng, Y.; Tin, M.S.; Lok, V.; Zhang, L.; Yuan, J.; Xu, W.; Zheng, Z.-J.; Wong, M.C. Cancer incidence and mortality in Asian countries: A trend analysis. Cancer Control 2022, 29, 10732748221095955. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; King, M.T.; Calvert, M.J.; Stockler, M.R.; Friedlander, M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 2018, 9, 353–367. [Google Scholar] [CrossRef]
- Begum, M.; Urquhart, I.; Lewison, G.; Fouad, F.; Sullivan, R. Research on lung cancer and its funding, 2004–2018. Ecancermedicalscience 2020, 14, 1132. [Google Scholar] [CrossRef]
- Aggarwal, A.; Lewison, G.; Idir, S.; Peters, M.; Aldige, C.; Boerckel, W.; Boyle, P.; Trimble, E.L.; Roe, P.; Sethi, T. The state of lung cancer research: A global analysis. J. Thorac. Oncol. 2016, 11, 1040–1050. [Google Scholar] [CrossRef]
- Marlow, L.A.; Waller, J.; Wardle, J. Does lung cancer attract greater stigma than other cancer types? Lung Cancer 2015, 88, 104–107. [Google Scholar] [CrossRef]
- Lilenbaum, R.C.; Cashy, J.; Hensing, T.A.; Young, S.; Cella, D. Prevalence of poor performance status in lung cancer patients: Implications for research. J. Thorac. Oncol. 2008, 3, 125–129. [Google Scholar] [CrossRef]
- Gajra, A.; Marr, A.S.; Ganti, A.K. Management of patients with lung cancer and poor performance status. J. Natl. Compr. Cancer Netw. 2014, 12, 1015–1025. [Google Scholar] [CrossRef]
- Sakuragi, T.; Oshita, F.; Nagashima, S.; Kasai, T.; Kurata, T.; Fukuda, M.; Yamamoto, N.; Ohe, Y.; Tamura, T.; Eguchi, K. Retrospective analysis of the treatment of patients with small cell lung cancer showing poor performance status. Jpn. J. Clin. Oncol. 1996, 26, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.R.; Previgliano, C.; Khandelwal, K.; Shi, R. Cachexia index in advanced non-small-cell lung cancer patients. Clin. Med. Insights Oncol. 2015, 9, CMO-S30891. [Google Scholar] [CrossRef] [PubMed]
- Fiteni, F.; Anota, A.; Westeel, V.; Bonnetain, F. Methodology of health-related quality of life analysis in phase III advanced non-small-cell lung cancer clinical trials: A critical review. BMC Cancer 2016, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Mannion, E.; Gilmartin, J.; Donnellan, P.; Keane, M.; Waldron, D. Effect of chemotherapy on quality of life in patients with non-small cell lung cancer. Support. Care Cancer 2014, 22, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, T.W.; Samsa, G.P.; Wolf, S.P.; Locke, S.C.; Cella, D.F.; Abernethy, A.P. Validation and real-world assessment of the Functional Assessment of Anorexia-Cachexia Therapy (FAACT) scale in patients with advanced non-small cell lung cancer and the cancer anorexia-cachexia syndrome (CACS). Support. Care Cancer 2015, 23, 2341–2347. [Google Scholar] [CrossRef]
- Ferrara, D.; Abenavoli, E.M.; Beyer, T.; Gruenert, S.; Hacker, M.; Hesse, S.; Hofmann, L.; Pusitz, S.; Rullmann, M.; Sabri, O. Detection of cancer-associated cachexia in lung cancer patients using whole-body [18F] FDG-PET/CT imaging: A multi-centre study. J. Cachexia Sarcopenia Muscle 2024, 15, 2375–2386. [Google Scholar] [CrossRef]
- Takaoka, T.; Yaegashi, A.; Watanabe, D. Prevalence of and survival with cachexia among patients with cancer: A systematic review and meta-analysis. Adv. Nutr. 2024, 15, 100282. [Google Scholar] [CrossRef]
- Boughey, J.C.; Snyder, R.A.; Kantor, O.; Zheng, L.; Chawla, A.; Nguyen, T.T.; Hillman, S.L.; Hahn, O.M.; Mandrekar, S.J.; Roland, C.L. Impact of the COVID-19 pandemic on cancer clinical trials. Ann. Surg. Oncol. 2021, 28, 7311–7316. [Google Scholar] [CrossRef]
| Characteristics of Clinical Trials | Number of Trials n (%) |
|---|---|
| Cancer type | |
| Non-Small Cell | 292 (87.2) |
| Small Cell | 43 (12.8) |
| Trial location | |
| Europe | 169 (50.4) |
| Asia | 95 (28.4) |
| North America | 69 (20.6) |
| Australasia | 2 (0.6) |
| Trial type | |
| Open | 236 (70.4) |
| Blinded | 99 (29.6) |
| Year commenced (actual or estimated a) | |
| 2012 | 25 (7.5) |
| 2013 | 16 (4.8) |
| 2014 | 18 (5.4) |
| 2015 | 34 (10.1) |
| 2016 | 16 (4.8) |
| 2017 | 31 (9.3) |
| 2018 | 30 (9.0) |
| 2019 | 33 (9.9) |
| 2020 | 52 (15.5) |
| 2021 | 31 (9.3) |
| 2022 | 38 (11.3) |
| 2023 | 11 (3.3) |
| Trial length (years) | |
| 0–1 | 5 (1.5) |
| 2 | 32 (9.6) |
| 3 | 66 (19.7) |
| 4 | 58 (17.3) |
| 5 | 66 (19.7) |
| 5–10 | 99 (29.6) |
| 10+ | 9 (2.7) |
| Lead investigator/funding source | |
| Industry | 190 (56.7) |
| Academia | 84 (25.1) |
| Clinic | 56 (16.7) |
| Government | 5 (1.5) |
| Allowed medication | |
| Yes | 10 (3.0) |
| None stated | 325 (97.0) |
| Allied health professional involvement | |
| Yes | 2 (0.6) |
| Not stated | 333 (99.4) |
| Performance status (at eligibility) | |
| Yes | 313 (93.4) |
| Not stated | 22 (6.6) |
| Number of trials using assessment tools | |
| Yes | 140 (41.8) |
| Not stated | 195 (58.2) |
| Number of trials with | |
| 1 assessment tool | 73 |
| 2 or more assessment tools | 67 |
| Performance Status | Frequency (%) | |
|---|---|---|
| Eastern Cooperative Oncology Group (ECOG) | 275 (82.1) | |
| WHO Performance Status | 27 (8.1) | |
| Karnofsky Performance Status | 6 (1.8) | |
| Medical evaluation and/or weight loss | 5 (1.5) | |
| None or not stated | 22 (6.6) | |
| Assessment tool a | 233 | Outcomes examined (number) b |
| European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30) | 101 (30.1) | Physical activity (6), Sleep/fatigue (3), Pain (2), Nausea (1), Vomiting (1), Diarrhoea (1), Constipation (1) and Appetite (1) |
| European Organisation for Research and Treatment of Lung Cancer (EORTC QLQ-LC13) | 61 (18.2) | Pain (3), Dyspnoea (3), Dysphagia (1) and Oral mucositis (1) |
| EuroQol Group 5 Dimensions 5 Dimension 5 Level (EQ-5D-5L) | 23 (6.9) | Physical activity (3), Pain (1) |
| Functional Assessment of Cancer Therapy–Lung (FACT-L (36 items)) | 6 (1.8) | Dyspnoea (1), Pain (1), Weight (1), Appetite (1), Sleep (1), Nausea (1), Fatigue (1) and Physical activity (1) |
| Non-Small Cell Lung Cancer Symptom Assessment Question (NSCLC-SAQ) | 4 (1.2) | Pain (2), Fatigue (2), Dyspnoea (1), Appetite (1) and Cough (1) |
| Lung Cancer Symptom Scale (LCS) c | 3 (0.9) | Pain (2), Fatigue (2), Dyspnoea (2), Appetite (2) and Physical activity (1) |
| European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire in Elderly (EORTC QLQ-ELD14) | 3 (0.9) | Physical activity (3), Pain (1) |
| M. D. Anderson Symptom Inventory Lung Cancer (MDASI-LC) | 2 (0.6) | Physical activity (3), Sleep (2), Fatigue (1), Pain (1), Nausea (1), Vomiting (1), Diarrhoea (1), Dry mouth (1), Dyspnoea (1), Constipation (1) and Appetite loss (1) |
| Others d | 14 (4.2) | |
| Not specified e | 16 (4.8) | |
| Not stated | 195 (58.2) |
| Variable | Cancer Type | Trial Location | Trial Lead Investigator/Funding Source | Assessment Tools | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Yes/No | Number n = 335 | Non-Small Cell n = 292 | Small Cell n = 43 | p-Value | Europe n = 169 | Asia n = 95 | North America n = 69 | Australasia n = 2 | p-Value | Industry n = 190 | Academia n = 84 | Clinic n = 56 | Government n = 5 | p-Value | Yes n = 140 | No n = 195 | p-Value | |
| Measures | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||||
| Overall survival | Yes | 323 (96.4) | 283 (96.9) | 40 (93.0) | 0.200 | 166 (98.2) | 90 (94.7) | 65 (94.2) | 2 (100) | 0.330 | 183 (96.3) | 80 (95.2) | 55 (98.2) | 5 (100) | 0.788 | 139 (99.3) | 184 (94.4) | 0.017 * | |
| No | 12 (3.6) | 9 (3.1) | 3 (7.0) | 3 (1.8) | 5 (5.3) | 4 (5.8) | 0 (0.0) | 7 (3.7) | 4 (4.8) | 1 (1.8) | 0 (0.0) | 1 (0.7) | 11 (5.6) | ||||||
| Toxicity/side effects a | Yes | 281 (83.9) | 245 (83.9) | 36 (83.7) | 0.976 | 148 (87.6) | 70 (73.7) | 61 (88.4) | 2 (100) | 0.015 * | 170 (89.5) | 66 (78.6) | 40 (71.4) | 5 (100) | 0.004 * | 131 (93.6) | 150 (76.9) | 0.000 * | |
| No | 54 (16.1) | 47 (16.1) | 7 (16.3) | 21 (12.4) | 25 (26.3) | 8 (11.6) | 0 (0.0) | 20 (10.5) | 18 (21.4) | 16 (28.6) | 0 (0.0) | 9 (6.4) | 45 (23.1) | ||||||
| Physical activity | Yes | 98 (29.3) | 89 (30.5) | 9 (20.9) | 0.199 | 67 (39.6) | 17 (17.9) | 14 (20.3) | 0 (0.0) | 0.000 * | 65 (34.2) | 21 (25.0) | 11 (19.6) | 1 (20.0) | 0.127 | 87 (62.1) | 11 (5.6) | 0.000* | |
| No | 237 (70.7) | 203 (69.5) | 34 (79.1) | 102 (61.8) | 78 (82.1) | 55 (87.5) | 2 (100.0) | 125 (65.8) | 63 (75.0) | 45 (80.4) | 4 (80.0) | 53 (37.9) | 184 (94.4) | ||||||
| Weight/BMI | Yes | 85 (25.4) | 75 (25.7) | 10 (23.3) | 0.733 | 49 (29.0) | 16 (16.8) | 20 (29.0) | 0 (0.0) | 0.113 | 66 (34.7) | 11 (13.1) | 6 (10.7) | 2 (40.0) | 0.000 * | 30 (21.4) | 55 (28.2) | 0.160 | |
| No | 250 (74.6) | 217 (74.3) | 33 (76.7) | 120 (71.0) | 79 (83.2) | 49 (71.0) | 2 (100.0) | 124 (65.3) | 73 (86.9) | 50 (89.3) | 3 (60.0) | 110 (78.6) | 140 (71.8) | ||||||
| Dietary limitations | Yes | 6 (1.8) | 6 (2.1) | 0 (0.0) | 0.343 | 4 (2.4) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0.644 | 2 (1.1) | 3 (3.6) | 1 (1.8) | 0 (0.0) | 0.533 | 2 (1.4) | 4 (2.1) | 0.672 | |
| No | 329 (98.2) | 286 (97.9) | 43 (100.0) | 165 (97.6) | 93 (97.9) | 69 (100) | 2 (10.0) | 188 (98.9) | 81 (96.4) | 55 (98.2) | 5 (100.0) | 138 (98.6) | 191 (97.9) | ||||||
| Caloric intake | Yes | 9 (2.7) | 9 (3.1) | 0 (0.0) | 0.243 | 4 (2.4) | 2 (2.1) | 2 (2.9) | 1 (50.0) | 0.001 * | 3 (1.6) | 4 (4.8) | 2 (3.6) | 0 (0.0) | 0.461 | 7 (5.0) | 2 (1.0) | 0.026 * | |
| No | 326 (97.3) | 283 (96.9) | 43 (100.0) | 165 (97.6) | 93 (97.9) | 67 (97.1) | 1 (50.0) | 187 (98.4) | 80 (95.2) | 54 (96.4) | 5 (100) | 133 (95.0) | 193 (99.0) | ||||||
| Lean muscle mass | Yes | 3 (0.9) | 3 (1.0) | 0 (0.0) | 0.504 | 1 (0.6) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0.497 | 2 (1.1) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0.876 | 2 (1.4) | 1 (0.5) | 0.380 | |
| No | 332 (99.1) | 289 (99.0) | 43 (100.0) | 168 (99.4) | 93 (97.9) | 69 (100) | 2 (100.0) | 188 (98.9) | 83 (98.8) | 56 (100) | 5 (100.0) | 138 (98.6) | 194 (99.5) | ||||||
| Symptoms | |||||||||||||||||||
| Appetite loss | Yes | 150 (44.8) | 133 (45.5) | 17 (39.5) | 0.459 | 91 (53.8) | 33 (34.7) | 26 (37.7) | 0 (0.0) | 0.006 * | 102 (53.7) | 27 (32.1) | 20 (35.7) | 1 (20.0) | 0.002 * | 105 (75.0) | 45 (23.1) | 0.000 * | |
| No | 185 (55.2) | 159 (54.5) | 26 (60.5) | 78 (46.2) | 62 (65.3) | 43 (62.3) | 2 (100.0) | 88 (46.3) | 57 (67.9) | 36 (64.3) | 4 (80.0) | 35 (25.0) | 150 (76.9) | ||||||
| Diarrhoea | Yes | 255 (76.1) | 226 (77.4) | 29 (67.4) | 0.153 | 140 (82.8) | 58 (61.1) | 55 (79.7) | 2 (100.0) | 0.001 * | 157 (82.6) | 59 (70.2) | 37 (66.1) | 2 (40.0) | 0.005 * | 128 (91.4) | 127 (65.1) | 0.000 * | |
| No | 80 (23.9) | 66 (22.6) | 14 (32.6) | 29 (17.2) | 37 (38.9) | 14 (20.3) | 0 (0.0) | 33 (17.4) | 25 (29.8) | 19 (33.9) | 3 (60.0) | 12 (8.6) | 68 (34.9) | ||||||
| Pain | Yes | 249 (74.3) | 218 (74.7) | 31 (72.1) | 0.719 | 137 (81.1) | 57 (60.0) | 53 (76.8) | 2 (100) | 0.002 * | 154 (81.1) | 57 (67.9) | 34 (60.7) | 4 (80.0) | 0.008 * | 127 (90.7) | 122 (62.6) | 0.000 * | |
| No | 86 (25.7) | 74 (25.3) | 12 (27.9) | 32 (18.9) | 38 (40.0) | 16 (23.2) | 0 (0.0) | 36 (18.9) | 27 (32.1) | 22 (39.3) | 1 (20.0) | 13 (9.3) | 73 (37.4) | ||||||
| Fatigue/insomnia | Yes | 250 (74.6) | 221 (75.7) | 29 (67.4) | 0.246 | 137 (81.1) | 57 (60.0) | 54 (78.3) | 2 (100) | 0.001 * | 156 (82.1) | 57 (67.9) | 35 (62.5) | 2 (40.0) | 0.002 * | 128 (91.4) | 122 (62.6) | 0.000 * | |
| No | 85 (25.4) | 71 (24.3) | 14 (32.6) | 32 (18.9) | 38 (40.0) | 15 (21.7) | 0 (0.0) | 34 (17.9) | 27 (32.1) | 21 (37.5) | 3 (60.0) | 12 (8.6) | 73 (37.4) | ||||||
| Constipation | Yes | 253 (75.5) | 225 (77.1) | 28 (65.1) | 0.089 | 138 (81.7) | 58 (61.1) | 55 (79.7) | 2 (100.0) | 0.001 * | 157 (82.6) | 58 (69.0) | 36 (64.3) | 2 (40.0) | 0.002 * | 126 (90.0) | 127 (65.1) | 0.000 * | |
| No | 82 (24.5) | 67 (22.9) | 15 (34.9) | 31 (18.3) | 37 (38.9) | 14 (20.3) | 0 (0.0) | 33 (17.4) | 26 (31.0) | 20 (35.7) | 3 (60.0) | 14 (10.0) | 68 (34.9) | ||||||
| Nausea | Yes | 258 (77.0) | 229 (78.4) | 29 (67.4) | 0.110 | 141 (83.4) | 60 (63.2) | 55 (79.7) | 2 (100) | 0.002 * | 158 (83.2) | 61 (72.6) | 37 (66.1) | 2 (40.0) | 0.006 * | 129 (92.1) | 129 (66.2) | 0.000 * | |
| No | 77 (23.0) | 63 (21.6) | 14 (32.6) | 28 (16.6) | 35 (36.8) | 14 (20.3) | 0 (0.0) | 32 (16.8) | 23 (27.4) | 19 (33.9) | 3 (60.0) | 11 (7.9) | 66 (33.8) | ||||||
| Vomiting | Yes | 255 (76.1) | 227 (77.7) | 28 (65.1) | 0.070 | 140 (82.8) | 58 (61.1) | 55 (79.7) | 2 (100) | 0.001 * | 158 (83.2) | 60 (71.4) | 35 (62.5) | 2 (40.0) | 0.001 * | 128 (91.4) | 127 (65.1) | 0.000 * | |
| No | 80 (23.9) | 65 (22.3) | 15 (34.9) | 29 (17.2) | 37 (38.9) | 14 (20.3) | 0 (0.0) | 32 (16.8) | 24 (28.6) | 21 (37.5) | 3 (60.0) | 12 (8.6) | 68 (34.9) | ||||||
| Dysphagia | Yes | 59 (17.6) | 53 (18.2) | 6 (14.0) | 0.500 | 36 (21.3) | 7 (7.4) | 16 (23.2) | 0 (0.0) | 0.016 * | 43 (22.6) | 9 (10.7) | 6 (10.7) | 1 (20.0) | 0.048 * | 23 (16.4) | 36 (18.5) | 0.630 | |
| No | 276 (82.4) | 239 (81.8) | 37 (86.0) | 133 (78.7) | 88 (92.6) | 53 (76.8) | 2 (100) | 147 (77.4) | 75 (89.3) | 50 (89.3) | 4 (80.0) | 117 (83.6) | 159 (81.5) | ||||||
| Dyspnoea | Yes | 67 (20.0) | 63 (21.6) | 4 (9.3) | 0.060 | 42 (24.9) | 13 (13.7) | 10 (14.5) | 2 (100.0) | 0.003 * | 44 (23.2) | 12 (14.3) | 10 (17.9) | 1 (20.0) | 0.383 | 67 (47.9) | 0 (0) | 0.000 * | |
| No | 268 (80.0) | 229 (78.4) | 39 (90.7) | 127 (75.1) | 82 (86.3) | 59 (85.5) | 0 (0.0) | 146 (76.8) | 72 (85.7) | 46 (82.1) | 4 (80.0) | 73 (52.1) | 195 (100.0) | ||||||
| Oral mucositis | Yes | 78 (23.3) | 69 (23.6) | 9 (20.9) | 0.696 | 46 (27.2) | 10 (10.5) | 22 (31.9) | 0 (0.0) | 0.004 * | 57 (30.0) | 12 (14.3) | 8 (14.3) | 1 (20.0) | 0.011 * | 28 (20.0) | 50 (25.6) | 0.228 | |
| No | 257 (76.7) | 223 (76.4) | 34 (79.1) | 123 (72.8) | 85 (89.5) | 47 (68.1) | 2 (100.0) | 133 (70.0) | 72 (85.7) | 48 (85.7) | 4 (80.0) | 112 (80.0) | 145 (74.4) | ||||||
| Year | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures (as %) | |||||||||||||
| Overall survival | 100 | 93.7 | 100 | 97.1 | 93.7 | 93.5 | 96.7 | 93.9 | 98.1 | 96.8 | 97.4 | 90.0 | 0.925 |
| Toxicity/side effects | 84.0 | 87.5 | 88.9 | 85.3 | 81.3 | 90.3 | 83.3 | 87.9 | 76.9 | 77.4 | 81.6 | 100 | 0.795 |
| Physical activity | 20.0 | 12.5 | 5.6 | 14.2 | 25.0 | 16.1 | 43.3 | 33.3 | 50.0 | 29.0 | 36.8 | 27.3 | 0.002 * |
| Weight/BMI | 32.0 | 56.3 | 61.1 | 55.9 | 50.0 | 45.2 | 26.7 | 9.1 | 7.7 | 0.0 | 0.0 | 9.1 | 0.000 * |
| Dietary limitations | 4.0 | 0.0 | 0.0 | 0.0 | 12.5 | 0.0 | 6.7 | 0.0 | 1.9 | 0 | 0.0 | 0.0 | 0.060 |
| Caloric intake | 4.0 | 0.0 | 0.0 | 0.0 | 6.3 | 3.2 | 6.7 | 0.0 | 3.8 | 3.2 | 0.0 | 0.0 | 0.860 |
| Lean muscle mass | 0.0 | 0.0 | 0.0 | 0.0 | 6.2 | 0.0 | 3.3 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.536 |
| Symptoms (as %) | |||||||||||||
| Appetite loss | 40.0 | 50.0 | 50.0 | 44.1 | 43.8 | 58.1 | 53.3 | 48.6 | 53.8 | 25.8 | 36.8 | 9.1 | 0.115 |
| Diarrhoea | 68.0 | 75.0 | 77.8 | 82.4 | 81.3 | 77.4 | 76.7 | 84.8 | 78.8 | 77.4 | 78.9 | 90.9 | 0.001 * |
| Pain | 72.0 | 62.5 | 77.8 | 73.5 | 81.3 | 74.2 | 76.7 | 78.8 | 76.9 | 74.2 | 78.9 | 36.4 | 0.433 |
| Fatigue/insomnia | 72.0 | 68.7 | 77.8 | 67.6 | 81.3 | 77.4 | 76.7 | 87.9 | 76.9 | 77.4 | 78.9 | 9.1 | 0.001 * |
| Constipation | 68.0 | 68.8 | 77.8 | 82.4 | 81.3 | 74.2 | 76.7 | 84.8 | 78.8 | 77.4 | 78.9 | 9.1 | 0.001 * |
| Nausea | 72.0 | 81.3 | 77.8 | 82.4 | 87.5 | 77.4 | 76.7 | 84.8 | 78.8 | 77.4 | 78.9 | 9.1 | 0.001 * |
| Vomiting | 72.0 | 75.0 | 77.8 | 82.4 | 87.5 | 74.2 | 76.7 | 84.8 | 76.9 | 77.4 | 78.9 | 9.1 | 0.001 * |
| Oral mucositis | 36.0 | 43.7 | 61.1 | 50.0 | 43.8 | 41.9 | 6.7 | 15.2 | 9.6 | 6.5 | 0.0 | 0.0 | 0.000 * |
| Dysphagia | 28.0 | 31.3 | 38.9 | 47.1 | 18.8 | 32.3 | 16.7 | 6.1 | 7.7 | 0.0 | 31.6 | 0.0 | 0.005 * |
| Dyspnoea | 16.0 | 0.0 | 5.6 | 14.7 | 6.3 | 12.9 | 26.7 | 30.3 | 34.6 | 12.9 | 0.0 | 0.0 | 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razmovski-Naumovski, V.; Tanous, A.; Valaire, R. What Cachexia-Related Outcomes Are Measured in Lung Cancer Chemotherapy Clinical Trials? Cancers 2025, 17, 2309. https://doi.org/10.3390/cancers17142309
Razmovski-Naumovski V, Tanous A, Valaire R. What Cachexia-Related Outcomes Are Measured in Lung Cancer Chemotherapy Clinical Trials? Cancers. 2025; 17(14):2309. https://doi.org/10.3390/cancers17142309
Chicago/Turabian StyleRazmovski-Naumovski, Valentina, Anthony Tanous, and Ross Valaire. 2025. "What Cachexia-Related Outcomes Are Measured in Lung Cancer Chemotherapy Clinical Trials?" Cancers 17, no. 14: 2309. https://doi.org/10.3390/cancers17142309
APA StyleRazmovski-Naumovski, V., Tanous, A., & Valaire, R. (2025). What Cachexia-Related Outcomes Are Measured in Lung Cancer Chemotherapy Clinical Trials? Cancers, 17(14), 2309. https://doi.org/10.3390/cancers17142309






