Redefining the Fight Against SCLC: Standards, Innovations, and New Horizons
Simple Summary
Abstract
1. Introduction
1.1. Epidemiology
1.2. Pathology
1.3. Diagnosis and Staging
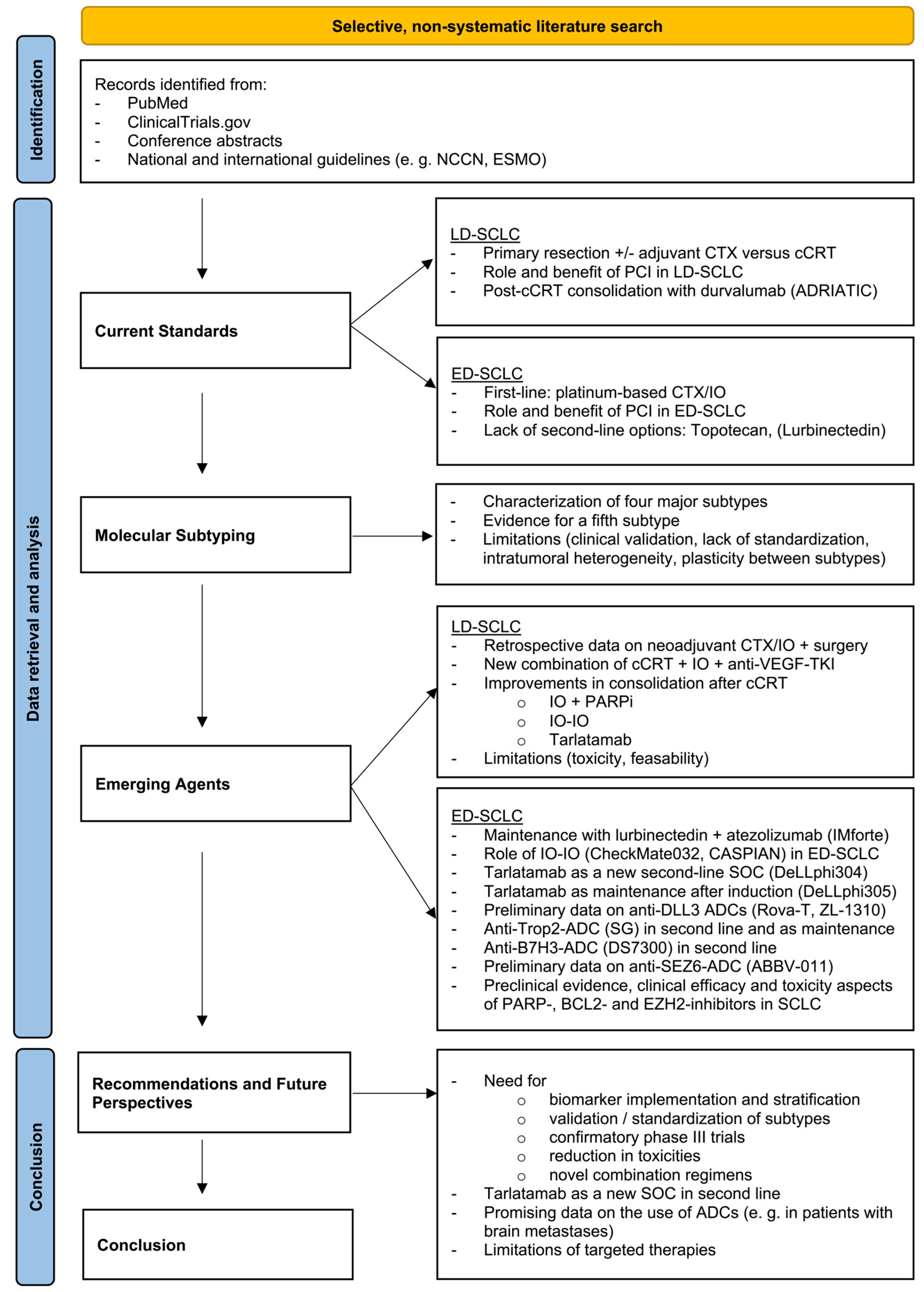
2. Methods
3. Molecular Subtyping
- -
- SCLC-A (ASCL1-dominant): Characterized by high expression of Achaete-Scute Family BHLH Transcription Factor 1 (ASCL1), this subtype is associated with classic neuroendocrine features and may be sensitive to B-Cell Lymphoma 2 (BCL-2) inhibition due to its dependency on anti-apoptotic pathways.
- -
- SCLC-N (NEUROD1-dominant): Defined by predominant expression of Neurogenic Differentiation Factor 1 (NEUROD1), this subtype exhibits features of neuronal differentiation and often harbors MYC gene amplifications, suggesting potential vulnerability to aurora kinase inhibitors and other MYC-targeted therapies.
- -
- SCLC-P (POU2F3-dominant): Marked by high expression of POU Class 2 Homeobox 3 (POU2F3), this non-neuroendocrine subtype represents a distinct tuft-cell-like lineage and may be sensitive to Insulin-like Growth Factor 1 Receptor (IGF1R) inhibitors or other novel targeted agents.
- -
- SCLC-I (Inflamed subtype): Distinguished by low expression of traditional lineage-defining transcription factors but high levels of immune cell infiltration and the upregulation of immune response-related genes, SCLC-I tumors appear particularly sensitive to immunotherapy.
4. Treatment for Limited Disease (LD-SCLC)
4.1. Current Treatment Standards in LD-SCLC
4.2. Novel Treatment Approaches in LD-SCLC
| Agent | Target/ Mechanism | Trial (Phase) | N | Population | Key Results | Safety Profile | Limitations | Clinical Implications | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Tarlatamab | DLL3/BiTE | DeLLphi-301 (Phase II) | 220 | ≥2 L ED-SCLC | ORR: 40%; PFS 4.9 mo; OS: 14.3 mo; 58% DOR ≥ 6 mo | CRS (51%), decreased appetite (29%), pyrexia (35%), ~3% treatment discontinuation | No control group; CRS/ICANS require careful monitoring; hospitalization | Favorable survival and tolerability, FDA approval | [43] |
| DeLLphi-304 (Phase III) | ~700 | Relapsed ED-SCLC after platinum-based CTX | Improvement in OS compared to local SOC (13.6 vs. 8.3 mo) | Lower grade ≥ 3 AEs (54% vs. 80%), fewer discontinuations | Indirect comparison to mix of chemo agents; CRS/ICANS monitoring not fully standardized | New SOC for second-line treatment in ED-SCLC | [44] | ||
| DeLLphi-305 (Phase III) | 550 | Maintenance after 1 L induction in ED-SCLC without tumor progression | recruiting | ||||||
| DeLLphi-306 (Phase III) | 400 | Consolidation after cCRT in LD-SCLC | recruiting | ||||||
| Rovalpituzumab Tesirine (Rova-T) | DLL3/ADC | NCT01901653 (Phase I/II) | 82 | ≥1 L SCLC | ORR 18% (38% in pts with high DLL3 expression) | Grade ≥ 3 TRAEs: thrombocytopenia (11%), pleural effusions (8%), elevated lipase (7%) | No control, small sample size; modest efficacy; high-grade toxicities | DLL3 expression linked to response, foundational for future ADCs | [45] |
| TAHOE (Phase III) | 444 | ≥1 L Advanced/ metastatic SCLC with high DLL3 expression | Inferior OS compared to topotecan (6.3 vs. 8.6 mo) → trial discontinuation | serosal effusions, photosensitivity, peripheral edema | No efficacy benefit; worse outcome vs. SOC; toxicity of ADC payload | First anti-DLL3 ADC tested in SCLC phase III; discontinuation of Rova-T program | [46] | ||
| MERU (Phase III) | 748 | Maintenance after 1 L platinum-based CTX in ED-SCLC | DLL3-high tumors: Significant improvement in PFS (4.0 vs. 1.4 mo) but not in OS (8.5 vs. 9.8 mo) → trial termination | ≥20% AEs in the Rova-T arm (pleural effusion, decreased appetite, peripheral edema, photosensitivity, fatigue, nausea, dyspnea) More grade ≥ 3 toxicities | No active comparator; high-grade AEs offset PFS gains | First phase III DLL3-targeted ADC in maintenance; discontinuation of Rova-T program | [47] | ||
| ZL-1310 | NCT06179069 (Phase I) | 112 | ≥1 L ED-SCLC; ±Atezolizumab ±Carboplatin | ORR 68%; pts with brain metastases: ORR 80%, DCR 100% | 39% grade ≥ 3 TRAEs (anemia, neutropenia, thrombocytopenia), one DLT | Early phase, small sample size; long-term safety and higher-dose toxicity unresolved | Highly promising early activity, rapid and intracranial responses; manageable early safety | [48] | |
| Sacituzumab Govitecan | Trop2/ADC | NCT03964727/ TROPiCS-03 (Phase II) | 227 (43 SCLC) | Recurrent ED-SCLC after 1 L platinum-based CTX and PD(L)1 directed therapy | ORR: 41.9% DOR: 4.7 mo PFS: 4.4 mo OS: 13.6 mo | 74.4% grade ≥ 3 TEAEs; no TEAE led to treatment discontinuation; 1 TEAE (neutropenic sepsis) led to death | Single-arm, small sample size; grade ≥ 3 events common; optimal patient selection unclear | Promising second-line option in ED-SCLC | [49] |
| Ifinatamab Deruxtecan (DS-7300) | B7-H3/ADC | NCT04145622 (Phase I/II); | 250 (22 SCLC) | Advanced/unresectable solid tumor (incl. SCLC), that is refractory to or intolerable with standard treatment, or for which no standard treatment is available. | ORR: 52.4%; PFS: 5.6 mo; OS: 12.2 mo | 36.4% grade ≥ 3 TEAEs (nausea, decreased appetite, constipation); 22.7% treatment discontinuation | Single-arm; small sample size; ILD/pneumonitis risk unclear; Dose/regimen optimization pending | First-in-human for anti-B7-H3 ADC in SCLC; broad efficacy across SCLC patients | [50] |
| NCT06203210/ IDeate-Lung02 (Phase III) | 540 | Relapsed SCLC after 1 L platinum-based CTX | recruiting | ||||||
| ABBV-011 | SEZ6/ADC | NCT03639194 (Phase I) | 99 | Relapsed/refractory ED-SCLC after 1 L platinum-based CTX | At 1.0 mg/kg: ORR: 25%; DOR: 4.2 mo; PFS: 3.5 mo | Fatigue (50%), nausea (42%), thrombocytopenia (41%), increased ASAT (22%), 2 cases of VOD | No comparator; modest response in selected cohort; grade ≥ 3 AEs in ~48%; biomarker cutoff and expression variability | First SEZ6-targeted ADC tested in SCLC; No further trials yet initiated | [51] |
| Olaparib | PARP inhibitor | NCT04728230, PRIO (Phase I/II) | 63 | 1 L ED-SCLC; +durvalumab with carboplatin/etoposide and/or radiotherapy | recruiting | ||||
| NCT04624204 KEYLYNK-013 (Phase III) | 672 | 1 L LD-SCLC; +pembrolizumab post cCRT | Active, not recruiting | ||||||
| Talazoparib | SWOG S1929 (Phase II) | 106 | Maintenance after 1 L in SLFN11-positive ED-SCLC; ±atezolizumab | Improvement in PFS (2.9 mo vs. 2.4 mo), but no difference in OS | 17% grade ≥ 3 non-hematologic TRAEs, higher rate of hematologic TRAEs (50%) | Phase II, small sample; No OS improvement; Hematologic toxicity in 50%; SLFN11 not validated as predictive for OS | Biomarker-selected, first of its kind in SCLC; requires larger studies to confirm clinical value | [52] | |
| Niraparib | NCT04701307 (Phase II) | 48 | ≥1 L SCLC or NECs; +dostarlimab | Active, not recruiting | |||||
| Navitoclax | BCL2 inhibitor | NCT00445198 (Phase II) | 39 | Recurrent/progressive SCLC after ≥1 L | Early efficacy signs (PR 2.6%, SD 23%) PFS 1.5 mo; OS 3.2 mo | Dose-limiting thrombocytopenia (41% grades 3–4) | Small, early-phase, no comparator; minimal reported efficacy; DLTs led to trial termination | Identified thrombocytopenia as key toxicity | [53] |
| Venetoclax | NCT0442221, NCT04543916 (Phase Ib/II) | N/A | 1 L ED-SCLC, relapsed/refractory SCLC | terminated/withdrawn | |||||
| Tazemetostat | EZH2 inhibitor | NCT05353439 (Phase I) | 60 | Relapsed/recurrent SCLC after platinum-based CTX; +topotecan/pembrolizumab | recruiting | ||||
| Mevrometostat | NCT03460977 (Phase I/II) | 343 | Relapsed/refractory SCLC, CRPC, and FL; ±SOC treatment | So far, no formal efficacy results have been reported for SCLC cohort | SCLC-specific safety outcomes unreported | Early-phase, small cohorts; unreported efficacy and safety data | First EZH1/2 inhibitor tested in SCLC | [54] | |
| Valemetostat | EZH1/2 inhibitor | NCT03879798 (Phase I/II) | 22 | Recurrent SCLC after platinum-based CTX | ORR: 21% (4/19); DOR: 4.6 mo PFS: 2.2 mo OS: 6.6 mo | ≥20% TRAEs were diarrhea, fatigue, nausea, and rash; 3 DLTs → early trial termination | Single-arm, small cohort; modest outcomes; DLTs occurred | Explored SLFN11, epigenetic markers, subtype shifts | [55] |
| Lurbinectedin | DNA damage | NCT02611024 (Phase I/II) | 320 (100 SCLC) | Relapsed/refractory solid tumors, including SCLC; +irinotecan | ORR: 52.7% DOR: 7.6 mo PFS: 5.0 mo OS: 12.7 mo (in pts with CTFI > 20 days) | 71.6% grade ≥ 3 TRAEs (neutropenia, anemia, diarrhea, fatigue); 31.1% serious AEs, 6.8% treatment discontinuations | Single-arm, open-label; high grade ≥ 3 AE rate | Proof-of-concept for the combination of lurbinectedin + irinotecan; led to LAGOON phase III trial | [56] |
| NCT05153239/ LAGOON (Phase III) | 705 | Relapsed SCLC after platinum-based CTX with CTFI ≥ 30 days; +irinotecan | Active, not recruiting | ||||||
| NCT05091567 IMforte (Phase III) | 660 | Maintenance after 1 L induction treatment in ED-SCLC without tumor progression | PFS: 5.4 mo OS: 13.2 mo | 25.6% grade 3/4 TRAEs; AEs led to treatment discontinuation in 6.2% | Open-label; moderate absolute improvements; PFS still limited; increased serious AEs and some fatal events | Significant survival benefits; potential new option for maintenance therapy | [57] | ||
5. Treatment for Extensive Disease (ED-SCLC)
5.1. Current Treatment Standards in ED-SCLC
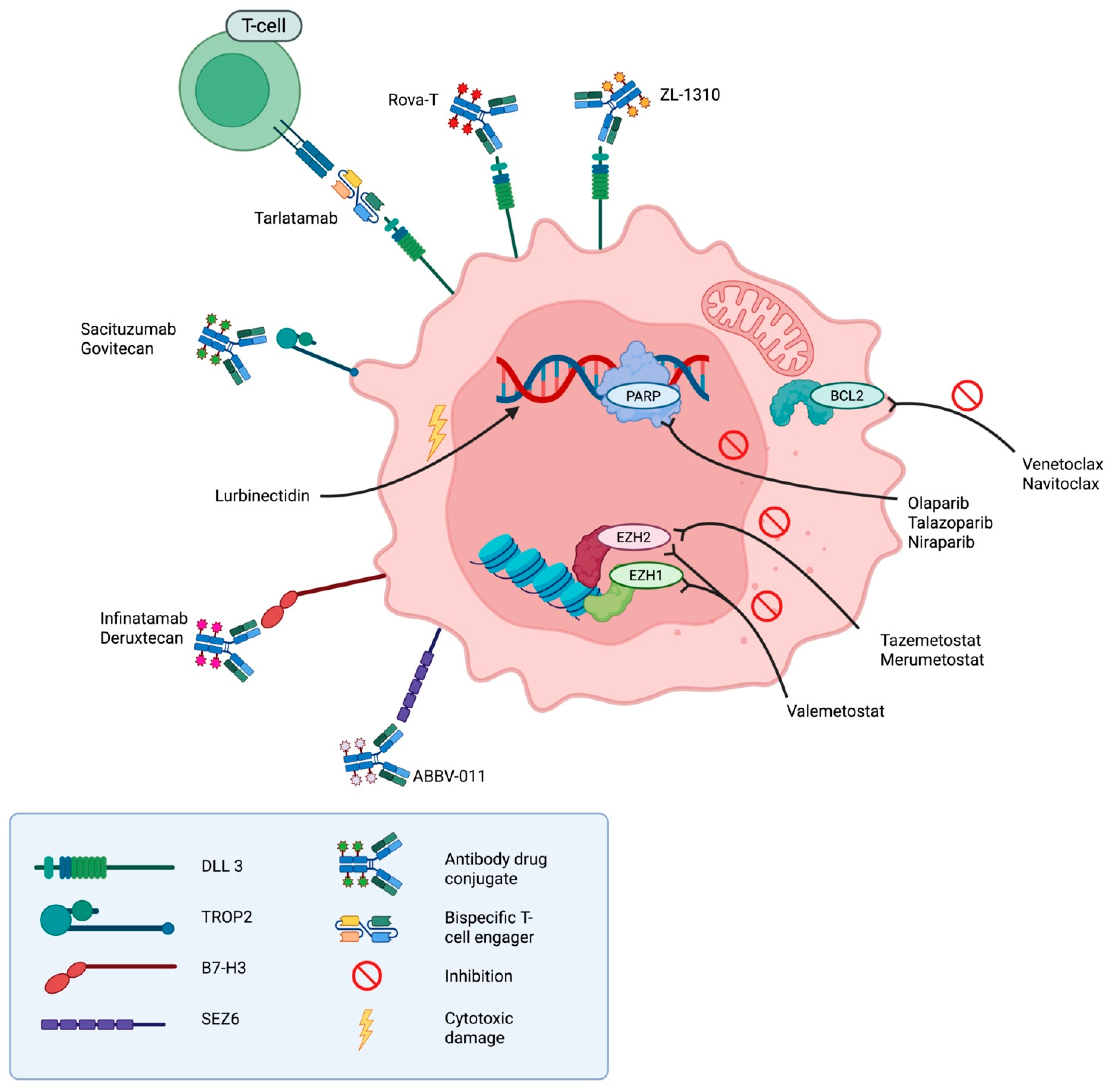
5.2. Novel Treatment Approaches in ED-SCLC
5.2.1. Lurbinectedin
5.2.2. Dual Checkpoint Inhibition in SCLC
5.2.3. Tarlatamab
5.2.4. Rovalpituzumab Tesirine (Rova-T)
5.2.5. ZL-1310
5.2.6. Sacituzumab Govitecan (SG)
5.2.7. Ifinatamab Deruxtecan (DS-7300)
5.2.8. ABBV-011
5.2.9. PARP Inhibitors
5.2.10. BCL2 Inhibitors
5.2.11. Epigenetic Modulators (EZH2 Inhibitors)
6. Recommendations and Future Perspectives
- A major obstacle remains the lack of durable treatment responses, particularly in the relapsed or refractory setting, where most available therapies offer only modest survival benefits. Rapid disease progression and the early development of treatment resistance further complicate clinical management.
- The absence of reliable predictive biomarkers significantly limits effective patient selection for emerging therapies. While molecular subtyping shows promise, its clinical implementation is hindered by inconsistent classification systems and the lack of standardized assays.
- Treatment-related toxicity remains a significant barrier, especially in combination regimens involving chemotherapy, immunotherapy, or targeted agents. Such regimens are often poorly tolerated by patients with limited performance status or significant comorbidities.
- Clinical trial development is also hampered by slow patient accrual, insufficient biomarker stratification, and early treatment discontinuations, collectively contributing to the slow pace of progress in this aggressive malignancy.
- The bispecific T-cell engager tarlatamab has emerged as a leading candidate, demonstrating improved survival and a favorable safety profile in a recent phase III trial. It is anticipated to become a new standard of care in the second-line setting, with potential use in earlier stages, including consolidation therapy for LD-SCLC, currently under investigation.
- The addition of lurbinectedin to immunotherapy as maintenance therapy in ED-SCLC remains under evaluation; however, its potential to become a new standard is uncertain given concerns that toxicity may outweigh any survival benefit.
- ADCs such as sacituzumab govitecan and ZL-1310 have shown promising activity in heavily pretreated populations, including patients with brain metastases. Newer-generation ADCs may offer effective alternatives for patients ineligible for more intensive treatment.
- By contrast, the future of targeted therapies such as PARP-, BCL-2-, and EZH2-inhibitors remains uncertain, due to modest clinical activity, toxicity concerns, and the early termination of several trials.
- Future efforts should prioritize the validation of predictive biomarkers (e.g., SLFN11, DLL3, BCL-2), development of rational combination regimens, expansion into earlier treatment settings, and long-term safety assessments of novel agents.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| ADC | Antibody-Drug Conjugate |
| AE | Adverse Event |
| ASCL1 | Achaete-Scute Family BHLH Transcription Factor 1 |
| BCL-2 | B-cell lymphoma 2 |
| BiTE | Bispecific T-cell Engager |
| B7-H3 | B7 Homolog 3/CD276 |
| cCRT | Concurrent Chemoradiotherapy |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| CRPC | Castration-Resistant Prostate Cancer |
| CRS | Cytokine-Release Syndrome |
| CRT | Chemoradiotherapy |
| CT | Computed Tomography |
| CTFI | Chemotherapy-Free Interval |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| CTX | Chemotherapy |
| DLCO | Diffusing Capacity of the Lung for Carbon Monoxide |
| DLL3 | Delta-like protein 3 |
| DLT | Dose-Limiting Toxicity |
| DCR | Disease Control Rate |
| DOR | Duration Of Response |
| ED-SCLC | Extensive-Disease small cell lung cancer |
| EMA | National Comprehensive Cancer Network |
| ESMO | European Society For Medical Oncology |
| EZH2 | Enhancer of Zeste Homolog 2 |
| FDA | United States Food and Drug Administration |
| FDG-PET | Fluorodeoxyglucose Positron Emission Tomography |
| FEV1 | Forced Expiratory Volume in 1 Second |
| FL | Follicular Lymphoma |
| HR | Hazard Ratio |
| ICANS | Immune Effector Cell-Associated Neurotoxicity Syndrome |
| IGFR1 | Insulin-like Growth Factor 1 Receptor |
| ILD | Interstitial Lung Disease |
| IO | Immunotherapy |
| irAE | Immune-Related Adverse Events |
| LD-SCLC | Limited-Disease small cell lung cancer |
| LDH | Lactate Dehydrogenase |
| MRI | Magnetic Resonance Imaging |
| MYC | Myelocytomatosis oncogene |
| NCAM | Neural Cell Adhesion Molecule, also known as CD56 |
| NCCN | National Comprehensive Cancer Network |
| NEC | Neuroendocrine Carcinoma |
| NEURO1 | Neurogenic Differentiation Factor 1 |
| NOTCH | Neurogenic locus notch homolog (NOTCH) signaling pathway gene |
| NSCLC | Non-Small Cell Lung Cancer |
| NSE | Neuron-Specific Enolase |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PARP | Poly ADP-ribose Polymerase |
| PARPi | Poly ADP-ribose Polymerase inhibitor |
| PCI | Prophylactic Cranial Irradiation |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PFS | Progression-Free Survival |
| POU2F3 | POU Class 2 Homeobox 3 |
| PR | Partial Response |
| PROTAC | Proteolysis-Targeting Chimera |
| PS | Performance Status |
| RB1 | Retinoblastoma 1 |
| SCLC | Small cell lung cancer |
| SD | Stable Disease |
| SEZ6 | Seizure-Related 6 Homolog |
| SG | Sacituzumab Govitecan |
| SIADH | Syndrome of Inappropriate Antidiuretic Hormone Secretion |
| SLFN11 | Schlafen Family Member 11 |
| SOC | Standarf Of Care |
| TEAE | Treatment-Emergent Adverse Event |
| TKI | Tyrosine Kinase Inhibitor |
| TP53 | Tumor protein p53 |
| TRAE | Treatment-Related Adverse Events |
| Trop-2 | Tumor-associated calcium signal transducer 2 |
| TTF-1 | Thyroid Transcription Factor-1 |
| UICC | Union for International Cancer Control |
| VC | Vital Capacity |
| VEGF | Vascular Endothelial Growth Factor |
| VOD | Veno-Occlusive Disease |
| YAP1 | Yes-associated protein 1 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.E.; Cohen, S.; Brennan, B.; Banerjee, M.; Kalemkerian, G.P. Epidemiology of SCLC in the United States From 2000 to 2019: A Study Utilizing the Surveillance, Epidemiology, and End Results Registry. JTO Clin. Res. Rep. 2025, 6, 100799. [Google Scholar] [CrossRef]
- Uprety, D.; Seaton, R.; Niroula, A.; Hadid, T.; Parikh, K.; Ruterbusch, J.J. Trends in the Incidence and Survival Outcomes in Patients with Small Cell Lung Cancer in the United States: An Analysis of the SEER Database. Cancer Med. 2025, 14, e70608. [Google Scholar] [CrossRef] [PubMed]
- Chansky, K.; Detterbeck, F.C.; Nicholson, A.G.; Rusch, V.W.; Vallières, E.; Groome, P.; Kennedy, C.; Krasnik, M.; Peake, M.; Shemanski, L.; et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2017, 12, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Tobacco Collaborators. Spatial, Temporal, and Demographic Patterns in Prevalence of Smoking Tobacco Use and Attributable Disease Burden in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- Wang, Q.; Gümüş, Z.H.; Colarossi, C.; Memeo, L.; Wang, X.; Kong, C.Y.; Boffetta, P. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J. Thorac. Oncol. 2023, 18, 31–46. [Google Scholar] [CrossRef]
- National Cancer Institute, Surveillance Research Program. SEER*Explorer: An Interactive Website for SEER Cancer Statistics [Internet]. 16 April 2025. Data Source (s): SEER Incidence Data, November 2024 Submission (1975–2022), SEER 21 Registries. 2025. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html (accessed on 25 May 2025).
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Peifer, M.; Fernández-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative Genome Analyses Identify Key Somatic Driver Mutations of Small-Cell Lung Cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef]
- Mollaoglu, G.; Guthrie, M.R.; Böhm, S.; Brägelmann, J.; Can, I.; Ballieu, P.M.; Marx, A.; George, J.; Heinen, C.; Chalishazar, M.D.; et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017, 31, 270–285. [Google Scholar] [CrossRef]
- Ruano-Raviña, A.; Provencio, M.; Calvo de Juan, V.; Carcereny, E.; Moran, T.; Rodriguez-Abreu, D.; López-Castro, R.; Cuadrado Albite, E.; Guirado, M.; Gómez González, L.; et al. Lung Cancer Symptoms at Diagnosis: Results of a Nationwide Registry Study. ESMO Open 2020, 5, e001021. [Google Scholar] [CrossRef]
- Giometto, B.; Grisold, W.; Vitaliani, R.; Graus, F.; Honnorat, J.; Bertolini, G. PNS Euronetwork Paraneoplastic Neurologic Syndrome in the PNS Euronetwork Database: A European Study from 20 Centers. Arch. Neurol. 2010, 67, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Gozzard, P.; Woodhall, M.; Chapman, C.; Nibber, A.; Waters, P.; Vincent, A.; Lang, B.; Maddison, P. Paraneoplastic Neurologic Disorders in Small Cell Lung Carcinoma: A Prospective Study. Neurology 2015, 85, 235–239. [Google Scholar] [CrossRef]
- Soomro, Z.; Youssef, M.; Yust-Katz, S.; Jalali, A.; Patel, A.J.; Mandel, J. Paraneoplastic Syndromes in Small Cell Lung Cancer. J. Thorac. Dis. 2020, 12, 6253–6263. [Google Scholar] [CrossRef] [PubMed]
- Iams, W.T.; Shiuan, E.; Meador, C.B.; Roth, M.; Bordeaux, J.; Vaupel, C.; Boyd, K.L.; Summitt, I.B.; Wang, L.L.; Schneider, J.T.; et al. Improved Prognosis and Increased Tumor-Infiltrating Lymphocytes in Patients Who Have SCLC with Neurologic Paraneoplastic Syndromes. J. Thorac. Oncol. 2019, 14, 1970–1981. [Google Scholar] [CrossRef]
- Dingemans, A.-M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Small Cell Lung Cancer [v.3.2025]. 2025. Available online: https://www.nccn.org/Guidelines/Guidelines-Detail?Category=1&id=1462 (accessed on 25 May 2025).
- Arriola, E.; Trigo, J.M.; Sánchez-Gastaldo, A.; Navarro, A.; Perez, C.; Crama, L.; Ponce-Aix, S. Prognostic Value of Clinical Staging According to TNM in Patients with SCLC: A Real-World Surveillance Epidemiology and End-Results Database Analysis. JTO Clin. Res. Rep. 2022, 3, 100266. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Crowley, J.; Van Houtte, P.; Postmus, P.E.; Carney, D.; Chansky, K.; Shaikh, Z.; Goldstraw, P. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals Regarding the Clinical Staging of Small Cell Lung Cancer in the Forthcoming (Seventh) Edition of the Tumor, Node, Metastasis Classification for Lung Cancer. J. Thorac. Oncol. 2007, 2, 1067–1077. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.E.; van Meerbeeck, J.; Rami-Porta, R.; Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 300–311. [Google Scholar] [CrossRef]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of Transcription Factor Programs and Immune Pathway Activation Define Four Major Subtypes of SCLC with Distinct Therapeutic Vulnerabilities. Cancer Cell 2021, 39, 346–360.e7. [Google Scholar] [CrossRef]
- Chen, P.; Sun, C.; Wang, H.; Zhao, W.; Wu, Y.; Guo, H.; Zhou, C.; He, Y. YAP1 Expression Is Associated with Survival and Immunosuppression in Small Cell Lung Cancer. Cell Death Dis. 2023, 14, 636. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Dwivedi, B.; Chen, Z.; Zhang, C.; Barwick, B.; Ernani, V.; Zhang, G.; Gilbert-Ross, M.; Carlisle, J.; Khuri, F.R.; et al. YAP1 Expression in SCLC Defines a Distinct Subtype with T-Cell-Inflamed Phenotype. J. Thorac. Oncol. 2021, 16, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Cai, L.; Girard, L.; Prall, O.W.J.; Rajan, N.; Khoo, C.; Batrouney, A.; Byrne, D.J.; Boyd, D.K.; Kersbergen, A.J.; et al. Molecular and Pathologic Characterization of YAP1-Expressing Small Cell Lung Cancer Cell Lines Leads to Reclassification as SMARCA4-Deficient Malignancies. Clin. Cancer Res. 2024, 30, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Baine, M.K.; Hsieh, M.-S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F.; et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J. Thorac. Oncol. 2020, 15, 1823–1835. [Google Scholar] [CrossRef]
- Mahadevan, N.R.; Knelson, E.H.; Wolff, J.O.; Vajdi, A.; Saigí, M.; Campisi, M.; Hong, D.; Thai, T.C.; Piel, B.; Han, S.; et al. Intrinsic Immunogenicity of Small Cell Lung Carcinoma Revealed by Its Cellular Plasticity. Cancer Discov. 2021, 11, 1952–1969. [Google Scholar] [CrossRef]
- Velut, Y.; Arqué, B.; Wislez, M.; Blons, H.; Burroni, B.; Prieto, M.; Beau, S.; Fournel, L.; Birsen, G.; Cremer, I.; et al. The Tumor Immune Microenvironment of SCLC Is Not Associated with Its Molecular Subtypes. Eur. J. Cancer 2024, 212, 115067. [Google Scholar] [CrossRef]
- Peressini, M.; Garcia-Campelo, R.; Massuti, B.; Martí, C.; Cobo, M.; Gutiérrez, V.; Dómine, M.; Fuentes, J.; Majem, M.; de Castro, J.; et al. Spatially Preserved Multi-Region Transcriptomic Subtyping and Biomarkers of Chemoimmunotherapy Outcome in Extensive-Stage Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 3036–3049. [Google Scholar] [CrossRef]
- Duplaquet, L.; Li, Y.; Booker, M.A.; Xie, Y.; Olsen, S.N.; Patel, R.A.; Hong, D.; Hatton, C.; Denize, T.; Walton, E.; et al. KDM6A Epigenetically Regulates Subtype Plasticity in Small Cell Lung Cancer. Nat. Cell Biol. 2023, 25, 1346–1358. [Google Scholar] [CrossRef]
- Jones, G.S.; Elimian, K.; Baldwin, D.R.; Hubbard, R.B.; McKeever, T.M. A Systematic Review of Survival Following Anti-Cancer Treatment for Small Cell Lung Cancer. Lung Cancer 2020, 141, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Shiono, A.; Imai, H.; Endo, S.; Katayama, K.; Sato, H.; Hashimoto, K.; Miura, Y.; Okazaki, S.; Abe, T.; Mouri, A.; et al. A Retrospective Evaluation of Therapeutic Efficacy and Safety of Chemoradiotherapy in Older Patients (Aged ≥ 75 Years) with Limited-Disease Small Cell Lung Cancer: Insights from Two Institutions and Review of the Literature. Radiol. Oncol. 2024, 58, 432–443. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Snee, M.; Ashcroft, L.; Appel, W.; Barlesi, F.; Bhatnagar, A.; Bezjak, A.; Cardenal, F.; Fournel, P.; Harden, S.; et al. Concurrent Once-Daily versus Twice-Daily Chemoradiotherapy in Patients with Limited-Stage Small-Cell Lung Cancer (CONVERT): An Open-Label, Phase 3, Randomised, Superiority Trial. Lancet Oncol. 2017, 18, 1116–1125. [Google Scholar] [CrossRef]
- Grønberg, B.H.; Killingberg, K.T.; Fløtten, Ø.; Brustugun, O.T.; Hornslien, K.; Madebo, T.; Langer, S.W.; Schytte, T.; Nyman, J.; Risum, S.; et al. High-Dose versus Standard-Dose Twice-Daily Thoracic Radiotherapy for Patients with Limited Stage Small-Cell Lung Cancer: An Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2021, 22, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Grønberg, B.H.; Halvorsen, T.O.; Fløtten, Ø.; Brustugun, O.T.; Brunsvig, P.F.; Aasebø, U.; Bremnes, R.M.; Tollåli, T.; Hornslien, K.; Aksnessæther, B.Y.; et al. Randomized Phase II Trial Comparing Twice Daily Hyperfractionated with Once Daily Hypofractionated Thoracic Radiotherapy in Limited Disease Small Cell Lung Cancer. Acta Oncol. 2016, 55, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A.; Arriagada, R.; Pignon, J.P.; Le Péchoux, C.; Gregor, A.; Stephens, R.J.; Kristjansen, P.E.; Johnson, B.E.; Ueoka, H.; Wagner, H.; et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N. Engl. J. Med. 1999, 341, 476–484. [Google Scholar] [CrossRef]
- Cheng, Y.; Spigel, D.R.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 391, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum-Etoposide versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients with Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as Second-Line Treatment for Patients with Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Aix, S.P.; Ciuleanu, T.E.; Navarro, A.; Cousin, S.; Bonanno, L.; Smit, E.F.; Chiappori, A.; Olmedo, M.E.; Horvath, I.; Grohé, C.; et al. Combination Lurbinectedin and Doxorubicin versus Physician’s Choice of Chemotherapy in Patients with Relapsed Small-Cell Lung Cancer (ATLANTIS): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Respir. Med. 2023, 11, 74–86. [Google Scholar] [CrossRef]
- Peters, S.; Pujol, J.-L.; Dafni, U.; Dómine, M.; Popat, S.; Reck, M.; Andrade, J.; Becker, A.; Moro-Sibilot, D.; Curioni-Fontecedro, A.; et al. Consolidation Nivolumab and Ipilimumab versus Observation in Limited-Disease Small-Cell Lung Cancer after Chemo-Radiotherapy—Results from the Randomised Phase II ETOP/IFCT 4-12 STIMULI Trial. Ann. Oncol. 2022, 33, 67–79. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Cho, B.C.; Felip, E.; Korantzis, I.; Ohashi, K.; Majem, M.; Juan-Vidal, O.; Handzhiev, S.; Izumi, H.; Lee, J.-S.; et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Mountzios, G.; Sun, L.; Cho, B.C.; Demirci, U.; Baka, S.; Gümüş, M.; Lugini, A.; Zhu, B.; Yu, Y.; Korantzis, I.; et al. Tarlatamab in Small-Cell Lung Cancer after Platinum-Based Chemotherapy. N. Engl. J. Med. 2025. [Google Scholar] [CrossRef]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A.; Robert, F.; et al. Rovalpituzumab Tesirine, a DLL3-Targeted Antibody-Drug Conjugate, in Recurrent Small-Cell Lung Cancer: A First-in-Human, First-in-Class, Open-Label, Phase 1 Study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef]
- Blackhall, F.; Jao, K.; Greillier, L.; Cho, B.C.; Penkov, K.; Reguart, N.; Majem, M.; Nackaerts, K.; Syrigos, K.; Hansen, K.; et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared with Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J. Thorac. Oncol. 2021, 16, 1547–1558. [Google Scholar] [CrossRef]
- Johnson, M.L.; Zvirbule, Z.; Laktionov, K.; Helland, A.; Cho, B.C.; Gutierrez, V.; Colinet, B.; Lena, H.; Wolf, M.; Gottfried, M.; et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients with Extensive-Stage-SCLC: Results from the Phase 3 MERU Study. J. Thorac. Oncol. 2021, 16, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Wu, Y.-L.L.C.; Wang, Z.; Rocha, P.; Wang, Q.; Du, Y.; Dy, G.K.; Dowlati, A.; Spira, A.; Dong, X.; et al. ZL-1310, a DLL3 ADC, in Patients with Extensive Stage Small Cell Lung Cancer: Ph1 Trial Update. JCO 2025, 43, 3041. [Google Scholar] [CrossRef]
- Dowlati, A.; Chiang, A.C.; Cervantes, A.; Babu, S.; Hamilton, E.; Wong, S.F.; Tazbirkova, A.; Sullivan, I.G.; van Marcke, C.; Italiano, A.; et al. Phase 2 Open-Label Study of Sacituzumab Govitecan as Second-Line Therapy in Patients with Extensive-Stage SCLC: Results From TROPiCS-03. J. Thorac. Oncol. 2025, 20, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Awad, M.; Koyama, T.; Gutierrez, M.; Falchook, G.S.; Piha-Paul, S.A.; Doi, T.; Satoh, T.; Okamoto, N.; Singh, J.; et al. OA05.05 Ifinatamab Deruxtecan (I-DXd; DS-7300) in Patients with Refractory SCLC: A Subgroup Analysis of a Phase 1/2 Study. J. Thorac. Oncol. 2023, 18, S54–S55. [Google Scholar] [CrossRef]
- Morgensztern, D.; Ready, N.; Johnson, M.L.; Dowlati, A.; Choudhury, N.; Carbone, D.P.; Schaefer, E.; Arnold, S.M.; Puri, S.; Piotrowska, Z.; et al. A Phase I First-in-Human Study of ABBV-011, a Seizure-Related Homolog Protein 6-Targeting Antibody-Drug Conjugate, in Patients with Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 5042–5052. [Google Scholar] [CrossRef]
- Karim, N.A.; Miao, J.; Reckamp, K.L.; Gay, C.M.; Byers, L.A.; Zhao, Y.-Q.; Redman, M.W.; Carrizosa, D.R.; Wang, W.-L.; Petty, W.J.; et al. Phase II Randomized Study of Maintenance Atezolizumab Versus Atezolizumab Plus Talazoparib in Patients with SLFN11 Positive Extensive-Stage SCLC: S1929. J. Thorac. Oncol. 2025, 20, 383–394. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; Ribeiro de Oliveira, M.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.; Penkov, K.D.; Tolcher, A.W.; Choudhury, A.D.; Doronin, V.; Aljumaily, R.; Calvo, E.; Frank, R.C.; Hamm, J.T.; Moreno Garcia, V.; et al. 488P Phase I Trial of PF-06821497, a Potent and Selective Inhibitor of Enhancer of Zeste Homolog 2 (EZH2), in Follicular Lymphoma (FL), Small Cell Lung Cancer (SCLC) and Castration-Resistant Prostate Cancer (CRPC). Ann. Oncol. 2022, 33, S763–S764. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Lai, W.V.; Makhnin, A.; Heller, G.; Eng, J.; Li, B.; Preeshagul, I.; Santini, F.C.; Offin, M.; Ng, K.; et al. A Phase I/II Study of Valemetostat (DS-3201b), an EZH1/2 Inhibitor, in Combination with Irinotecan in Patients with Recurrent Small-Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 3697–3703. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Falcon Gonzalez, A.; Navarro Mendivil, A.F.; Sanchez Gastaldo, A.; Simoes da Rocha, P.F.; Cote, G.M.; Bockorny, B.; Molina Cerrillo, J.; Artal, A.; Baena Espinar, J.; et al. 1790P Phase II Data of Lurbinectedin (LUR) and Irinotecan (IRI) in Relapsed Small Cell Lung Cancer (SCLC) Patients (Pts) with Chemotherapy-Free Interval (CTFI)>30 Days (d). Ann. Oncol. 2024, 35, S1064–S1065. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Borghaei, H.; Liu, S.V.; Peters, S.; Herbst, R.S.; Stencel, K.M.; Majem, M.; Czyz, G.; Caro, R.B.; Lee, K.H.; et al. Lurbinectedin (Lurbi) + Atezolizumab (Atezo) as First-Line (1 L) Maintenance Treatment (Tx) in Patients (Pts) with Extensive-Stage Small Cell Lung Cancer (ES-SCLC): Primary Results of the Phase 3 IMforte Trial. In Proceedings of the 2025 American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA, 31 May–5 June 2025. Abstract 8006. 2025. [Google Scholar]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Leal, J.F.M.; Martínez-Díez, M.; García-Hernández, V.; Moneo, V.; Domingo, A.; Bueren-Calabuig, J.A.; Negri, A.; Gago, F.; Guillén-Navarro, M.J.; Avilés, P.; et al. PM01183, a New DNA Minor Groove Covalent Binder with Potent in Vitro and in Vivo Anti-Tumour Activity. Br. J. Pharmacol. 2010, 161, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Santamaría Nuñez, G.; Robles, C.M.G.; Giraudon, C.; Martínez-Leal, J.F.; Compe, E.; Coin, F.; Aviles, P.; Galmarini, C.M.; Egly, J.-M. Lurbinectedin Specifically Triggers the Degradation of Phosphorylated RNA Polymerase II and the Formation of DNA Breaks in Cancer Cells. Mol. Cancer Ther. 2016, 15, 2399–2412. [Google Scholar] [CrossRef]
- Allavena, P.; Belgiovine, C.; Digifico, E.; Frapolli, R.; D’Incalci, M. Effects of the Anti-Tumor Agents Trabectedin and Lurbinectedin on Immune Cells of the Tumor Microenvironment. Front. Oncol. 2022, 12, 851790. [Google Scholar] [CrossRef]
- Ready, N.E.; Ott, P.A.; Hellmann, M.D.; Zugazagoitia, J.; Hann, C.L.; de Braud, F.; Antonia, S.J.; Ascierto, P.A.; Moreno, V.; Atmaca, A.; et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J. Thorac. Oncol. 2020, 15, 426–435. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without Tremelimumab, plus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer: 3-Year Overall Survival Update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef]
- Yamato, M.; Hasegawa, J.; Maejima, T.; Hattori, C.; Kumagai, K.; Watanabe, A.; Nishiya, Y.; Shibutani, T.; Aida, T.; Hayakawa, I.; et al. DS-7300a, a DNA Topoisomerase I Inhibitor, DXd-Based Antibody-Drug Conjugate Targeting B7-H3, Exerts Potent Antitumor Activities in Preclinical Models. Mol. Cancer Ther. 2022, 21, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Wiedemeyer, W.R.; Gavrilyuk, J.; Schammel, A.; Zhao, X.; Sarvaiya, H.; Pysz, M.; Gu, C.; You, M.; Isse, K.; Sullivan, T.; et al. ABBV-011, A Novel, Calicheamicin-Based Antibody-Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors. Mol. Cancer Ther. 2022, 21, 986–998. [Google Scholar] [CrossRef]
- Barayan, R.; Ran, X.; Lok, B.H. PARP Inhibitors for Small Cell Lung Cancer and Their Potential for Integration into Current Treatment Approaches. J. Thorac. Dis. 2020, 12, 6240–6252. [Google Scholar] [CrossRef] [PubMed]
- Lochmann, T.L.; Floros, K.V.; Naseri, M.; Powell, K.M.; Cook, W.; March, R.J.; Stein, G.T.; Greninger, P.; Maves, Y.K.; Saunders, L.R.; et al. Venetoclax Is Effective in Small-Cell Lung Cancers with High BCL-2 Expression. Clin. Cancer Res. 2018, 24, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, L.; Wiegand, J.; Zhang, P.; Zajac-Kaye, M.; Kaye, F.J.; Zheng, G.; Zhou, D. PROTAC-Mediated Dual Degradation of BCL-xL and BCL-2 Is a Highly Effective Therapeutic Strategy in Small-Cell Lung Cancer. Cells 2024, 13, 528. [Google Scholar] [CrossRef]
- Shi, M.-X.; Ding, X.; Tang, L.; Cao, W.-J.; Su, B.; Zhang, J. PROTAC EZH2 Degrader-1 Overcomes the Resistance of Podophyllotoxin Derivatives in Refractory Small Cell Lung Cancer with Leptomeningeal Metastasis. BMC Cancer 2024, 24, 504. [Google Scholar] [CrossRef]
| TNM/UICC Stage | Tumor Characteristics | 5-Year Survival Rate |
|---|---|---|
| Limited Disease | ||
| Stage I (IA, IB) | Early-stage tumor: T1–T2 (≤5 cm or >5 cm but confined to one lobe), N0, M0 | ~13–25% |
| Stage II (IIA, IIB) | Locally advanced tumor: T2–T3 (infiltrating adjacent structures), N0 or N1, M0 | ~17–21% |
| Stage III (IIIA, IIIB, IIIC) | Advanced local tumor: T3–T4 (e.g., invasion of chest wall, vessels, or other lobes), N1–N3, M0 | ~9–13% |
| Extensive Disease | ||
| Stage IV | Presence of distant metastases (M1) | <5% |
| Trial | N | Interventional Arm | Control Arm | Indication | Key Results | Limitations | Clinical Implications | Reference |
|---|---|---|---|---|---|---|---|---|
| IMpower133 (phase I/III) | 403 | Atezolizumab + carboplatin/ etoposide | Placebo + carboplatin/ etoposide | First-line, ED-SCLC | Significant improvement in OS: 12.3 vs. 10.3 months | Limited biomarker stratification | First-line anti-PD-L1 treatment option in ED-SCLC (SOC) | [37] |
| CASPIAN (phase III) | 805 | Durvalumab + platinum/etoposide (±tremelimumab) | Platinum/ etoposide | First-line, ED-SCLC | Significant improvement in OS: 13.0 vs. 10.5 months | Addition of tremelimumab without extra benefit | First-line anti-PD-L1 treatment option in ED-SCLC (SOC) | [38] |
| ASTRUM-005 (phase III) | 585 | Serplulimab + carboplatin/ etoposide | Placebo + carboplatin/ etoposide | First-line, ED-SCLC | Significant improvement in OS: 15.4 vs. 10.9 months | No direct comparison to PD-L1 inhibitors | Novel anti-PD1 first-line treatment option in ED-SCLC | [39] |
| ADRIATIC (phase III) | 600 | Durvalumab (±tremelimumab) | Placebo | Consolidation post-cCRT, LD-SCLC | Significant improvement in OS: 55.9 vs. 33.4 months | Data on dual checkpoint inhibition still pending | SOC for consolidation therapy post-cCRT in LD-SCLC | [36] |
| ATLANTIS (phase III) | 613 | Lurbinectedin + doxorubicin | Topotecan or CAV (cyclophosphamide, doxorubicin, vincristine) | Second-line SCLC | No significant improvement in OS: 8.6 vs. 7.6 months | No survival benefit; heterogeneity in patient selection; no approval | Signs of better tolerability; CTFI proved as prognostic/predictive | [40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemper, M.; Reitnauer, L.E.; Lenz, G.; Evers, G.; Bleckmann, A. Redefining the Fight Against SCLC: Standards, Innovations, and New Horizons. Cancers 2025, 17, 2256. https://doi.org/10.3390/cancers17132256
Kemper M, Reitnauer LE, Lenz G, Evers G, Bleckmann A. Redefining the Fight Against SCLC: Standards, Innovations, and New Horizons. Cancers. 2025; 17(13):2256. https://doi.org/10.3390/cancers17132256
Chicago/Turabian StyleKemper, Marcel, Lea Elisabeth Reitnauer, Georg Lenz, Georg Evers, and Annalen Bleckmann. 2025. "Redefining the Fight Against SCLC: Standards, Innovations, and New Horizons" Cancers 17, no. 13: 2256. https://doi.org/10.3390/cancers17132256
APA StyleKemper, M., Reitnauer, L. E., Lenz, G., Evers, G., & Bleckmann, A. (2025). Redefining the Fight Against SCLC: Standards, Innovations, and New Horizons. Cancers, 17(13), 2256. https://doi.org/10.3390/cancers17132256






